Abstract
Pregnancy at high altitude is associated with a reduction in birth weight of ∼100 g/1000 m of ascent. The underlying mechanisms are unclear but may involve alteration in energy-demanding activities, such as protein synthesis. To test this hypothesis, both in vivo and in vitro approaches were used. Placental tissues from pregnant women residing at 3100 m were studied, and placental cells were incubated under hypoxia. In the 3100-m placentas, we observed dilation of endoplasmic reticulum (ER) cisternae, increased phosphorylation of eukaryotic initiation factor 2 subunit α (P-eIF2α), reduced AKT phosphorylation, and reduced P-4E-BP1 but increased 4E-BP1 protein compared to sea level controls. These findings suggest the presence of ER stress and protein synthesis inhibition. Hypoxia (1% O2) reduced proliferation of trophoblast-like JEG-3 cells, BeWo cells, and placental fibroblasts by ∼40, ∼60, and ∼18%, respectively. Sublethal dosage of salubrinal, an eIF2α phosphatase inhibitor, increased P-eIF2α and reduced BeWo cell and placental fibroblast proliferation by ∼50%. Administration of the PI-3K inhibitor LY294002 also reduced JEG-3 proliferation. Our results demonstrate that exposure to chronic hypobaric hypoxia causes mild placental ER stress, which, in turn, modulates protein synthesis and slows proliferation. These effects may account for the reduced placental villous volume, and contribute to the low birth weight that typifies high-altitude populations.—Yung, H. W., Cox, M., Tissot van Patot, M., Burton, G. J. Evidence of endoplasmic reticulum stress and protein synthesis inhibition in the placenta of non-native women at high altitude.
Keywords: AKT-mTOR signaling, birth weight, hypoxia, pregnancy
Pregnancy at high altitude represents an experiment of nature in which the fetoplacental unit is exposed to chronic hypobaric hypoxia. Many studies have confirmed that it is associated with an increased incidence of small for gestational age (SGA) babies, reflected in a fall in birth weight of ∼100 g/1000 m (1–3). The effect is dependent on the ethnicity and high-altitude ancestry of the population concerned, being greatest in recent migrants of nonindigenous origin (4, 5), but it is independent of other risk factors, such as smoking and socioeconomic class (2).
Chronic hypoxia is considered a key component of the pathophysiology of the growth restriction in high-altitude pregnancies. This is supported by the finding that incubation of chicken eggs in a hypoxic environment is sufficient to reduce weight at hatching (6). Maternal arterial Po2 decreases from 95 mmHg at sea level to ∼50 mmHg at 3500–4000 m (7, 8). This decrease is compensated for by an increase in the maternal hematocrit and hemoglobin concentration, which is equivalent in both indigenous and migrant populations (8, 9). However, oxygen delivery to the fetoplacental unit is also dependent on uterine arterial blood flow, and ultrasound studies have revealed that flow is significantly greater in indigenous populations compared to recent migrants (9, 10). Consequently, uterine artery oxygen delivery was found to be almost twofold greater at 36 wk of gestation in women of Andean ancestry compared to Europeans at high altitude in Bolivia (9).
Cells sense and respond to hypoxia through a variety of mechanisms, including oxygen-sensitive transcription factors, such as the hypoxia-inducible factor (HIF) family, changes in redox potential and stress response signaling pathways activated by reactive oxygen species (ROS), alterations in oxygen-sensitive ion channels, and through raised concentrations of ATP metabolites. Recently, it has been recognized that the unfolded protein response (UPR) can also be activated through hypoxia (11). Protein synthesis is an energy-demanding processes, estimated to account for ∼30% of total placental oxygen consumption (12). In addition, protein folding is an oxidative process that requires oxygen as a substrate. Hence, it is beneficial to suppress nonessential protein synthesis during periods of hypoxia. A reduction of placental protein synthesis during gestation at high altitude would be predicted to reduce placental size through effects on cell proliferation. Although placental weight is often not changed at high altitude, stereological studies have revealed that the volumetric composition is altered, with a reduction in absolute villous volume and surface area, and a corresponding increase in the volume of maternal blood within the intervillous space (13, 14).
Prolonged hypoxia in vitro is associated with a biphasic mechanism for reducing nonessential protein synthesis. The initial reduction is a result of increased phosphorylation of eukaryotic initiation factor 2 subunit α (eIF2α), followed later by dephosphorylation of eukaryotic initiation factor 4 subunit E binding protein (4E-BP1) (15).
At least 4 kinases phosphorylate eIF2α in response to a variety of stimuli or stresses, including heme-regulated inhibitor (HRI) for hypoxia and oxidative stress, and PKR-like endoplasmic reticulum kinase (PERK) for endoplasmic reticulum (ER) stress (16). Hypoxia induces ER stress through the generation of ROS, which triggers a homeostatic adaptation to conserve energy in order to ensure cell survival under a low-oxygen environment (17, 18). Increased levels of phosphorylated eIF2α inhibit protein synthesis and cell proliferation (19), while mutation of the eIF2α regulatory residue Ser51 to Ala increases basal protein synthesis rate by ∼30% (20). Impairment of ER stress response pathways, through loss of function mutation of PERK or eIF2α, reduces cell survival under hypoxia (17).
Translation initiation is also regulated by 4E-BP1, which in its unphosphorylated state binds to the eIF4E subunit of the cap-binding protein complex and so inhibits protein synthesis (15). Interestingly, a recent publication by Yamaguchi et al. (21) demonstrated that activating transcription factor 4 (ATF4), downstream of eIF2α, mediates induction of 4E-BP1. This induction is important for pancreatic β-cell survival under conditions of ER stress and provides a mechanistic link between the two systems of hypoxic control. The phosphorylation status of 4E-BP1 is regulated by the protein kinase B (AKT)–mammalian target of rapamycin (mTOR) pathway, which enhances phosphorylation and consequently facilitates protein translation. AKT-mTOR signaling is the central regulatory pathway for cell survival, metabolism, growth, and proliferation. Full activation of AKT depends on the phosphorylation level at both the Thr308 and Ser473 residues (22). Thr308 is the target substrate of 3-phosphoinositide-dependent protein kinase 1 (PDK1; ref. 22), while Ser473 is phosphorylated by mTOR complex 2 (mTORC2; ref. 23). AKT regulates mTOR signaling by two mechanisms, directly by phosphorylating mTOR at Ser2448 (24) or indirectly by phosphorylating its inhibitory protein, tuberous sclerosis complex 2 (TSC2)/tuberin, at Thr1462 (25). mTOR complex 1 (mTORC1), in turn, regulates protein translation via 4E-BP1 (26) and p70 ribosomal protein S6 kinase 1 (S6K1; ref. 27). The feedback loop of mTORC2 to phosphorylate AKT Ser473 could provide a fine-tuning mechanism in the control of protein translation. We recently demonstrated that ER stress attenuates AKT protein translation (28) and that ER stress modulates AKT downstream substrate specificity in a severity-dependent manner (29). Hence, reinforcing feed-forward cycles may easily be established under conditions of stress.
