Abstract
α-Actinins are actin-binding proteins that can be broadly divided into Ca2+-sensitive cytoskeletal and Ca2+-insensitive sarcomeric isoforms. To date, little is known about functional differences between the isoforms due to their indistinguishable activities in most in vitro assays. To identify functional differences in vivo between sarcomeric isoforms, we employed computational and molecular approaches to characterize the zebrafish (Danio rerio) genome, which contains orthologoues of each human α-actinin gene, including duplicated copies of actn3. Each isoform exhibits a distinct and unique pattern of gene expression as assessed by mRNA in situ hybridization, largely sharing similar expression profiles as seen in humans. The spatial conservation of expression of these genes from lower invertebrates to humans suggests that regulation and subsequent functions of these genes are conserved during evolution. Morpholino-based knockdown of the sarcomeric isoform, actn2, leads to skeletal muscle, cardiac, and ocular defects evident over the first week of development. Remarkably, despite the high degree of sequence conservation between actn2 and actn3, the phenotypes of α-actinin-2 deficient zebrafish can be rescued by overexpression of α-actinin-2 but not by α-actinin-3 mRNAs from zebrafish or human. These data provide functional evidence that the primary sequences of α-actinin-2 and α-actinin-3 evolved differences to optimize their functions.—Gupta, V., Discenza, M., Guyon, J. R., Kunkel, L. M., Beggs, A. H. α-Actinin-2 deficiency results in sarcomeric defects in zebrafish that cannot be rescued by α-actinin-3 revealing functional differences between sarcomeric isoforms.
Keywords: skeletal muscle, cytoskeletal, myofibrillogenesis, gene duplication, evolution
α-Actinins are actin-crosslinking proteins belonging to the ancient gene family of spectrin-related proteins (1–3). α-Actinins have been highly conserved throughout evolution and are largely collinear proteins that share 3 conserved functional domains: an N-terminal actin-binding domain, a central rod domain containing several spectrin-like repeats, and a C-terminal domain that contains 2 potential Ca2+ binding EF-hand motifs. The actin-binding domain and Ca2+ binding domains are the most highly conserved, whereas the central rod domain includes a variable number of spectrin-like repeats whose internal structure also harbors the greatest degree of variation among the isoforms. Functionally, α-actinins form antiparallel homodimers with the actin-binding domains on each end of the molecule, allowing crosslinking of actin molecules (4–6).
α-Actinins have been identified in organisms as primitive as cynobacterium Spirulina platensis; in insects, including Drosophila melanogaster; in the nematode Caenorhabditis elegans; and in mammals (4, 6–9). The level of α-actinin sequence conservation among these diverse taxa is striking. For instance, the human sarcomeric α-actinin-2 and Drosophila melanogaster isoforms are 69% identical and 82% similar along their whole length, with even higher conservation within the N-terminal actin-binding domain (10). The most notable difference is the number of α-actinin genes and spliced isoforms in different taxa. Primitive organisms tend to have a single α-actinin gene and transcript, whereas alternate gene splicing or intergenic gene duplication has resulted in multiple muscle and nonmuscle specific genes and isoforms in higher invertebrates and vertebrates (9). Humans are typical of mammalia, with 4 different α-actinin genes, 2 of which encode cytoskeletal isoforms (ACTN1 and ACTN4), while 2 are striated muscle-specific sarcomeric genes (ACTN2 and ACTN3) (8, 10, 11). Human ACTN1 is also alternatively spliced to form smooth muscle or brain specific isoforms (12).
The major, and best characterized, functional difference among α-actinin isoforms is calcium sensitivity for actin-binding, due to variable degrees of degeneracy at the calcium-binding EF-hand domains (10, 11). The binding of α-actinin to actin by nonmuscle cytoskeletal isoforms is dependent on the calcium concentration, whereas this interaction is independent of calcium concentration in sarcomeric striated and smooth muscle isoforms. Remarkably, this difference in calcium sensitivity is the exception, as few other clear structure-function relationships have been identified to date. α-Actinins have been shown to bind an impressive variety of different proteins, yet despite the great diversity of tissue-specific locations and functions of different isoforms, and some degree of sequence variation, the different isoforms have indistinguishable binding activities in most in vitro assays (1, 3).
Interestingly, mutations of the α-actinin-3 gene are compatible with normal life span, resulting only in a mild phenotype of a shift in muscle metabolism toward the more efficient aerobic pathway and an increase in intrinsic endurance performance (13). In Actn3-knockout mouse muscles, up-regulation of α-actinin-2 was observed, which suggests compensation by α-actinin-2 and significant genetic redundancy between these closely related isoforms (14, 15). Moreover, presence of only one sarcomeric gene, for α-actinin-2, in chickens also supports the model of functional redundancy.
In vitro studies in cell culture have shown that deletion mutants of α-actinin-2 lacking the EF-hand domain results in formation of nemaline like bodies and disruption of thick and thin filaments, suggesting its possible role in skeletal muscle maintenance and disease (16). A single amino acid change of Q8R in ACTN2 has been implicated in a human patient with dilated cardiomyopathy and a recent genome-wide linkage analysis has also suggested an association of ACTN2 variants with cardiomyopathy in humans (17, 18). α-Actinin-2 variants are also associated with dilated cardiomyopathy in dogs (19). Even though mutations in different sarcomeric α-actinins are associated with a different phenotype/disease, all in vitro assays show indistinguishable activities of these α-actinin isoforms, raising the question of whether functional differences are related only to differences in spatiotemporal expression patterns of the genes or whether structural differences are also important (15). The high degree of evolutionary conservation for each isoform, and observations in human populations and knockout mouse models, suggests differential physiological requirements for different isoforms, but a rapid assay system to characterize these differences is lacking.
In recent years, the zebrafish Danio rerio has emerged as an excellent genetic system to study muscle development and diseases. Orthologues for many of the human muscular dystrophy associated genes have been identified in zebrafish, making this a relevant organism for modeling human muscle disease (20–22). A previous study at 1 and 4 days postfertilization (dpf) identified that α-actinins are conserved in zebrafish and show highly distinct expression at these stages (23). We further extend examination of the α-actinin family in zebrafish by studying the spatiotemporal expression in a broader developmental context (1 cell to 7 dpf), and refine the expression patterns of α-actinin-2 using a new isoform-specific antibody. This study of earlier developmental stages reveals that, while some family members like α-actinin-2 and α-actinin-3 showed highly restricted cell-type specific expression during early embryogenesis, other family members displayed ubiquitous expression in all cell-types. Later in development (1 dpf), when distinct boundaries of α-actinin expression become established, the dynamic changes in expression continue until organogenesis is complete (4–5 dpf). To identify physiological differences in the function of sarcomeric isoforms, we tested the ability of different α-actinin isoforms to complement each other in vivo, using zebrafish as a model system. These results demonstrate an inability of closely related sarcomeric isoforms to rescue each other, despite apparent redundancy in primates, providing insight into the functional basis for evolutionary conservation of the distinct isoforms.
MATERIALS AND METHODS
Fish and embryo maintenance
Fish were bred and maintained as described previously (24). Wild-type embryos were obtained from the Oregon AB line and were staged by hours or days postfertilization at 28.5°C. All animal work was performed with approval from the Children's Hospital Boston Animal Care and Use Committee (11-05-1955R).
Computational analysis
Zebrafish orthologues to the human protein sequences were identified as described previously (22). Human α-actinins were compared with the zebrafish genome sequence in the Sanger Institute database (Zv 9.0; Sanger Institute, Hinxton, UK) as well as using the University of California–Santa Cruz (UCSC) genome browser (UCSC, Santa Cruz, CA, USA). Synteny between zebrafish and human α-actinins was determined using U.S. National Center for Biotechnology Information (NCBI) Entrez GeneView (NCBI, Bethesda, MD, USA). Genomic loci for neighboring genes were determined as above, using the human protein sequence and tBLASTn algorithm to search the Zebrafish genome.
Multiple sequence alignment was performed using MEGA 5.0 (25). The FASTA sequences were imported, and a full amino acid sequence alignment was performed using the ClustalW algorithm with BLOSUM matrix. The phylogenetic tree was generated with MEGA 5.0 using the neighbor-joining (NJ) method. Each phylogeny was evaluated using the bootstrap method with 1000 replications. The distances used in phylogenetic tree construction are measures of the genetic distance between nodes on the tree. The genetic distance is measured based on the minimum number of substitutions required to convert one sequence into other.
Whole-mount in situ hybridization
Isoform-specific riboprobes were constructed from the 3′ untranslated regions (UTRs) of each gene using adult zebrafish RNA. The PubMed accession numbers and primers used were as follows: Actn1 (XM_001335509), forward 5′-CTCGAGGCATCCATAATGAAATTGCTAAAA-3′, reverse 5′-GGATCCAGCCTGATTAGCAAACTGTCTG-3′; Actn2 (BC110103), forward 5′-CTCGAGGCCGAGTACTGTATCAGTCGAA-3′, reverse 5′-GGATCCAGTTAGGCTTTGTTCTCTTTATTTAGC-3′; Actn3a (BC054911), forward 5′-CTCGAGCAGGCGCGCTTGATTATATC-3′, reverse 5′-GGATCCCCTCCAATAGTTAAGCCATTTGC-3′; Actn3b (BC065595), forward 5′-CTCGAGGTCTGGTTCTTTAAAAGAGATGTTTGG-3′, reverse 5′-GGATCCTAGAGTTTTGAGCTGTTTTATTCTGGG-3′; Actn4 (BC054901), forward 5′-CTCGAGTGTTATTTG GCATAGTTGTATGTCG-3′, reverse 5′-GGATCCACACAGACCCGTAGCTACTCAAT-3′. Total RNA was extracted from adult zebrafish muscle tissue using Trizol (Invitrogen, Carlsbad, CA, USA). cDNAs were synthesized and amplified using single-step Superscript One-step RT-PCR with platinum Taq kit (Invitrogen, Carlsbad, CA, USA) using gene specific primers. Amplified DNA fragments were cloned in pZEM7Z(+) using XhoI and BamHI sites, respectively. Sense or antisense digoxigenin-labeled riboprobes were synthesized by in vitro transcription using DIG RNA labeling kits (Roche Applied Sciences, Indianapolis, IN, USA). Whole-mount in situ hybridization was performed as described previously (26). Imaging was performed using a Nikon SMZ1500 microscope with a Spot camera system (Nikon, Tokyo, Japan). For sectioning, embryos were fixed in 4% paraformaldehyde overnight at 4°C after in situ hybridization. Embryos were subsequently washed with 20% sucrose and embedded in Tissue-Tek optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA). Sections (8 μm thick) were cut and mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) and visualized using a Nikon Eclipse 90i microscope.
Morpholino (MO) knockdown and mRNA rescue
Two splice-site-blocking MOs, targeting different exon-intron boundaries, and a translational blocking MO were designed to knock down zebrafish actn2 transcripts (Genetools, Philomath, OR, USA). The splice-site-blocking MOs and paired negative control mismatch MOs were as follows: exon1-intron1 (E1-I1), 5′-AAGGCCAAACTCTCTTTACCTTCCT-3′; exon1-intron1 negative control mismatch (E1-I1m), 5′-AAcGCgAAACTCTgTTTACgTTgCT-3′; exon2-intron2 (E2-I2), 5′-AGAAAGTTCAGTGTGTTAACCTGAG-3′; exon2-intron2 negative control mismatch (E2-I2m), 5′-AcAAAcTTCAGTcTGTTAAgCTcAG-3′; TRA, 5′-CTGAAAGCTCGATCTGATTCATCAT-3′; TRA negative control mismatch (TRAm), 5′-CTcAAAcCTCGATgTGATTgATgAT-3′. MO against human β-globulin was used as a negative control for all injections. MOs were dissolved in 1× Danieau's buffer, and 1–2 nl (1–10 ng) was injected into 1-cell embryos.
For rescue experiments, the full-length zebrafish actn2, actn3a, and actn3b as well as human ACTN2 and ACTN3 coding sequences were cloned into a PCSDest destination vector (a gift from Nathan Lawson, University of Massachusetts Medical School, Worcester, MA, USA) using Gateway technology (Invitrogen). mRNA was synthesized in vitro using mMessage kits (Ambion, Austin, TX, USA). 50–200 pg of mRNA was injected into embryos at the 1-cell stage.
Immunofluorescence
Indirect immunofluorescence staining was performed on frozen sections as described previously (21). Primary antibodies used in this work were F59 [recognizes myosin heavy chain in slow fibers till 24 hours postfertilization (hpf), diluted to 1:50; Developmental Studies Hybridoma Bank, Iowa City, IA, USA), F310 (against myosin light chain; 1:250 dilution; Developmental Studies Hybridoma Bank), MF20 (recognizes atrial and ventricular meromyosin; 1:25; Developmental Studies Hybridoma Bank), and zebrafish Actn2 (1:500 dilution). Antibody to zebrafish α-actinin-2 (designated F6523) was raised in rabbit using a synthetic peptide representing the N-terminal 18 aa residues (MMNQIELSVPYDNGYEID). FITC-anti-mouse or Cy-3-anti-rabbit secondary antibodies were used at 1:250 dilution (Jackson ImmunoResearch, West Grove, PA, USA). Imaging was performed using a Nikon Eclipse 90i microscope or spinning disc confocal microscope (Perkin Elmer Ultraview Vox; Perkin Elmer, Wellesley, MA, USA). Whole-mount immunofluorescence was performed as described previously (21). Embryos were mounted in 70% glycerol and visualized using a Nikon Eclipse 90i microscope.
Western blotting
Zebrafish embryos were homogenized in buffer containing Tris-Cl (20 mM, pH 7.6), NaCl (50 mM), EDTA (1 mM), Nonidet P-40 (0.1%) and complete protease inhibitor cocktail (Roche Applied Sciences, Indianapolis, IN, USA). Western blotting was performed as described previously (21). Primary antibodies used were rabbit anti-Actn2 (F6523; 1:250) and mouse anti-β-actin (A5441; 1:2000; Sigma, St. Louis, MO, USA). Protein bands were quantified using Quantity One software (Bio-Rad, Hercules, CA, USA).
Real-time RT-PCR
To detect actn2 knockdown in 3-dpf zebrafish embryos, RNA was prepared using RNeasy fibrous tissue mini kits (Qiagen, Valencia, CA, USA). cDNA was prepared using high capacity RNA-to-cDNA mastermix kits (Ambion, Austin, TX, USA). Real-time PCR was performed using a Taqman assay for actn2 exon1-exon2 (Applied Biosystems, Austin, TX, USA). Gapdh was used as the control to normalize actn2 expression. Actn2-knockdown quantification was performed using the 2−ΔΔCt method.
Touch-evoked escape response assay
Embryonic locomotor assays were performed as described previously (27). Briefly, mechanosensory stimuli were delivered to 3-dpf embryo tails using insect pins. Time-lapse images of zebrafish embryos were taken at different time intervals using a Nikon smz1500 microscope with SPOT camera system. The length of time for each fish to leave the frame of view was averaged across fish (n=4 or 5).
Electron microscopy
Zebrafish embryos were fixed in formaldehyde-glutaraldehyde-picric acid in cacodylate buffer overnight at 4°C, followed by osmication and uranyl acetate staining. Subsequently, embryos were dehydrated in a series of ethanol washes and finally embedded in TAAB epon (Marivac Ltd., Halifax, NS, Canada). Sections (95 nm) were cut with a Leica ultracut microtome (Leica Microsystems, Wetzlar, Germany), picked up on 100-μm Formvar-coated Cu grids, and stained with 0.2% lead citrate. Sections were viewed and imaged under a Philips Tecnai BioTwin Spirit Electron Microscope (Philips, Amsterdam, The Netherlands) at the Harvard Medical School Electron Microscopy Core.