We have previously reported an association between reduced placental villous volume, birth weight, and evidence of altered metabolism in non-native women residing at 3100 m (30, 31). In this study, we investigated evidence of ER stress and protein synthesis inhibition in placentas obtained from these pregnancies. In addition, we tested whether hypoxia is sufficient to trigger the same changes in placental cells in vitro. The importance of P-eIF2α and P-AKT in the regulation of placental cell proliferation was also tested using the eIF2α phosphatase inhibitor salubrinal, and a phosphatidylinositol 3-kinase (PI-3K) inhibitor.
MATERIALS AND METHODS
All chemicals and tissue culture reagents were purchased from Sigma-Aldrich (Gillingham, UK) and Invitrogen (Paisley, UK), respectively, except where otherwise mentioned. Salubrinal was from Chem-Bridge Corp. (San Diego, CA, USA). The majority of the antibodies, including anti-P-eIF2α (Ser51), anti-eIF2α, anti-P-4E-BP1(Ser65), anti-P-4E-BP1(Thr37/46), anti-4E-BP1, anti-AMP-activated kinase (AMPK) (Thr172), anti-AMPK, anti-P-TSC2 (Thr1462), anti-TSC2, anti-P-p38 kinase (Thr180/Tyr-182), anti-p38 kinase, anti-phosphorylated heat-shock protein 27 (P-HSP27) (Ser82), anti-HSP27, anti-P-AKT(Ser473), anti-AKT, anti-phosphorylated glycogen synthase kinase 3β (P-GSK3β) (Ser9), and anti-GSK3β were from Cell Signaling Technology (NEB, Hitchin, UK). Anti-P-PERK (Thr980), anti-PERK, and anti-P-AKT (Thr308) were from Santa Cruz Biotechnology (New Insight Technology, Wembley, UK), and anti-glucose-regulated protein 78 (GRP78) was from Transduction Laboratories (BD Biosciences, Oxford, UK). Anti-β-actin was from Sigma-Aldrich. Anti-4-HNE was from Alexis Biochemicals (Enzo Life Sciences, Exeter, UK).
Placental sample collection
The placentas were obtained from patients who provided written informed consent, and the procecdures were approved by the local ethics committees at the University of Colorado Health Sciences Center (Aurora, CO, USA) and University College Hospital London (London, UK), as well as the Cambridge Local Research Ethics Committee. Exclusion criteria included smoking, renal disease, cardiac disease, diabetes, chronic hypertension, pregnancy-induced hypertension, or any other complications of pregnancy, and known risk factors for these conditions. All placentas were from term deliveries, either by elective caesarean section or vaginal delivery. The high-altitude placentas were collected at St. Vincent's General Hospital (Leadville, CO, USA; 3100 m) from stable, but recently migrant, populations of European descent. The sea-level placentas were collected at the Rosie Hospital (Cambridge, UK) and University College Hospital (London, UK). The samples were collected immediately after delivery by the same team at each site, eliminating differences in tissue handling. Samples were obtained using a systematic random system by which the placenta was divided into 5 areas. Two full-thickness samples were taken from each area, washed in PBS to remove excess blood contamination, snap-frozen in liquid nitrogen within 10 min of delivery, and then maintained at −80°C until further processed. Samples for immunohistochemistry were immediately submersed in formalin to be fixed for 12–24 h. After being treated with 70% ethanol for 24 h, placental samples were further dissected before being embedded in paraffin.
Cell culture
The human choriocarcinoma cell line JEG-3 cells, primary human placental fibroblasts, and primary human cytotrophoblasts were gifts from Professor Ashley Moffett (University of Cambridge). The human choriocarcinoma cell line BeWo cells were a gift from Dr. Stephen Charnock-Jones (University of Cambridge). In brief, JEG-3 cells were cultured in RPMI 1640 medium supplemented with 5% heat-inactivated FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml). BeWo cells were cultured in DMEM/F12 medium supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine and the same antibiotics. Placental fibroblasts were cultured in DMEM and 10% HI-FBS with the same antibiotics. All cells were cultured in a humidified incubator at 37°C in a 5% CO2 atmosphere.
Hypoxic incubation
Cells were seeded at low density with normal growth medium with serum and immediately put inside a hypoxic chamber (X vivo; BioSpherix, Lacona, NY, USA) containing 1% O2-5% CO2 balanced in nitrogen for 3 d. The control cells were incubated at 20% O2-5% CO2 under standard culture conditions.
Cell viability assay
The percentage of cell death was determined by costaining with 2 nuclear dyes: Hoechst 33342 (Sigma-Aldrich) and propidium iodide (PI; Sigma-Aldrich), as described previously (28). In brief, cells were incubated with 5 μg/ml of Hoechst 33342 and 5 μg/ml of PI for 10 min. The numbers of Hoechst 33342-positive cells (blue) and PI-positive cells (red/pink) were counted separately under UV fluorescence using a Leitz DM1L microscope (Leica Microsystems, Wetzlar, Germany). All fragmented or highly condensed nuclei were scored as apoptotic, whereas PI-positive cells with normal nuclear size were scored as necrotic.
Western blot analysis
Only elective nonlabor caesarean placentas were used for the Western blot analysis, which reduced the sample size to 6 placentas from sea level and 3 from 3100 m. To compensate for the small sample size, tissues from 2 and 4 regions/placenta were used from sea-level and 3100-m donor, respectively. The samples were divided evenly into 2 groups, with each group containing 12 samples, 6 from sea level (1 sample/placenta) and 6 from 3100 m (2 samples/placenta).
Details of the procedures were described previously (28). In brief, bicinchoninic acid was used to determine protein concentration in the tissue lysate. Equal amounts of proteins were resolved into SDS-PAGE and transferred to a nitrocellulose membrane. After incubation with primary and secondary antibodies, enhanced chemiluminescence (ECL; GE Healthcare, Little Chalfont, UK) and X-ray film (Kodak, Hempstead, UK) were used to detect the bands. Multiple exposure times were employed when necessary. The unsaturated bands were scanned using HP Scanjet G4050 (HP, Bracknell, UK) and band intensity was quantified by ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA). The two groups of samples were analyzed separately.
Immunohistochemistry
After fixation in paraformaldehyde (12–24 h), the tissues were passed through ascending concentrations of ethanol prior to paraffin embedding, and were subsequently sectioned (7 μm). After dewaxing and rehydration, endogenous peroxidases were inhibited by incubation with H2O2 (3%, 30 min) followed by blocking with nonimmune serum (20 min). Primary antibody anti-4HNE was applied (4°C for overnight), and binding was detected using Vectastain Elite ABC kits (Vector Laboratories, Peterborough, UK) and SigmaFast DAB (Sigma-Aldrich), according to the manufacturers' instructions. Sections were then counterstained with hematoxylin. Negative controls were performed by replacement with equal concentrations of nonimmune or isotype-matched irrelevant control.