Statistical analysis
Data were statistically analyzed by parametric Student's t test (2 tailed) and were considered significant when P < 0.05. All data analyses were performed using XLSTAT software.
RESULTS
α-Actinin genes in zebrafish
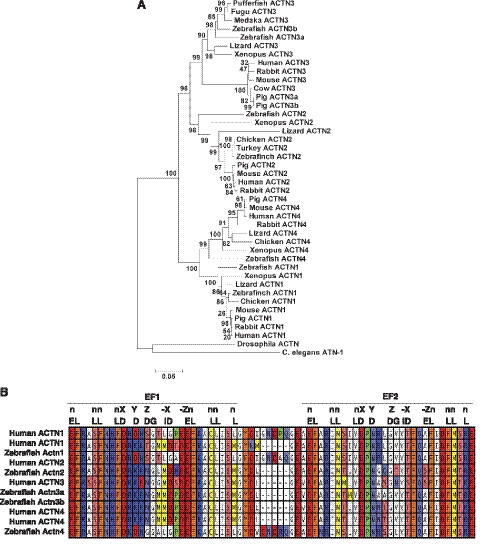
Human and mouse α-actinin protein sequences were used to query the zebrafish genome using tBLASTn in Ensembl (Zv9). This resulted in identification of 9 putative α-actinin genes in the zebrafish genome. The identity of each of these loci as a transcriptional unit was evaluated by detection of homologous zebrafish expressed sequence tags, and orthology with human genes was predicted by identification of conserved syntenic relationships using both the Ensembl and UCSC genome browsers. This analysis confirmed the validity of 5 of the 9 candidate α-actinin genes in zebrafish as true human orthologoues (Table 1). The predicted exon structure of each gene was examined, and full-length amino acid sequences for each of the 5 genes were assembled. Each predicted protein was 895–901 aa long and completely collinear with human α-actinins, with the exception of variable sequences at the N terminus. In addition to single zebrafish orthologues for ACTN1, ACTN2, and ACTN4, our search also identified 2 potential orthologues of ACTN3, on chromosomes 7 and 21 (Table 1). Overexpression of the complete open reading frames of these computationally predicted zebrafish α-actinins in C2C12 cells also resulted in protein products of expected sizes, confirming the validity of the computational analysis (Supplemental Fig. S1A).
Table 1.
Zebrafish α-actinin genes and synteny with human orthologues
| Ensembl gene ID (NCBI Refseq) | Fish chromosome | Potential human orthologue | Synteny with humans |
|---|---|---|---|
| ENSDARG000000007219 (NM_001168286) | 13 | ACTN1 | SLC8A3 |
| ENSDARG00000071090 (BC110103) | 17 | ACTN2 | RYR2, CHRM3, FMN2, GREM2 |
| ENSDARG00000001431 (BC054911) | 7 | ACTN3 | CCS, RBM14, PITPNM1, NRXN2 |
| ENSDARG00000013755 (BC065595) | 21 | ACTN3 | BBS1, DPP3, RBM30 |
| ENSDARG00000025364 (BC054901) | 12 | ACTN4 | SYDE1, CYP4F3 |
To further understand the relationship between the different α-actinins in zebrafish and with α-actinins of other species, these 5 predicted amino acid sequences were subjected to a ClustalW alignment against a collection of known α-actinin proteins using the BLOSUM matrix with phylogenetic analysis in MEGA 5.0 to build a tree of proposed phylogenetic relationship (Fig. 1A). The products of two actn3 genes in zebrafish are more closely related to each other than either is to other vertebrate α-actinins. This duplicated copy of α-actinin-3 was not found in other teleost genomes. In addition, we did not see duplication of any adjacent chromosomal segments that are normally seen in genomic duplications. Therefore, from the syntenic and phylogenetic analysis, we conclude that zebrafish actn3a and actn3b are coorthologues to mammalian ACTN3, which arose from a gene duplication event. Differential calcium sensitivity of cytoskeletal and sarcomeric α-actinins is regulated by the structure of the C-terminal EF-hands that contain helix-loop-helix Ca2+ binding motifs. The ability of EF-hands to bind to calcium is dependent on the presence of a consensus amino acid sequence (16 residues) that forms the Ca2+ binding lattice (Fig. 1B). The EF-hand is predicted to be functional (Ca2+-sensitive) if ≥12 aa match with the consensus sequence (28). Sequence analysis of the predicted EF-hand Ca2+ binding domains revealed that the ACTN1 and ACTN4 orthologues are both predicted to encode Ca2+-sensitive EF-hands with characteristic 5 residue spacers found in other cytoskeletal isoforms. No evidence was found for smooth muscle isoforms of actn1 in zebrafish, although they may be present but underrepresented in the sequence databases. Analysis of ACTN2 and ACTN3 orthologues showed that they encoded Ca2+-insensitive EF-hands (Table 2). Therefore, like the mammalian α-actinins, zebrafish actn1 and actn4 are predicted to be cytoskeletal, and actn2, actn3a, and actn3b appear to be sarcomeric in nature.
Figure 1.
The zebrafish genome encodes 5 α-actinins; 2 cytoskeletal and 3 sarcomeric isoforms. A) Phylogenetic analysis showing relationships between zebrafish α-actinins and other vertebrate α-actinins. Tree was constructed by neighbor-joining method. Numbers on nodes represent distance. Scale bar shows the distance scale and represents the number of differences between the sequences (25). B) Alignment of zebrafish and human EF-hand regions. E = E; L = L, I, F, V, M, A; D = D, N, E, Q, S, T; I = I, V; G =G. See Table 2 for quantification.
Table 2.
Number of amino acid matches to EF-hand consensus sequence
| Protein | Matches to EF-hand consensus |
|
|---|---|---|
| EF1 | EF2 | |
| Human ACTN1a | 12/16 | 13/16 |
| Human ACTN1b | 11/16 | 13/16 |
| Zebrafish Actn1 | 12/16 | 13/16 |
| Human ACTN2 | 11/16 | 14/16 |
| Zebrafish Actn2 | 11/16 | 14/16 |
| Human ACTN3 | 11/16 | 13/16 |
| Zebrafish Actn3a | 11/16 | 14/16 |
| Zebrafish Actn3b | 11/16 | 14/16 |
| Human ACTN4a | 11/16 | 14/16 |
| Human ACTN4b | 11/16 | 14/16 |
| Zebrafish Actn4 | 13/16 | 14/16 |
Matches at ≥12 of the 16 conserved residues are considered necessary for functionality.
Zebrafish α-actinins are expressed differentially in a spatiotemporal way
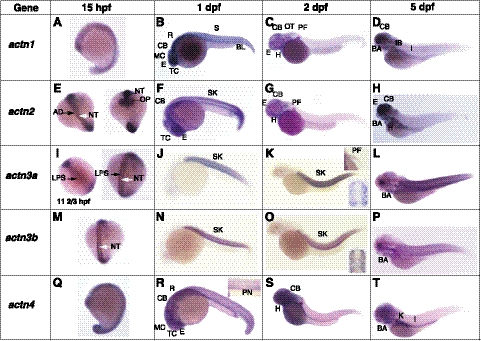
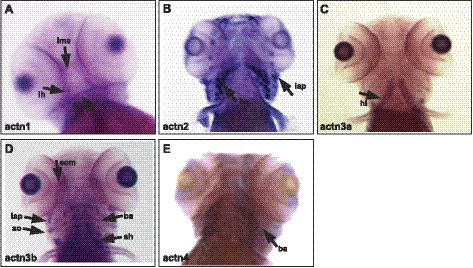
To understand the biological functions of zebrafish α-actinins, temporal and spatial expression of α-actinin mRNAs during embryonic and larval development were further examined using whole-mount in situ hybridization with isoform-specific probes (summarized in Table 3). During zebrafish development, actn1 expression was detected as early as the 1-cell stage by both in situ hybridization and RT-PCR, suggesting maternal expression of the gene (Supplemental Fig. S1B). Maternal expression of genes decreases by the end of gastrulation, so expression of actn1 in subsequent stages is clearly zygotic in origin. At the early 12- to 13-somite stage (15 hpf), actn1 transcripts were detected ubiquitously, with higher expression in the developing nervous system and eye (Fig. 2A). By the end of d 1, high expression was still observed in all parts of the brain, including the mesencephlon, telecephlon, and rhombocephlan (on lateral views), as well as in the cerebellum (Fig. 2B). Low levels of actn1 expression were also observed in the skin. By d 2 onward, actn1 expression predominated in the cerebellum and rhombecephalon, whereas expression in other parts of the brain was seen to subside (Fig. 2C, D). These results reveal dynamic expression of actn1 occurring during early nervous system development in zebrafish. In addition to the nervous system, we also observed actn1 expression in the otic capsule (ear primodia) and pectoral fin buds from 2 dpf. By 5 dpf, actn1 expression was also established in cranial muscles, more specifically the intermandibularis anterior, interhyoides, and hyodes inferior (Fig. 3A).
Table 3.
Expression of α-actinins in zebrafish development
| Location | Gene |
||||
|---|---|---|---|---|---|
| actn1 | actn2 | actn3a | actn3b | actn4 | |
| Brain | |||||
| Mesocephalon | + | − | − | − | + |
| Telencephalon | + | + | − | − | + |
| Rhombecephalon | + | − | − | − | + |
| Cerebellum | + | + | − | − | + |
| Eye | + | + | − | − | + |
| Otic capsule | + | − | − | − | − |
| Heart | + | + | − | − | + |
| Pectoral fin | + | + | + | − | − |
| Branchial arches | + | + | + | + | + |
| Cranial muscles | |||||
| Intermandibularis posterior | − | − | + | + | − |
| Intermandibularis anterior | + | − | − | − | − |
| Interhyoideus | + | − | − | − | − |
| Hyoideus inferior | + | − | + | + | − |
| Sternohyoideus | − | − | − | − | − |
| Levator arcus palitini | − | + | − | + | − |
| Extraocular muscles | − | − | − | + | − |
| Skeletal muscles | |||||
| Fast muscles | − | − | + | + | − |
| Slow muscles | − | + | + | + | − |
| Kidney | − | − | − | − | + |
| Intestines | + | − | − | − | − |
Figure 2.
Gene expression patterns of α-actinins during zebrafish development detected using whole-mount in situ hybridization at 15 hpf (A, E, I, M, Q), 1 dpf (B, F, J, N, R), 2 dpf (C, G, K, O, S), and 5 dpf (D, H, L, P, T). A–D) actn1 expression (lateral view). E–H) actn2 expression (E, left, dorsal view; right; ventral view; F–H, lateral view). I–L) actn3a expression (I, left, dorsal view; right, ventral view; J–L, lateral view; K, top inset, pectoral fin; bottom inset, skeletal muscles). M–P) actn3b expression (M, dorsal view, N–P, lateral view; O, inset, skeletal muscles). (Q–T) actn4 expression (lateral view; R, inset, pronepharic duct). A, atrium; CB, cerebellum; CNS, central nervous system; E, eye; MC, mesocephalon; TC, telencephalon; R, rhombecephalon; S, somites; SK, skeletal muscles; PF, pectoral fin; IB, intestinal blub; I; intestine, H; heart, AD; adaxial cells, NC; notochord, OP; optic primordial, LPS; lateral presomitic cells, PN; pronephric duct, K; kidney, L; lens, PL; photoreceptor layer, V; ventricle,. Note that strong apparent staining in the lens of embryos at 5 dpf represents naturally occurring pigmentation.
Figure 3.
Expression of α-actinins in zebrafish craniofacial muscles. α-Actinins exhibit diverse expression patterns in craniofacial muscles in zebrafish (5 dpf). A) actn1 expression was detected in intermandibularis anterior (ima), interhyoides (ih), and hyodes inferior (hh). B) actn2 transcripts were strongly expressed in levator arcus palatini (lap) and branchial archs (ba). C) actn3a expression was observed in hyoideus inferior (hi). D) actn3b showed a wide expression pattern in craniofacial muscles including extraocular muscles (eom), levator arcus palatini (lap), adductor operculi (ao), sternohyoides (sh) and branchial arches (ba). E) actn4 expression was restricted to branchial arches (ba).
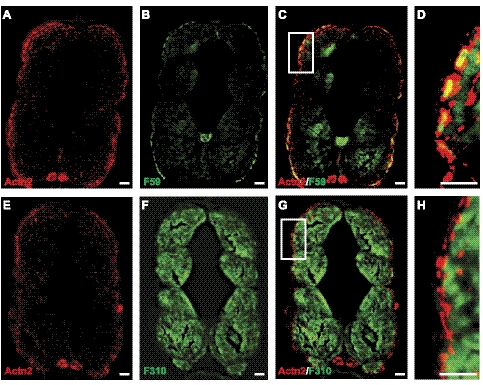
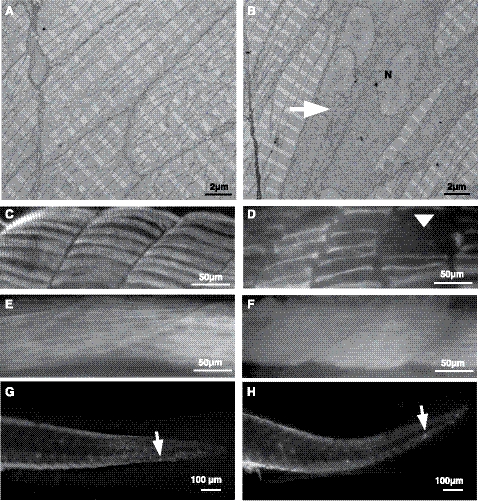
By RT-PCR, actn2 transcripts were observed from the 1-cell stage, but their levels decreased sharply by the end of gastrulation (8 hpf), indicating they were also likely the result of maternal expression (Supplemental Fig. S1B). Subsequently, zygotic expression of actn2 was first observed as somitogenesis began (11 hpf). By in situ hybridization, expression of actn2 transcripts was detectable at the 12- to 13-somite stage (15 hpf) in bilateral diffuse stripes flanking the prospective notochord, in a pattern consistent with staining of adaxial muscle cells, which are precursors to slow myofibers (Fig. 2E and ref. 29). By 1 dpf, these cells have migrated and differentiated to form the surface layer of slow muscle fibers, which remained a significant site of actn2 expression (Fig. 2F). Between 2 and 3 dpf, the slow fibers become marginalized and limited to a single cell layer at the periphery of the trunk. Transcripts of actn2 were difficult to detect at this stage by in situ hybridization (Fig. 2G, H). The expression of actn2 in slow muscle fibers was also investigated by coimmunostaining for the protein together with slow or fast muscle markers in zebrafish muscles (4 dpf). Immunostaining with the zebrafish α-actinin-2 specific antibody F6523 revealed labeling of skeletal muscle in a pattern restricted to the location of these slow myofibers at the periphery of the trunk (Fig. 4A, E). This finding was confirmed by double-immunofluorescence for α-actinin-2 and the slow muscle marker (F59 antibody) or fast muscle marker (F310 antibody), which showed colocalization of α-actinin-2 with F59 (Fig. 4C, D) but not F310 (Fig. 4G, H). These data demonstrate that α-actinin-2 is a predominantly slow muscle-specific isoform in mature zebrafish muscles. By in situ hybridization, high levels of actn2 expression were also seen in craniofacial muscles by 5 dpf (Fig. 3B). In particular, actn2 transcripts were detected in levator arcus palatine, as reported previously (23). In addition, our 3′ UTR-specific probe detected strong expression of actn2 in the branchial arches. This expression of α-actinin-2 was confirmed at the protein level by immunofluorescence that detected the α-actinin-2 expression in branchial arches (Supplemental Fig. S2F, arrows).