RT-PCR analysis of X-box binding protein 1 (XBP1) mRNA splicing
Total RNA was isolated from placental tissues using an RNeasy mini kit (Qiagen, Crawley, UK), as described previously (28). Tissues were homogenized with lysis buffer using a FastPrep 24 homogenizer (MP Bio, Derby, UK). Total RNA (1 μg) was used for first-strand cDNA synthesis using Superscript First-Strand Synthesis System (Invitrogen). PCR was used to amplify XBP-1 mRNA, (30 cycles at 94°C for 30 s; 58°C for 30 s; and 72°C for 1 m). Forward and reverse primer sequences were 5≪-CTGGAACAGCAAGTGGTAGA-3 ≪ and 5≪-CTGGGTCCTTCTGGGTAGAC-3≪, respectively. PCR products were resolved by agarose gel (2%) electrophoresis with ethidium bromide and documented on a UVP Gel Documentation system (UVP, Cambridge, UK); 398- and 424-bp fragments represented spliced and unspliced XBP-1 transcripts, respectively. JEG-3 cells treated with tunicamycin (5 μg/ml, 24 h) were used as a positive control.
Quantitative real-time RT-PCR
Quantitative RT-PCR was performed by TaqMan with Absolute QPCR ROX mix (Thermo Fisher Scientific, Loughborough, UK) using 7500 Fast real-time PCR System (Applied Biosystems, Warrington, UK). The human 4E-BP1 (Hs00607050_m1) and 18S ribosomal RNA (Hs99999901_s1) probes were from Applied Biosystems (Foster City, CA, USA).
Electron microscopy
Placentas were fixed immediately in 2.5% glutaraldehyde in 0.1 M cacodylate buffer for 4 h, postfixed in 1% osmium, and embedded in Araldite resin. Thin sections were stained with lead citrate and uranyl acetate, and viewed in a Philips CM100 microscope (Philips, Amsterdam, The Netherlands).
Statistical analysis
Differences between averages or means were tested using either a 2-tailed Student's t test or nonparametric Mann-Whitney U test, with a value of P < 0.05 considered significant.
RESULTS
A total of 33 nonpathological placentas were collected from patients at sea level and 3100 m. At sea level, of the 14 placentas collected, 6 were from labored caesarean deliveries, and 8 were from elective nonlabored Caesarean deliveries. At 3100 m altitude, of the 19 placentas collected, 15 were from vaginal deliveries, 1 was from a labored caesarean, and 3 were from elective nonlabored caesarean deliveries. Clinical characteristics of the patients are summarized in Table 1. There was no significant difference in maternal age and gestational age between the two groups. However, there was a significant reduction in birth weight (∼300 g) in the high-altitude group, and placental weight trended lower as well.
Table 1.
Clinical characteristics of all the placental samples collected
| Parameter | Sea level | 3100 m | P |
|---|---|---|---|
| n | 14 | 19 | |
| Maternal age (yr) | 29.3 ± 5.7 | 28.2 ± 6.1 | 0.78 |
| Gestational age (wk) | 39.2 ± 1.4 | 39.8 ± 1.7 | 0.29 |
| Birth weight (g) | 3554 ± 375 | 3240 ± 382 | 0.025* |
| Range | 2740–4000 | 2610–3840 | |
| Median | 3640 | 3220 | |
| Placental weight (g) | 595 ± 117 | 548 ± 99 | 0.23 |
| Range | 460–900 | 280–680 | |
| Median | 560 | 570 |
Values are expressed as means ± se. Reduction in birth weight by ∼300 g at high altitude was considered significant.
P < 0.05.
The process of labor is a powerful inducer of oxidative stress (32), which activates phosphorylation of eIF2α during mild hypoxia (28). Therefore, only the placentas delivered by elective nonlabored caesarean section (sea level, n=6; 3100 m, n=3) were used for further analysis, and their clinical characteristics are presented in Table 2. Because of high variability, there was no significant difference in either fetal or placental weight between the sea-level and 3100-m placental samples chosen for Western blot analysis.
Table 2.
Clinical characteristics of the placental samples used for the experiments
| Parameter | Sea level | 3100 m | P |
|---|---|---|---|
| n | 6 | 3 | |
| Maternal age (yr) | 28.5 ± 5.6 | 34 ± 1 | 0.063 |
| Gestational age (wk) | 38.3 ± 0.4 | 39.9 ± 1.2 | 0.13 |
| Birth weight (g) | 3470 ± 419.4 | 3263.3 ± 502.4 | 0.58 |
| Range | 2830–4000 | 2920–3840 | |
| Placental weight (g) | 581.7 ± 148.3 | 530 ± 34.6 | 0.33 |
| Range | 460–900 | 510–570 |
Values are expressed as means ± se.
Elevation of eIF2α phosphorylation in high-altitude placentas
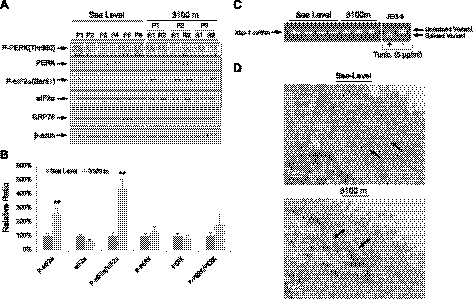
The hypoxic ER stress response resulting in suppressed protein synthesis is mediated by P-eIF2α (18). On ER stress, eIF2α is phosphorylated by PERK. We observed a significant 4.6-fold elevation of P-eIF2α in the high-altitude samples, and an associated trend for a rise in P-PERK/PERK (Fig. 1A, B). The levels of GRP78 protein, and of the spliced variant of Xbp-1 mRNA, were unchanged (Fig. 1C). To further demonstrate the existence of ER stress, electron microscopy was used to examine the ultrastructure of the ER cisternae. Moderate dilation of the cisternae was observed in the syncytiotrophoblast of the 3100-m placentas, but not in the sea-level controls (Fig. 1D). These results indicated the presence of placental ER stress, but that the level of stress was relatively mild (28).
Figure 1.
Evidence of ER stress in placentas from high altitude. A) Phosphorylation of PERK and eIF2α and total protein level of PERK, eIF2α, and GRP78 were measured by Western blot analysis. β-Actin was used as loading control. B) Densitometric quantification of band intensity. Levels of phosphorylated and total protein are presented as a ratio to the sea-level control (100%). Data are means ± se from all 24 samples. **P < 0.01. C) Splicing of Xbp-1 mRNA was analyzed by RT-PCR; positive and negative controls were set up by treatment of the JEG-3 cells in the presence or absence of 5 μg/ml tunicamycin for 24 h. D) Electron microscopy showed dilation of ER cisternae at high altitude, but not at sea level. Arrows indicate ER cisternae. Scale bars = 500 nm.