Figure 4.
α-Actinin-2 is expressed in slow fibers in zebrafish skeletal muscle. A, B) Immunofluorescence was performed on the transverse sections of wild-type zebrafish (5 dpf) with α-actinin-2 antibody (F6523) against zebrafish protein (A) or F59 antibody that strongly labels superficial layer of slow muscle cells and weakly labels deep fast muscle cells (B). C) Immunolabeling with both F6523 and F59 antibody showed colocalization of 2 proteins (yellow signal). D) High-magnification view of boxed area in C. E–H) In contrast, immunolabeling with fast muscle marker F310 did not show any colocalization with F6523. E) F6523 antibody. F) F310 antibody. G) F6523 and F310 antibody. H) High-magnification view of boxed area in G. Scale bars = 30 μm (A–C, E–G); 10 μm (D, H).
In situ hybridization also revealed early actn2 expression, beginning at 1 dpf at multiple sites in the developing brain and throughout the developing optic vesicle (Fig. 2E–H). Immunofluorescence with α-actinin-2-specific antibody confirmed the expression of this protein in zebrafish brain (diencephalon; Supplemental Fig. S2F, arrowhead). In the eye, actn2 expression was observed continuously beginning at 1 dpf, but immunodetection of α-actinin-2 protein at 5 dpf demonstrated that this isoform is specifically expressed in the photoreceptor layer of the retina, as well as in a few cells in the developing lens region (Fig. 2F–H and Supplemental Fig. S2A). Similar to our findings of α-actinin-2 expression in the eye, a previous report has also identified expression of α-actinin in the outer retinal layer. However, lack of an isoform-specific antibody in that work prevented identification of the different α-actinins (30). Expression of actn2 was also seen in the developing heart from the earliest stages of cardiogenesis at 1 dpf (Fig. 2F–H). α-Actinin-2 protein was expressed in both the ventricle and atrium, as observed by immunostaining in the zebrafish heart (Supplemental Fig. S2E). Thus, actn2 is expressed at multiple sites during development and early expression in these organs suggests a crucial role for actn2 in organogenesis.
Maternally derived expression of actn3a transcripts (seen at 0 hpf) was supplemented by early embryonic expression that was first observed as early as 4 hpf in the blastula sphere stage (Supplemental Fig. S1B). By the 5-somite stage (11-2/3 hpf), in situ hybridization revealed actn3a expression in a pair of longitudinal rows on either side of the prospective notochord (Fig. 2I, left). As somitogenesis progressed, actn3a expression was observed as laterally projected bands, or dots, along the mediolateral axis in the somites of developing embryos (Fig. 2I, right). In contrast to adaxial cell staining observed for actn2, this staining pattern is most consistent with expression in lateral presomitic cells (29). Expression of actn3a was observed in all somites by the end of 1 dpf (Fig. 2J), and by 2 dpf, expression was mainly restricted to skeletal muscle in a pattern consistent with expression predominantly in fast muscle fibers (Fig. 2K, inset). By 3 dpf, expression of actn3a was also observed in developing pectoral fin musculature, which contains only fast myofibers at this stage (Fig. 2K, inset). In addition, actn3a expression was also observed in developing cranial muscles (Fig. 3C), in the hyoideus inferior muscles in particular.
In contrast to all the other α-actinins examined, expression of actn3b was not detected until late gastrulation (8 hpf; Supplemental Fig. S1B). However, as somitogenesis progressed, actn3b expression was seen in a pattern similar to that for actn3a (Fig. 2M). By 1 dpf, actn3b was strongly expressed in all somites (Fig. 2N). Interestingly, unlike actn3a, which was restricted to fast myofibers, actn3b expression was observed in both fast and slow muscle fibers (Fig. 2O, inset) and RT-PCR of adult skeletal muscle revealed robust expression throughout development. Actn3b expression was also seen in the extraocular muscles (Fig. 3D), and expression was seen in the branchial arches, lavatory arcus palatini, adductor hyoideus as well as sternohyoideus that plays a major role in hyoid depression and through a series of mechanical linkages, in mouth opening and suspensorail abduction (Fig. 3D).
Expression of actn4 transcripts was detected from the 1-cell stage, suggesting that actn4 is a maternally expressed gene (Supplemental Fig. S1B) but embryonic expression was detectable by the 4-hpf blastula stage and continued throughout gastrulation and later during development (Supplemental Fig. S1B). In situ hybridization revealed ubiquitous expression of actn4 until late in somitogenesis (13-somite stage; Fig. 2Q) when it became localized in developing brain, eyes, somites, fin bud, heart, kidney, and pronephric ducts by the end of 1 dpf (Fig. 2R). Expression levels varied depending on tissue type at this stage, with higher expression observed in brain and developing eyes and lower expression in somites (Fig. 2S). By the end of d 5, detectable expression of actn4 was restricted to telencephalon and cerebellum in brain, and in kidney and pronephric ducts (Fig. 2T). Low expression of actn4 was also detected in branchial arches in craniofacial muscles (Fig. 3E).
Targeted knockdown of actn2 results in developmental defects in eye, heart, and skeletal muscle
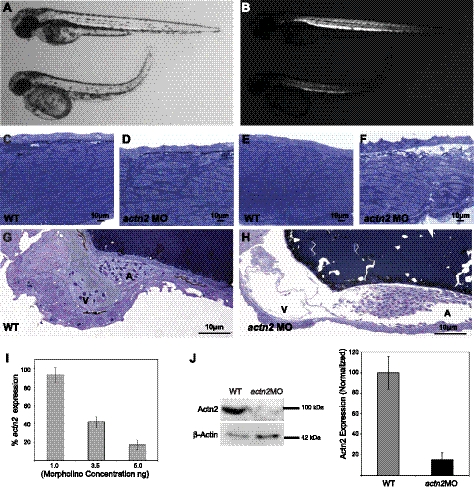
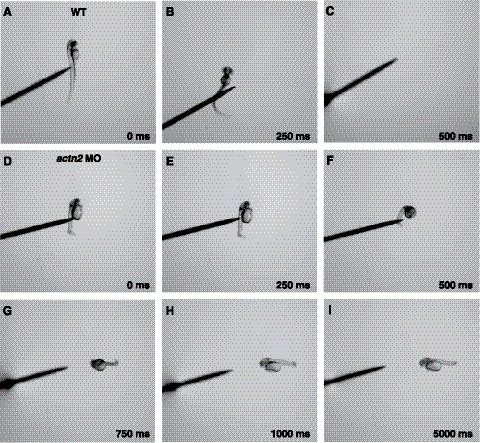
To understand the functional role of actn2 in zebrafish development, we ablated the gene product using 2 independent splice site MOs, exon1-intron1 and exon2-intron2, and a translational start-site MO, TRA. In preparatory dose-response studies, embryos injected with 1 and 2.5 ng of actn2 MOs were morphologically indistinguishable from embryos injected with control MOs, but doses in the range of 3.5–5.0 ng resulted in consistent abnormalities of multiple organs as most effective knockdown of actn2 transcripts was achieved (Fig. 5). Subsequent studies employed 5 ng of MO, as 100% embryos gave a phenotype without any side effects (as checked by mismatch control) in comparison to the controls (Fig. 5I). Knockdown of actn2 using each of the 3 MOs resulted in similar highly specific and dose-dependent phenotypes in comparison to uninjected or mismatch-injected controls. The actn2 morphant fish routinely hatched 12–16 h later than siblings injected with control MOs. Morphant fish were largely immotile, and their touch-evoked escape response was blunted; instead of rapidly swimming out of the field of view, they twitched and moved only several lengths when stimulated with a needle, which suggests a significant degree of overall muscle weakness (Fig. 6). Morphants consistently exhibited smaller eyes, enlarged hearts with reduced heartbeat, and abnormal organization of skeletal muscles (Fig. 5). Quantitative real-time RT-PCR analysis showed up to 85% reduction of actn2 mRNA in the morphant zebrafish embryos injected with 5 ng of MO (Fig. 5I) in comparison to controls. By Western blotting, levels of α-actinin-2 protein in morphants were decreased to ∼15% of normal (Fig. 5J).
Figure 5.
Knockdown of α-actinin-2 results in skeletal muscle, heart, and eye phenotypes in zebrafish. A) Representative actn2 morphants injected with 5 ng of exon2-intron2 MO (bottom) show smaller body and eyes and an enlarged heart in comparison to wild-type control at 3 dpf (top; n=15). B) Decreased birefringence is observed in actn2 morphant fish (bottom) in comparison to the wild-type fish. C–F) Longitudinal sections of zebrafish skeletal muscles and heart were stained with toluidine blue. C, E) Wild-type muscles show well-differentiated myofibers with peripherally localized nuclei. D, F) Actn2 morphant muscle fibers are smaller, with many fibers lacking normal striations and containing multiple centrally localized nuclei (arrow). G, H) In comparison to wild-type control (G), actn2 morphant embryos show an enlarged heart with enlarged ventricle and highly enlarged atrium at 3 dpf (H). I) Quantitative RT-PCR analysis of actn2 expression showed a decrease with increasing MO concentration. J) Knockdown of actn2 (5 ng of exon2-intron2 MO) results in highly reduced levels of the protein detected using F6523 antisera. β-Actin was used as a loading control (n=50 embryos used for Western blotting). All experiments were performed in triplicate.
Figure 6.
actn2 morphant embryos exhibit slow swimming in response to touch. A–C) Mechanosensory stimulation induced a wild-type embryo (3 dpf) to swim away rapidly (within 250–500 ms). A) t = 0 ms. B) t = 250 ms. C) t = 500 ms. D–I) Touch-induced actn2 morphant embryos (3 dpf) fail to swim outside the frame even after 5000 ms (n=4–5). D) t = 0 ms. E) t = 250 ms. F) t = 500 ms. G) t = 750 ms. H) t = 1000 ms. I) t = 5000 ms.
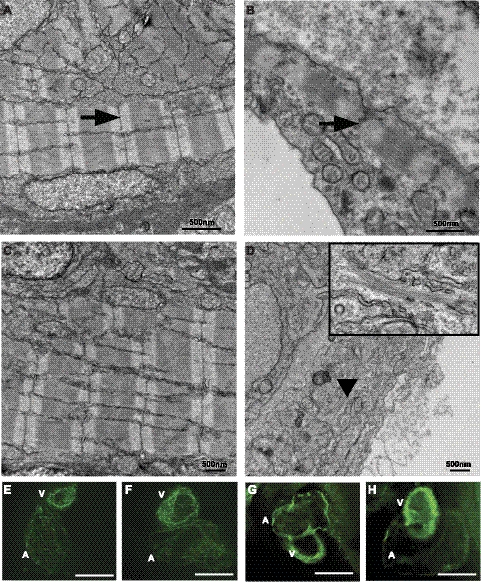
Skeletal muscles of the actn2 morphant embryos exhibited a significant decrease in birefringence in comparison to control-injected embryos, which suggests myofibrillar disorganization throughout the major axial skeletal muscles (Fig. 5B). The average length of actn2 morphants was reduced 33% (2.5±0.35 mm, P<0.01) in comparison to controls (3.5±0.25 mm, P<0.01; n=15). Further, the total number of somites at 24 hpf was reduced in actn2-knockdown fish from 28–30 in control embryos to 21–23 in morphants. Histological analysis of longitudinal sections revealed disorganized muscle fibers, with occasional fibers completely lacking in myofibrillar organization (Fig. 5C–F). Nuclei of these fibers were rounder in shape in comparison to the elongated nuclei present in normal myofibers. This phenotype exhibited a gradient of severity from anterior to posterior with the last few somites containing very few fibers with significant myofibrillar organization. Electron microscopy confirmed the presence of many fibers with reduced sarcomeric organization and rounded multiple nuclei in skeletal muscles of actn2 morphants (Fig. 7A, B). No histological evidence for a dystrophic process associated with active degeneration and regeneration or myofibrillogenesis in the affected fibers was seen, suggesting that lack of α-actinin-2 may block or lead to defective sarcomerogenesis at an early stage in deficient skeletal muscle fibers.
Figure 7.
α-Actinin-2 deficiency results in reduced myofibrillar apparatus in slow muscle fibers. A, B) Electron microscopy of wild-type myofibers (A) demonstrates normal sarcomeric organization at 3 dpf, while actn2 morphants (B) show many muscle fibers lacking highly organized sarcomeric organization (arrow) with multiple nuclei (N). C, D) Indirect immunofluorescence with the slow muscle marker, F59, shows that wild-type muscles are well organized (C), whereas fewer and thinner slow myofibers were seen in actn2 morphants (D); arrowhead indicates missing myofibers. E, F) Wild-type (E) as well as actn2 morphant muscle (F) shows similar staining with fast muscle marker, F310. G, H) Immunostaining with activated caspase-3 antibody showed no significant differences between wild-type (G) and actn2 MO muscles (H).
The cardiac phenotype of actn2 morphant fish first presented between 1 and 2 dpf as an appreciable reduction in heart rate. By 3 dpf, morphants exhibited clearly enlarged hearts, and the rate was reduced from 201 ± 3.8 beats per minute (bpm) in control fry to 144 ± 3.5 bpm in morphants (n=15, P<0.001). Whole-mount histological sections of actn2 morphants demonstrated enlarged pericardial cavities containing enlarged hearts that exhibited both artial and ventricular dilation, with remarkably thin walls (Fig. 5G, H). Electron microscopy of control fish revealed well-organized sarcomeric assemblies in both ventricular and atrial cardiomyocytes at 3 dpf (Fig. 8A, C); however, the size and number of these structures were greatly reduced in ventricular (Fig. 8B) as well as atrial cardiomyocytes (Fig. 8D) of actn2 morphant fish. To investigate further whether actn2 deficiency affects sarcomeric structures in heart, whole-mount immunofluorescence was performed using MF20, which recognizes sarcomeric myosin heavy chain (Fig. 8E, F) and actin (Fig. 8G, H). While a reduction in staining for both actin and myosin proteins was observed, a considerable amount of immunoreactivity in a striated pattern remained in morphant hearts, demonstrating that either some residual α-actinin-2 was expressed in actn2 morphants, or some myofibrillogenesis could proceed in the absence of this protein.
Figure 8.
α-Actinin-2 deficiency results in reduced sarcomeric assemblies in zebrafish heart. A, C) In wild-type hearts, well-organized sarcomeric organization is observed in ventricle (A) and atrium (C). B, D) Actn2 morphants exhibit myofibers with small Z discs (B, ventricle) and reduced myofibrillar apparatus with scattered thick and thin filaments with no Z lines or Z discs (D, atrium; inset, high magnification). E–H) Whole-mount immunofluorescence of wild-type (E) and actn2 morphant (F) hearts with MF20 antibody that recognizes both atrial and ventricular myosin and with actin antibody (G, wild-type; H, actn2 MO).
The actn2-knockdown fish also had a profound ocular phenotype. Grossly, morphants had smaller eyes in comparison to control fish at 3 dpf. At 3 dpf, wild-type zebrafish retina is composed of the photoreceptor, inner nuclear, and ganglion cell layers, with α-actinin-2 expression prominent in the photoreceptor layer, and to a lesser extent, in the lens (Supplemental Fig. S2A). In actn2 morphants, the photoreceptors appeared to be absent or undifferentiated, and the inner layers were disorganized. By 3 dpf, normal lens contains a single layer of epithelial cells surrounding lens fibers that have differentiated and lost their nuclei (Supplemental Fig. S2B), but the actn2 morphants lacked the crystalline organization of normal lens, and many of the fiber cells retained their nuclei (Supplemental Fig. S2C).