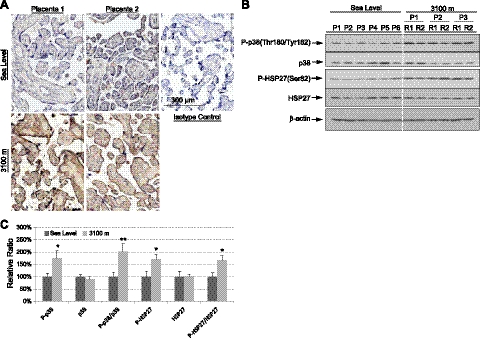
Hypoxia causes oxidative stress, a strong stimulus for ER stress (28, 33), and ROS increase phosphorylation of PERK, and thus of eIF2α (18). To examine whether oxidative stress was positively associated with the higher P-eIF2α observed, lipid peroxidation was determined in the sea-level and high-altitude placental sections using antibodies directed against 4-hydroxynonenal (HNE). There was strong positive immunoreactivity in the high-altitude placentas, with the majority of the staining in the syncytiotrophoblast, yet little in the sea-level samples (Fig. 2A). Activation of p38 kinase and its downstream substrate HSP27 are closely correlated with oxidative stress (34). Phosphorylation of p38 kinase and HSP27 were elevated significantly by >1.7-fold in the high-altitude placentas, further confirming the existence of oxidative stress (Fig. 2B, C).
Figure 2.
Increase of oxidative stress in high-altitude placentas. A) Placental sections were immunohistochemically stained with anti-4HNE antibody, an indicator of lipid peroxidation. The majority of staining was in the syncytiotrophoblast. Scale bar = 300 μm. B) Phosphorylation of p38 kinase and HSP27 and total protein level of p38 kinase and HSP27 were measured by Western blot analysis. β-Actin was used as loading control. C) Densitometric quantification of band intensity. Levels of phosphorylated and total protein are presented as a ratio to the sea-level control (100%). Data are means ± se from all 24 samples. *P < 0.05; **P < 0.01.
Elevation of 4E-BP1 in high-altitude placentas
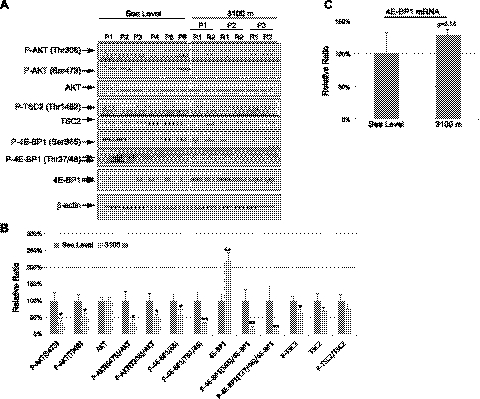
Because the next phase of protein translation inhibition in response to prolonged hypoxia is mediated by hypophosphorylation of 4E-BP1, we determined levels of phosphorylated and total 4E-BP1 in the placental samples. Phosphorylation of 4E-BP1 at Ser65, which causes the greatest reduction in binding affinity to eIF4E (35), was reduced, yet total 4E-BP1 was increased by ∼2.2-fold, in placentas from 3100 m, as compared with sea-level controls (Fig. 3A, B). An increase of 4E-BP1 protein concentration could be due to both increased translation and transcription, as the 4E-BP1 transcript showed a trend to increase, although this did not reach statistical significance (Fig. 3C).
Figure 3.
Reduction of AKT-mTOR signaling in high-altitude placentas. A) Proteins were extracted from nonlabored elective Caesarean placentas at both sea level and 3100 m. Western blot analysis was used to measure phosphorylated and total protein levels of P-AKT (Ser473), P-AKT (Thr308), AKT, P-TSC2 (Thr1462), TSC2, P-4E-BP1 (Ser65), P-4E-BP1 (Thr37/46), and 4E-BP-1. β-Actin was used as a loading control. B) Densitometric quantification of band intensity. Levels of phosphorylated and total protein are presented as a ratio to the sea-level control (100%). Data are means ± se from all 24 samples. *P < 0.05; **P < 0.01. C) No change in 4E-BP1 transcript level. qRT-PCR was used to measure the 4E-BP1 mRNA level by TaqMan; 18S was used as a house-keeping gene.
Dephosphorylation of 4E-BP1, and thus reduction of cap-binding translation, occurs via dephosphorylation of AKT and mTORC1. Therefore, we investigated whether the reduction of placental weight at high altitude is also linked to changes in AKT-mTORC1 signaling. We observed a significant reduction of P-AKT at both Thr308 and Ser473 residues in placentas from 3100 m compared to sea-level controls (Fig. 3A, B). TSC2 binds to mTOR complex and inhibits mTOR activity, and phosphorylation of TSC2 by AKT at residue Thr1462 prevents this process (25). Loss of AKT phosphorylation was associated with a significant reduction of P-TSC2 in the high-altitude placentas. Furthermore, Thr37 and Thr46 phosphorylation sites of 4E-BP-1 are direct target substrates for mTORC1 (26). We observed ∼80% reduction in P-4E-BP1 (Thr37/46) in the high-altitude placentas (Fig. 3A, B). As Ser473 residue of AKT is a direct phosphorylation site for mTORC2 (36), these results suggested a loss of both mTORC1 and mTORC2 activity in the high-altitude placentas.
In summary, increased phosphorylation of eIF2α and up-regulation of 4E-BP1 total protein level in association with its hypophosphorylation indicate protein synthesis inhibition. Loss of AKT-mTOR signaling is likely to contribute to the 4E-BP1 hypophosphorylation.
Hypoxia induces eIF2α phosphorylation, reduces AKT phosphorylation, and slows cell proliferation
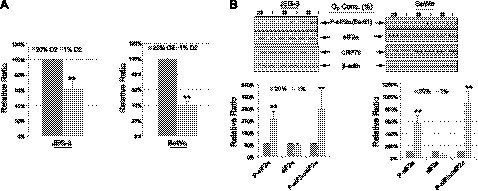
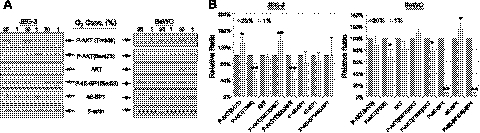
To investigate further the links between increased P-eIF2α, lower P-AKT, and the smaller placental size at 3100 m, we investigated the effects of hypoxia in vitro on these pathways and on cell proliferation in two human choriocarcinoma cell lines, JEG-3 and BeWo. Cells were exposed to 1 or 20% O2 for JEG-3 cells and BeWo cells for 3 d. Cell proliferation assays showed there was a ∼40 and ∼60% reduction of cell proliferation rate in JEG-3 and BeWo cells, respectively, in response to 1% O2 (Fig. 4A). Hypoxia treatment induced phosphorylation of eIF2α by 2.7- and ∼7-fold in JEG-3 and BeWo cells, respectively (Fig. 4B). However, there was no change in the ER chaperone GRP78 in either cell type (Fig. 4B), similar to the results found in the high-altitude placentas.
Figure 4.
Hypoxia reduces trophoblast cell proliferation and induces eIF2α phosphorylation. A) Both JEG-3 and BeWo cells were cultured at either 20 or 1% O2 in normal culture medium for 3 d. Number of cells was counted using a hemocytometer. Experiments were done in duplicate and repeated 3 times. Data presented are means ± sd; the value of 20% O2 is used as a control and expressed as 100%. B) Top panel: proteins were isolated from both JEG-3 cells and BeWo cells after 3 d incubation and subjected to Western blot analysis. Anti-P-eIF2α, anti-eIF2α, and anti-GRP78 antibodies were used. β-Actin was used as a loading control. Bottom panel: densitometric quantification of band intensity. Levels of phosphorylated and total protein are presented as a ratio to the untreated control (100%). Data are means ± sd from 3 independent experiments. **P < 0.01.
Hypoxia inhibited the phosphorylation of AKT at the Thr308 residue in both cell types, but not at the Ser473 residue. The latter slightly increased in JEG-3 cells, but remained constant in BeWo cells (Fig. 5). Acute hypoxia caused a 90% reduction in P-4E-BP1 and a slight increase of total 4E-BP1 protein in BeWo cells (Fig. 5) but had no effect on JEG-3 cells.
Figure 5.
Differential effect of hypoxia on AKT and 4E-BP1 phosphorylation in JEG-3 and BeWo cells. JEG-3 cells and BeWo cells were incubated either at 20 or 1% O2 for 3 d. A) Proteins were isolated and subjected to Western blot analysis to measure P-AKT (Thr308), P-AKT (Ser473), AKT, P-4E-BP1 (Ser65) and 4E-BP1. β-Actin was used as loading control. B) Densitometric quantification of band intensity. Levels of phosphorylated and total protein are presented as a ratio to the untreated control (100%). Data are means ± sd from 3 independent experiments. *P < 0.05; **P < 0.01.
The same experiments were also performed on primary human placental fibroblasts. Hypoxia appeared to have relatively mild effects, causing an ∼18% reduction in cell proliferation rate and a small, but statistically significant, increase in the ratio of P-eIF2α/eIF2α. Although hypoxia inhibited AKT phosphorylation at both Thr308 and Ser473 residues by ∼30%, it did not affect either phosphorylation or expression of 4E-BP1 (Supplemental Fig. S1A–C). These results suggest a differential susceptibility of placental cell types to hypoxia.
Elevation of eIF2α phosphorylation or reduction of AKT phosphorylation is sufficient to slow cell proliferation
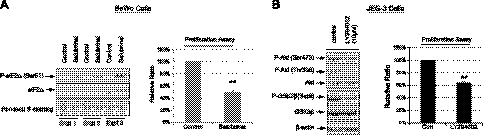
Protein synthesis is one of the crucial factors determining cell proliferation rate (37). We have previously demonstrated that a phosphatase inhibitor, salubrinal, which blocks eIF2α dephosphorylation specifically (38), increases P-eIF2α and is associated with 50% reduction in JEG-3 cell proliferation rate without induction of cell death (39). Here, we further tested this effect on BeWo cells. Application of salubrinal to BeWo increased phosphorylation of eIF2α greatly (Fig. 6A, left panel). Notably, there was an ∼50% in reduction cell proliferation rate (Fig. 6A, right panel), indicating that phosphorylation of eIF2α is sufficient to slow cell proliferation. A similar reduction of cell proliferation was also observed in placental fibroblasts (Supplemental Fig. S1D).
Figure 6.
Inhibition of AKT activity and increased phosphorylation of eIF2α reduce proliferation in placental cells. A) Left panel: BeWo cells were incubated with the eIF2α phosphatase inhibitor salubrinal (17.5 μM) for 3 d. Western blot analysis was used to measure eIF2α phosphorylation. No cell death was detected after incubation. Right panel: proliferation rate of BeWo cells was reduced under salubrinal treatment. B) Left panel: JEG-3 cells were incubated with sublethal dosage (10 μM) of the PI-3K inhibitor LY294002 for 2 d. Western blot analysis was used to show the inhibitory effect of LY294002 on AKT phosphorylation; GSK-3 phosphorylation was used as a readout of AKT activity. Right panel: proliferation rate of JEG-3 cells was reduced under LY294002 incubation. For cell proliferation assay, the number of cells was counted using a hemocytometer. Experiments were done in duplicate and repeated 3 times. Data presented are means ± sd, with the value of the untreated cells used as a control and expressed as 100%. **P < 0.01.
To further confirm that AKT activity is also crucial in the regulation of JEG-3 cell proliferation, cells were treated with the PI-3K inhibitor LY294002, which inhibits AKT phosphorylation. At a concentration of 10 μM LY294002, it did not induce cell death (data not shown), but it was sufficient to reduce the phosphorylation of AKT at both Ser473 and Thr308 residues. Phosphorylation of the downstream substrate of AKT, GSK-3β, was also decreased, further confirming the inhibition of AKT activity by LY294002 (Fig. 6B, left panel). Again, there was ∼40% reduction in cell proliferation rate (Fig. 6B, right panel). These results confirmed that the loss of AKT phosphorylation observed in the high-altitude placentas and the in vitro hypoxia model could also modulate cell proliferation, resulting in a smaller placenta that, in turn, restricts fetal growth. Indeed, the Akt1-deficient mouse displays a smaller placenta and fetus (39, 40).
DISCUSSION
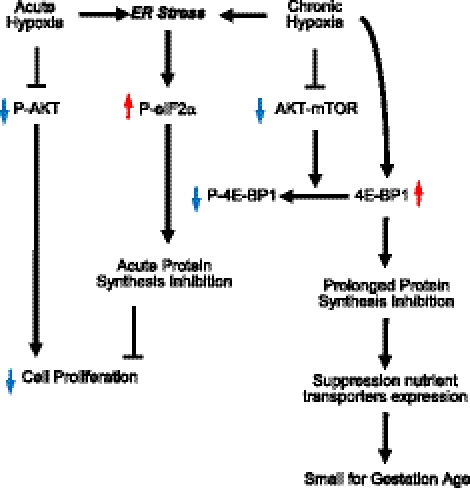
Our results demonstrate the presence of active pathways for the modulation of protein synthesis in the placentas from high-altitude pregnancies, which is likely to be the result of ER stress. In vitro studies further confirmed that hypoxia activates similar pathways in placental cells, resulting in protein synthesis inhibition and a subsequent reduction in cell proliferation. Together, these data suggest that placental hypoxia-induced protein synthesis inhibition could be one of the mechanistic explanations for the pathophysiology of SGA babies (Fig. 7). Although this conclusion is drawn from a small sample size, the remarkable consistency of the increased P-eIF2α and 4E-BP1 total protein levels, the two most important components regulating cap-dependent translation, observed across 4 different regions of each of the high-altitude placentas, suggest these findings are unlikely to be an artifact.
Figure 7.
Schematic diagram showing how protein synthesis inhibition may be linked to the growth restriction (SGA) caused by chronic hypoxia in high-altitude pregnancies.
Dilation of the endoplasmic reticulum cisternae has previously been reported in Nepalese placentas, although the full significance of the finding was not realized at that time (41). Protein synthesis is the most energy-demanding cellular process, and up to 35% of intracellular ATP production is used by the translation machinery in eukaryotic cells (42). During cell growth and proliferation, one of the primary limiting factors is the rate of protein translation. Total protein translation must reach a critical threshold level before cell replication is allowed to occur. In response to mitogenic signals, a series of signaling cascades may be triggered in order to activate protein translation machinery, while withdrawal of extracellular cues results in a reduction in protein synthesis and exit from the cell cycle (37).