α-Actinin-2 is required for maintenance of slow muscle fibers
We next investigated whether the reduced myofibrillar organization in actn2-deficient myofibers is fiber type specific by performing immunofluorescence on control and actn2 morphant embryos at the end of somitogenesis, using antibodies specific for fast and slow muscle muscle. Immunostaining with anti-slow myosin heavy chain, F59 antibody, which recognizes slow type 1 muscle fibers, revealed a reduction in number of slow muscle fibers in actn2 knockdowns in comparison to controls (Fig. 7C, D). Moreover, the few slow fibers that were detected in actn2 morphant fish were thinner in diameter when compared to the control. However, immunostaining embryos with fast muscle specific antibody (F310) did not show any difference between control and actn2 morphant fish in numbers or organization of fast myofibers (Fig. 7E, F). To assess whether the decrease in slow myosin staining in actn2 morphants was a result of decreased myofibrillogenesis or due to degeneration of slow muscle fibers, we conducted whole-mount staining with activated caspase-3 antibody (a marker of apoptosis), which did not show any significant increase in staining in the muscles of actn2 morphants (Fig. 7G, H). Similarly, TUNEL staining patterns in actn2 morphant and control embryos at 3 dpf were indistinguishable, indicating no increase in degenerating muscle fibers (data not shown). These data support the notion that actn2 deficiency selectively affects myofibrillogenesis of slow muscle fibers in zebrafish, as the absence of intact myofibrils does not appear to be associated with dystrophic histology, indicating muscle degeneration, or with increased rates of apoptosis.
α-Actinin-2, but not α-actinin-3 mRNAs, can rescue actn2 morphant fish
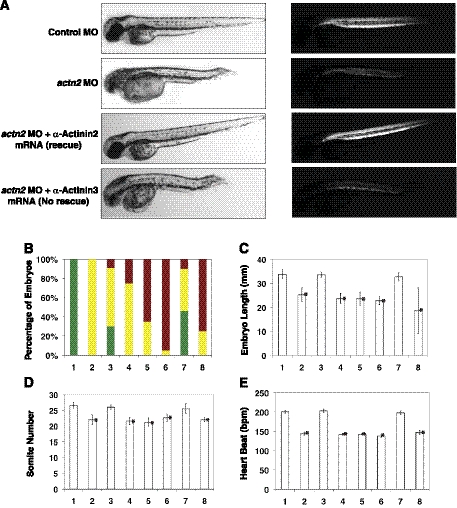
To confirm that each of the phenotypes described above was a direct consequence of α-actinin-2 deficiency and not an off-target effect of the MOs, we rescued actn2 exon2-intron2 morphants by coinjection of actn2 mRNA that was already spliced, and hence, resistant to the effects of the MOs (Fig. 9). Control fish injected with actn2 mRNA alone were indistinguishable from wild-type, while virtually all morphants exhibited the triad of abnormal musculature, enlarged heart, and abnormal eyes (Fig. 9A). When zebrafish actn2 mRNA was coinjected along with the exon2-intron2 MO, up to 30% of fry were rescued and developed with normal gross morphology. Furthermore, these fish exhibited normal birefringence, indicating restored sarcomeric organization in much of their skeletal muscles. Individual measurements of morphant length, somite number, and heart rate confirmed that all these aspects were largely rescued by actn2 mRNA (Fig. 9B–E).
Figure 9.
α-Actinin-2 and α-actinin-3 are not functionally redundant. A) α-Actinin-2 mRNA but not α-actinin-3 mRNA rescues the phenotypes caused by actn2 deficiency in zebrafish at 3 dpf. Left panel: pictures of live zebrafish embryos in normal light. Right panel: same embryos in polarized light (i.e., birefringence assay). Rescue/nonrescue pictures are representative of studies with both zebrafish and human α-actinin mRNAs. Three independent experiments were performed in each group. B) Percentage of rescue by overexpression of actn2 or actn3a/b mRNA. Green bars indicate proportion of morphologically normal fry; yellow bars, living fry with abnormal musculature and enlarged heart and eyes, red bars, dead fry. Percentages are normalized to proportion of viable embryos in the WT control clutches. C–E) Effect of mRNA overexpression on rescue of average somite length (C), somite number (D), and heartbeat (E). Bar 1, WT control; bar 2, actn2 MO; bar 3, actn2 MO + zebrafish actn2 mRNA; bar 4, actn2 MO + zebrafish actn3a mRNA; bar 5, actn2 MO+actn3b mRNA; bar 6, actn2 MO + zebrafish actn3a and actn3b mRNA; bar 7, actn2 MO + human ACTN2 mRNA; bar 8, actn2 MO + human ACTN3 mRNA. Data are means ± se, n=15. *P < 0.005; Student's t test, 2-tailed.
Many biochemical studies have demonstrated identical functional and binding characteristics between α-actinin-2 and α-actinin-3, yet the two proteins have maintained temporally and spatially distinct expression patterns throughout evolution, suggesting distinct structural and functional properties. We used the actn2 knockdown and rescue system to determine whether α-actinin-3 is capable of substituting for α-actinin-2 by coinjecting zebrafish actn3a and actn3b mRNAs individually or together with the actn2 exon2-intron2 MO. Remarkably, despite the high degree of sequence conservation between these isoforms, α-actinin-3a and/or α-actinin-3b overexpression had no measurable affect on ameliorating the developmental defects associated with actn2 knockdown (Fig. 9). Furthermore, overexpression of α-actinin-3 in actn2 morphant fish resulted in increased mortality (70–90%) relative to actn2 morphants alone and to morphants coinjected with actn2 mRNA (Fig. 9B). Coinjecting actn2 morphant fish with human ACTN2 mRNA also resulted in rescuing the phenotypes associated with α-actinin-2 deficiency in zebrafish. However, human ACTN3 overexpression did not result in rescue of any phenotypes observed in actn2-knockdown fish. Therefore, our studies show that, despite a great degree of sequence similarity, α-actinin-3 does not functionally substitute for α actinin-2 in vivo.
DISCUSSION
Evolutionary conservation of zebrafish α-actinins
Identification of only a single sarcomeric α-actinin (α-actinin-2) in all lower vertebrates previously led to assumptions that 2 sarcomeric α-actinins were unique to mammals. Identification of orthologs of all 4 human α-actinin genes in zebrafish, lizards, rabbits, and pigs suggests that, in addition to mammals, other vertebrates have also retained all 4 copies of α-actinin genes. Throughout evolution, the α-actinins have maintained a high sequence similarity to the ancestral α-actinin (Fig. 1). The high evolutionary conservation of amino acid sequence of α-actinins suggests strong evolutionary pressure consistent with conservation of function in all vertebrates. Therefore, studies on the α-actinin gene family using zebrafish as an in vivo model may provide important insight into their mechanistic roles in human development and diseases.
Our analysis identified the presence of an extra copy of actn3 that seems to be a result of a localized gene duplication event rather than genome duplication, as had been suggested in a previous study (23). None of the neighboring syntenic genes appeared to be coduplicated with actn3a and actn3b, nor was a duplicated actn3 identified in any other teleost genome. Interestingly, our survey of available genomes revealed that the porcine (Sus scrofa) ACTN3 gene is also duplicated, although the functional consequences of such duplication in the porcine genome remain unexplored (Fig. 1A). In support of a subfunctionalization mechanism being responsible for retention for the duplicated zebrafish genes, actn3b showed delayed expression relative to the other skeletal muscle-specific isoforms, suggesting that actn2 and actn3a may be required for formation and/or maintenance of primary muscle fibers, while actn3b may be required for skeletal muscle maintenance. Further, the extremely early induction of actn3a expression (by 4 hpf), suggests a unique nonmuscle role for this isoform well before the differentiation of any myogenic precursors. Retention of actn3b expression in fast muscle fibers along with actn3a could be explained by a dosage compensation theory where presence of an additional copy, and thus increased protein levels, may provide additional selective advantage to zebrafish whose bulk of skeletal muscles are composed of fast myofibers.