The majority of ATP production takes place in mitochondria, where oxygen molecules are used as the final electron acceptor. Therefore, limitation of oxygen availability during chronic hypoxia at high altitude likely affects this process, resulting in a potential energy crisis. Our previously published data showed that although there is a significant reduction of the ATP:ADP ratio in the same high-altitude placentas, the concentrations of ATP and ADP show no significant difference between sea level and high altitude (31). This may reflect a metabolic reprogramming, with an increase in anaerobic glycolysis and a reduction in mitochondrial oxygen consumption (43). Interestingly, we have recently found that the protein levels of at least one subunit of mitochondrial complex I and/or complex IV are reduced in both the high-altitude placentas and placental cells maintained at 1% O2 (44). Mitochondrial respiratory activity can also be inhibited by phosphocreatine, which is used as an energy reserve to maintain ATP levels (45). Indeed, there is a trend toward higher concentrations of phosphocreatine in these high-altitude placentas (31), suggesting the placentas are preconditioned to store energy for use during hypoxic stress. Similar observations have been reported in the rat hippocampus, in which phosphocreatine concentrations increase under chronic hypoxia (46). We did not observe any increase in phosphorylation of AMPK in the high-altitude placentas (Supplemental Fig. S2), indicating the placentas are unlikely to be suffering an energy crisis because of chronic hypoxia. This may reflect successful restoration of energy supply and demand as a result of activation of these homeostatic pathways.
To achieve the maximal efficiency in energy savings, regulatory mechanisms for protein synthesis have been evolutionarily established at the initiation stage and are regulated by a family of eIFs (47). Phosphorylation of eIF2α at Ser51 directly halts the translation initiation as it acts as a competitive inhibitor of eIF2B, thus blocking recycling of eIF2. Indeed, we observed elevation of P-eIF2α in the high-altitude placentas, as well as in response to hypoxic incubation of JEG-3 and BeWo (Figs. 1 and 4B). This mechanism does not block translation initiation for all mRNAs. Those mRNAs containing small upstream open reading frames (uORFs) within their 5′-UTR regions or internal ribosome entry site (IRES) sequences are selectively translated independent of eIF2α regulation (48, 49). It has been shown that many stress-response and essential genes, including ATF4, ER chaperones (GRP78 and GRP94), N-myc downstream regulated gene 1 (NDRG1) and vascular endothelial growth factor A (VEGF-A) are able to bypass this regulation and increase their expression on ER stress (49–52). This phenomenon may explain the increase of total 4E-BP1 protein that we observed (Fig. 3), for ATF4 can induce expression of 4E-BP1 (21).
Koritzinsky et al. (15) reported a biphasic hypoxic stress response in the protein synthesis inhibition pathways, in which the P-PERK/P-eIF2α pathway is initiated within 1 h of anoxia, and by 16 h, there is a transition to predominant control by the P-AKT/4E-BP1 pathway. Both pathways remain active, but the P-PERK/P-eIF2α pathway activity is reduced. We did observe a dramatic inhibition of P-4E-BP1 phosphorylation and a significant increase of total 4E-BP1 in BeWo cells under hypoxia for 3 d, but not in JEG-3 and placental fibroblasts. This difference is likely due to the differential susceptibility of the cells to the hypoxic environment, possibly reflecting their endocrine activities. This speculation is supported by the cell proliferation assay, in which there was an 18, 40, and 60% suppression of cell proliferation in placental fibroblasts, JEG-3 cells, and BeWo cells, respectively.
It is apparent that after the initial eIF2α response, translation initiation is primarily regulated by AKT-mTOR-4E-BP1 signaling (15). 4E-BP1 regulates cap-dependent translation initiation by binding to eIF4E, preventing recruitment of mRNA to ribosomes (53). Phosphorylation of 4E-BP1 by mTORC1 blocks this interaction (54). In high-altitude placentas, there was a significant decrease in AKT phosphorylation and P-4E-BP1 (Fig. 3), suggesting loss of AKT-mTOR signaling. Interestingly, we also observed a 2.2-fold increase of total 4E-BP1 in high-altitude placenta, suggesting a long-term role of 4E-BP1 in the prolonged inhibition of protein synthesis. High 4E-BP1 levels protect pancreatic β cells from ER stress-induced cell death (21), suggesting that the increase seen in the high-altitude placenta may perform a similar function.
Increased angiogenesis is an important adaptation under hypoxia, and studies have shown increased vascularity in the high-altitude placenta of indigenous populations (41, 55, 56). For many years, much emphasis has been placed on the role of HIF1α. However, ER stress has recently been identified as a regulator of angiogenesis through a HIF1α-independent pathway by direct regulation of VEGF mRNA expression (57, 58). IRE1 is another proximal sensor involved in ER stress pathways. Knockout of IRE1α genes reduces VEGF mRNA expression, as well as protein levels, and compromises placental vascularization in the labyrinthine zone of the mouse placenta (58). We did not observe any spliced variant of XBP-1 mRNA in the 3100-m placentas, indicating that IRE1α is not activated. This is consistent with the fact that the fractional volume of the villi occupied by the fetal capillaries was the same as in sea-level placentas (30). Differences in the ER response to hypoxia between indigenous and non-native populations could potentially explain the lack of an angiogenic response in the placentas studied here.
Taken together, ER stress appears on the one hand to conserve energy consumption, and on the other hand to promote angiogenesis for greater oxygen delivery. A recent publication from Bastide et al. (52) showed that the VEGF-A 5′UTR contains an uROF within an IRES that controls its translation, allowing VEGF-A to bypass P-eIF2α regulation on ER stress. Therefore, could the mild ER stress we observe at 3100 m be simply considered a homeostatic response, slowing cell proliferation through protein synthesis inhibition and thereby resulting in a lower birth weight that matches the maternal oxygen supply? This view is supported by the findings of Postigo et al. (8), who reported that fetal oxygen delivery is constant between altitude levels and ethnic groups when normalized to fetal weight.
Furthermore, we have observed more severe ER stress in placentas associated with intrauterine growth restriction of maternal vascular origin, in particular, when complicated with preeclampsia (39). In those instances, we saw evidence of activation of the IRE1α and apoptotic pathways, along with greater dilation of the ER cisternae. This differential activation of the ER stress response pathways can be recapitulated in vitro by applying increasing doses of tunicamycin to JEG-3 cells. Low doses cause only increased phosphorylation of eIF2α, whereas higher doses result in increased expression of the chaperone protein, GRP78, and activation of the IRE1α and the apoptotic pathways (39). Hence, we speculate that there is a spectrum of placental ER stress in vivo, stimulating a mild homeostatic response in the chronically hypoxic placenta at altitude and a more severe response with pathological consequences, in cases of deficient maternal spiral arterial conversion.
Supplementary Material
Acknowledgments
This work was supported by the Wellcome Trust (084804/2/08/Z).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 4E-BP1
- eukaryotic initiation factor 4 subunit E binding protein
- AKT
- protein kinase B
- AMPK
- AMP-activated kinase
- ATF4
- activating transcription factor 4
- eIF2α
- eukaryotic initiation factor 2 subunit α
- ER
- endoplasmic reticulum
- GRP78
- glucose-regulated protein 78
- GSK3β
- glycogen synthase kinase 3β
- HIF
- hypoxia-inducible factor
- HNE
- 4-hydroxynonenal
- HRI
- heme-regulated inhibitor
- HSP27
- heat-shock protein 27
- IRE1α
- inositol-requiring enzyme 1α
- mTOR
- mammalian target of rapamycin
- mTORC1
- mammalian target of rapamycin complex 1
- mTORC2
- mammalian target of rapamycin complex 2
- PDK1
- 3-phosphoinositide-dependent protein kinase 1
- PERK
- PKR-like endoplasmic reticulum kinase
- PI-3K
- phosphatidylinositol 3-kinase
- ROS
- reactive oxygen species
- S6K1
- S6 kinase 1
- SGA
- small for gestational age
- TSC2
- tuberous sclerosis complex 2
- UPR
- unfolded protein response
- VEGF-A
- vascular endothelial growth factor A
- XBP-1
- X-box binding protein 1.
REFERENCES
- 1. Moore L. G., Niermeyer S., Zamudio S. (1998) Human adaptation to high altitude: regional and life-cycle perspectives. Am. J. Physiol. Anthropol. Suppl. 27, 25–64 [DOI] [PubMed] [Google Scholar]
- 2. Giussani D. A., Phillips P. S., Anstee S., Barker D. J. (2001) Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr. Res. 49, 490–494 [DOI] [PubMed] [Google Scholar]
- 3. Keyes L. E., Armaza J. F., Niermeyer S., Vargas E., Young D. A., Moore L. G. (2003) Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr. Res. 54, 20–25 [DOI] [PubMed] [Google Scholar]
- 4. Moore L. G., Young D., McCullough R. E., Droma T., Zamudio S. (2001) Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am. J. Hum. Biol. 13, 635–644 [DOI] [PubMed] [Google Scholar]
- 5. Julian C. G., Vargas E., Armaza J. F., Wilson M. J., Niermeyer S., Moore L. G. (2007) High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch. Dis. Child. Fetal Neonatal. Ed. 92, F372–F377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giussani D. A., Salinas C. E., Villena M., Blanco C. E. (2007) The role of oxygen in prenatal growth: studies in the chick embryo. J. Physiol. 585, 911–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krampl E., Lees C., Bland J. M., Espinoza Dorado J., Moscoso G., Campbell S. (2000) Fetal biometry at 4300 m compared to sea level in Peru. Ultrasound Obstet. Gynecol. 16, 9–18 [DOI] [PubMed] [Google Scholar]
- 8. Postigo L., Heredia G., Illsley N. P., Torricos T., Dolan C., Echalar L., Tellez W., Maldonado I., Brimacombe M., Balanza E., Vargas E., Zamudio S. (2009) Where the O2 goes to: preservation of human fetal oxygen delivery and consumption at high altitude. J. Physiol. 587, 693–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Julian C. G., Wilson M. J., Lopez M., Yamashiro H., Tellez W., Rodriguez A., Bigham A. W., Shriver M. D., Rodriguez C., Vargas E., Moore L. G. (2009) Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1564–R1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore L. G., Zamudio S., Zhuang J., Sun S., Droma T. (2001) Oxygen transport in Tibetan women during pregnancy at 3,658 m. Am. J. Physiol. Anthropol. 114, 42–53 [DOI] [PubMed] [Google Scholar]
- 11. Wouters B. G., Koritzinsky M. (2008) Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat. Rev. Cancer 8, 851–864 [DOI] [PubMed] [Google Scholar]
- 12. Carter A. M. (2000) Placental oxygen consumption. Part I: in vivo studies–a review. Placenta 21(Suppl. A), S31–S37 [DOI] [PubMed] [Google Scholar]
- 13. Jackson M. R., Mayhew T. M., Haas J. D. (1987) The volumetric composition of human term placentae: altitudinal, ethnic and sex differences in Bolivia. J. Anat. 152, 173–187 [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson M. R., Mayhew T. M., Haas J. D. (1987) Morphometric studies on villi in human term placentae and the effects of altitude, ethnic grouping and sex of newborn. Placenta 8, 487–495 [DOI] [PubMed] [Google Scholar]
- 15. Koritzinsky M., Magagnin M. G., van den Beucken T., Seigneuric R., Savelkouls K., Dostie J., Pyronnet S., Kaufman R. J., Weppler S. A., Voncken J. W., Lambin P., Koumenis C., Sonenberg N., Wouters B. G. (2006) Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 25, 1114–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wek R. C., Jiang H. Y., Anthony T. G. (2006) Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 34, 7–11 [DOI] [PubMed] [Google Scholar]
- 17. Bi M., Naczki C., Koritzinsky M., Fels D., Blais J., Hu N., Harding H., Novoa I., Varia M., Raleigh J., Scheuner D., Kaufman R. J., Bell J., Ron D., Wouters B. G., Koumenis C. (2005) ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 24, 3470–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu L., Wise D. R., Diehl J. A., Simon M. C. (2008) Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J. Biol. Chem. 283, 31153–31162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koromilas A. E., Roy S., Barber G. N., Katze M. G., Sonenberg N. (1992) Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science 257, 1685–1689 [DOI] [PubMed] [Google Scholar]
- 20. Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S., Kaufman R. J. (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165–1176 [DOI] [PubMed] [Google Scholar]
- 21. Yamaguchi S., Ishihara H., Yamada T., Tamura A., Usui M., Tominaga R., Munakata Y., Satake C., Katagiri H., Tashiro F., Aburatani H., Tsukiyama-Kohara K., Miyazaki J., Sonenberg N., Oka Y. (2008) ATF4-mediated induction of 4E-BP1 contributes to pancreatic beta cell survival under endoplasmic reticulum stress. Cell Metab. 7, 269–276 [DOI] [PubMed] [Google Scholar]
- 22. Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 23. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 24. Nave B. T., Ouwens M., Withers D. J., Alessi D. R., Shepherd P. R. (1999) Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem. J. 344, 427–431 [PMC free article] [PubMed] [Google Scholar]
- 25. Inoki K., Li Y., Zhu T., Wu J., Guan K. L. (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657 [DOI] [PubMed] [Google Scholar]
- 26. Gingras A. C., Gygi S. P., Raught B., Polakiewicz R. D., Abraham R. T., Hoekstra M. F., Aebersold R., Sonenberg N. (1999) Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 13, 1422–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown E. J., Beal P. A., Keith C. T., Chen J., Shin T. B., Schreiber S. L. (1995) Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature 377, 441–446 [DOI] [PubMed] [Google Scholar]
- 28. Yung H. W., Korolchuk S., Tolkovsky A. M., Charnock-Jones D. S., Burton G. J. (2007) Endoplasmic reticulum stress exacerbates ischemia-reperfusion-induced apoptosis through attenuation of Akt protein synthesis in human choriocarcinoma cells. FASEB J. 21, 872–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yung H. W., Charnock-Jones D. S., Burton G. J. (2011) Regulation of AKT phosphorylation at Ser473 and Thr308 by endoplasmic reticulum stress modulates substrate specificity in a severity dependent manner. PLoS One 6, e17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Patot M. C., Valdez M., Becky V., Cindrova-Davies T., Johns J., Zwerdling L., Jauniaux E., Burton G. J. (2009) Impact of pregnancy at high altitude on placental morphology in non-native women with and without preeclampsia. Placenta 30, 523–528 [DOI] [PubMed] [Google Scholar]
- 31. Tissot van Patot M. C., Murray A. J., Beckey V., Cindrova-Davies T., Johns J., Zwerdlinger L., Jauniaux E., Burton G. J., Serkova N. J. (2010) Human placental metabolic adaptation to chronic hypoxia, high altitude: hypoxic preconditioning. Am. J. Physiol. Regul. Integr Comp. Physiol. 298, R166–R172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cindrova-Davies T., Yung H. W., Johns J., Spasic-Boskovic O., Korolchuk S., Jauniaux E., Burton G. J., Charnock-Jones D. S. (2007) Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am. J. Pathol. 171, 1168–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malhotra J. D., Kaufman R. J. (2007) Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 9, 2277–2293 [DOI] [PubMed] [Google Scholar]
- 34. Cindrova-Davies T., Spasic-Boskovic O., Jauniaux E., Charnock-Jones D. S., Burton G. J. (2007) Nuclear factor-κB, p38, and stress-activated protein kinase mitogen-activated protein kinase signaling pathways regulate proinflammatory cytokines and apoptosis in human placental explants in response to oxidative stress: effects of antioxidant vitamins. Am. J. Pathol. 170, 1511–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karim M. M., Hughes J. M., Warwicker J., Scheper G. C., Proud C. G., McCarthy J. E. (2001) A quantitative molecular model for modulation of mammalian translation by the eIF4E-binding protein 1. J. Biol. Chem. 276, 20750–20757 [DOI] [PubMed] [Google Scholar]
- 36. Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S. Y., Huang Q., Qin J., Su B. (2006) SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127, 125–137 [DOI] [PubMed] [Google Scholar]
- 37. Zetterberg A., Larsson O., Wiman K. G. (1995) What is the restriction point? Curr. Opin. Cell Biol. 7, 835–842 [DOI] [PubMed] [Google Scholar]
- 38. Boyce M., Bryant K. F., Jousse C., Long K., Harding H. P., Scheuner D., Kaufman R. J., Ma D., Coen D. M., Ron D., Yuan J. (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307, 935–939 [DOI] [PubMed] [Google Scholar]
- 39. Yung H. W., Calabrese S., Hynx D., Hemmings B. A., Cetin I., Charnock-Jones D. S., Burton G. J. (2008) Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am. J. Pathol. 173, 451–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Z. Z., Tschopp O., Hemmings-Mieszczak M., Feng J., Brodbeck D., Perentes E., Hemmings B. A. (2003) Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J. Biol. Chem. 278, 32124–32131 [DOI] [PubMed] [Google Scholar]
- 41. Soma H., Hata T., Oguro T., Fujita K., Kudo M., Vaidya U. (2005) Characteristics of histopathological and ultrastructural features of placental villi in pregnant Nepalese women. Med. Mol. Morphol. 38, 92–103 [DOI] [PubMed] [Google Scholar]
- 42. Siems W. G., Schmidt H., Gruner S., Jakstadt M. (1992) Balancing of energy-consuming processes of K 562 cells. Cell Biochem. Funct. 10, 61–66 [DOI] [PubMed] [Google Scholar]
- 43. Illsley N. P., Caniggia I., Zamudio S. (2010) Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int. J. Dev. Biol. 54, 409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Colleoni F., Yung H. W., Padmanabhan N., Cetin I., Tissot van Patot M. C., Burton G. J., Murray A. J. (2011) Hypoxia induces electron transport chain dysfunction in human placental mitochondria. Placenta 32, A46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Walsh B., Tonkonogi M., Soderlund K., Hultman E., Saks V., Sahlin K. (2001) The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J. Physiol. 537, 971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Raman L., Tkac I., Ennis K., Georgieff M. K., Gruetter R., Rao R. (2005) In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Brain Res. Dev. Brain Res. 156, 202–209 [DOI] [PubMed] [Google Scholar]
- 47. Pestova T. V., Kolupaeva V. G., Lomakin I. B., Pilipenko E. V., Shatsky I. N., Agol V. I., Hellen C. U. (2001) Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. U. S. A. 98, 7029–7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jan E., Thompson S. R., Wilson J. E., Pestova T. V., Hellen C. U., Sarnow P. (2001) Initiator Met-tRNA-independent translation mediated by an internal ribosome entry site element in cricket paralysis virus-like insect viruses. Cold Spring Harb. Symp. Quant. Biol. 66, 285–292 [DOI] [PubMed] [Google Scholar]
- 49. Lu P. D., Harding H. P., Ron D. (2004) Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167, 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim Y. K., Jang S. K. (2002) Continuous heat shock enhances translational initiation directed by internal ribosomal entry site. Biochem. Biophys. Res. Commun. 297, 224–231 [DOI] [PubMed] [Google Scholar]
- 51. Choi S. J., Oh S. Y., Kim J. H., Sadovsky Y., Roh C. R. (2007) Increased expression of N-myc downstream-regulated gene 1 (NDRG1) in placentas from pregnancies complicated by intrauterine growth restriction or preeclampsia. Am. J. Obstet. Gynecol. 196, 45 e41–e47 [DOI] [PubMed] [Google Scholar]
- 52. Bastide A., Karaa Z., Bornes S., Hieblot C., Lacazette E., Prats H., Touriol C. (2008) An upstream open reading frame within an IRES controls expression of a specific VEGF-A isoform. Nucleic Acids Res. 36, 2434–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gingras A. C., Raught B., Sonenberg N. (1999) eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68, 913–963 [DOI] [PubMed] [Google Scholar]
- 54. Gingras A. C., Raught B., Gygi S. P., Niedzwiecka A., Miron M., Burley S. K., Polakiewicz R. D., Wyslouch-Cieszynska A., Aebersold R., Sonenberg N. (2001) Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 15, 2852–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reshetnikova O. S., Burton G. J., Milovanov A. P. (1994) Effects of hypobaric hypoxia on the fetoplacental unit: the morphometric diffusing capacity of the villous membrane at high altitude. Am. J. Obstet. Gynecol. 171, 1560–1565 [DOI] [PubMed] [Google Scholar]
- 56. Espinoza J., Sebire N. J., McAuliffe F., Krampl E., Nicolaides K. H. (2001) Placental villus morphology in relation to maternal hypoxia at high altitude. Placenta 22, 606–608 [DOI] [PubMed] [Google Scholar]
- 57. Drogat B., Auguste P., Nguyen D. T., Bouchecareilh M., Pineau R., Nalbantoglu J., Kaufman R. J., Chevet E., Bikfalvi A., Moenner M. (2007) IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer Res. 67, 6700–6707 [DOI] [PubMed] [Google Scholar]
- 58. Iwawaki T., Akai R., Yamanaka S., Kohno K. (2009) Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc. Natl. Acad. Sci. U. S. A. 106, 16657–16662 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.