α-Actinin family members exhibit distinct spatiotemporal expression patterns during zebrafish development
The expression patterns of all 5 α-actinins revealed spatial and temporal partitioning, indicative of unique functional roles for each isoform. A study has previously identified that α-actinin family members are conserved and show distinct expression in zebrafish (23). Here, we find that actn1 is highly expressed in the central nervous system till 1 dpf, suggesting it may be required for cell fate and patterning of the developing nervous system. At the molecular level, mammalian α-actinin-1 has been shown to interact by protein–protein interactions with membrane receptors and other proteins involved in synaptic transmission in brain (31). Similar expression of α-actinin-1 in zebrafish brain suggests conserved roles of α-actinin-1 in zebrafish and mammals.
α-Actinin-4 expression in zebrafish was seen in brain, eyes, developing heart, and pronephric ducts and later in kidney and liver. The main function of the ichthyoid pronephric duct and kidney is osmoregulation. This expression of actn4 in zebrafish kidney is a novel and potentially important finding as mutation of α-actinin-4 in human and mice leads to a kidney disease, familial focal segmental glomerulosclerosis (32, 33), suggesting that zebrafish may be useful to model this disease.
Our studies show that during early zebrafish development, actn2 expression is dynamic with expression predominantly in brain, eye, heart, and skeletal muscles. In skeletal muscles, the expression of actn2 is primarily seen in slow muscle fibers during development. In both the previous study (23) and our own in situ hybridizations, actn2 transcripts were undetectable in trunk skeletal muscle after 1 dpf; however, use of our new α-actinin-2 specific antibody, F6523, revealed stable expression of this isoform in slow myofibers. In mammalian CNS, actn2 expression is observed in dendritic processes of neurons in the stratium, hippocampus, cortex, and substantia nigra, where it is believed to contribute to synaptic plasticity (34, 35). Considering the similar expression patterns of actn2 in zebrafish and mammalian nervous systems, it is attractive to hypothesize that it may play similar roles in CNS development and function across species. Finally, expression of actn2 was also present in developing zebrafish heart, suggesting its involvement in the development and/or function of cardiac muscle, similar to the situation in higher vertebrates.
α-Actinin-2 deficiency results in sarcomeric abnormalities in cardiac and skeletal muscles
In zebrafish skeletal muscles, we first detected actn2 expression around 10 dpf, which suggests that, after desmin, α-actinin-2 is among the first sarcomeric proteins to be expressed during zebrafish somitogenesis. Although we cannot know whether the sarcomeric disorganization seen in actn2 morphant muscles is due to reduced myofibrillogenesis or loss of stability and increased turnover, α-actinin-2 clearly plays an important role in sarcomeric structure. This condition could be due to loss of protein–protein interactions, as α-actinin has been shown to interact directly or indirectly with many of the proteins that eventually form mature myofibers as well as with elements of intermediate filaments, sarcoplasmic reticulum, costameres, and signaling molecules (36). In cardiac muscles of actn2 morphant zebrafish, decreased organization of thin and thick filaments as well as smaller size contractile apparatus were observed. The presence of some normal appearing sarcomeric structures could be explained by either incomplete knockdown or the partial rescue of actn2 deficiency by other isoforms of α-actinin (actn1 and actn4) that are coexpressed with actn2 in zebrafish heart and can form Z-discs under certain conditions (38). Reduced sarcomeric assemblies in actn2-deficient heart illustrates the requirement for α-actinin-2 in formation and maintenance of mature myocardium, which is not replaceable by other α-actinin isoforms, including α-actinin-3, as demonstrated by our rescue experiments (Fig. 9).
Actn2 and actn3 have functionally distinct activities in vivo
Previous studies in an Actn3-knockout mouse model showed that up-regulation of endogenous α-actinin-2 acted as a compensatory mechanism, resulting in a milder phenotype in these mice (15). However, we did not find the reverse to be true in the zebrafish system. No up-regulation of actn3a or actn3b was observed in actn2-deficient embryos, as observed by quantitative RT-PCR (data not shown). Further, despite the fact that each of the different α-actinin isoforms can localize at Z lines in cell culture systems (38), overexpression of actn3a and/or actn3b in developing zebrafish embryos was not sufficient to rescue the phenotype caused by actn2 deficiency. Similarly, human ACTN3 could not rescue the phenotypes associated with actn2 knockdown in fish, although human ACTN2 was effective. The difference in functional requirement of these two sarcomeric α-actinins is not a manifestation of differential spatial or temporal expression patterns, as ubiquitous overexpression of α-actinin-3 did not rescue the phenotypes of actn2 deficient zebrafish as efficiently as rescue with α-actinin-2 mRNA.
Therefore, these results lead us to believe that since early in vertebrate evolution, the primary sequences of α-actinin-2 and α-actinin-3 evolved differences to optimize their function in fast and slow muscle fibers. Future studies exploring the mechanisms of differential molecular and physiological properties of the sarcomeric α-actinins will significantly help us to understand the role of α-actinins in development and diseases of skeletal and cardiac muscles.
Supplementary Material
Acknowledgments
The authors gratefully thank Dr. Genri Kawahara and Dr. Yukio Nakamura (Children's Hospital Boston, Boston, MA, USA) for sharing reagents. The authors thank Ryan Darnall for technical help in these studies and Dr. Yi Zhao for help with zebrafish genomic analysis. The authors thank members of the A.H.B. and L.M.K. laboratories for critically reading the manuscript. The authors also thank Chris Lawrence and Jason Best (Zebrafish Facility, Children's Hospital Boston) for sharing their wonderful expertise with zebrafish. Antibodies F59 and F310 (developed by F. E. Stockdale) and MF20 (developed by D. A. Fischman) were obtained from the Developmental Studies Hybridoma Bank supported under the auspices of the U.S. National Institute for Child Health and Human Development and maintained by The University of Iowa Department of Biology (Iowa City, IA, USA). Confocal microscopy was performed at Children's Hospital Boston Intellectual and Developmental Disability Research Center imaging core, supported by U.S. National Institutes of Health (NIH) grant NIH-P30-HD-18655.
This work was supported by funding from the NIH, National Institute of Arthritis and Musculoskeletal and Skin Disease (R01 AR044345), the Muscular Dystrophy Association (MDA201302), and the Lee and Penny Anderson Family Foundation to A.H.B. and a William Hearst Foundation fellowship to V.G.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- dpf
- days postfertilization
- hpf
- hours postfertilization
- MO
- morpholino
- UTR
- untranslated region.
REFERENCES
- 1. Sjoblom B., Salmazo A., Djinovic-Carugo K. (2008) Alpha-actinin structure and regulation. Cell. Mol. Life Sci. 65, 2688–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Otey C. A., Carpen O. (2004) Alpha-actinin revisited: a fresh look at an old player. Cell Motil. Cytoskeleton 58, 104–111 [DOI] [PubMed] [Google Scholar]
- 3. Broderick M. J., Winder S. J. (2005) Spectrin, alpha-actinin, and dystrophin. Adv. Protein Chem. 70, 203–246 [DOI] [PubMed] [Google Scholar]
- 4. Schleicher M., Noegel A., Schwarz T., Wallraff E., Brink M., Faix J., Gerisch G., Isenberg G. (1988) A Dictyostelium mutant with severe defects in alpha-actinin: its characterization using cDNA probes and monoclonal antibodies. J. Cell Sci. 90(Pt. 1), 59–71 [DOI] [PubMed] [Google Scholar]
- 5. Chan Y., Tong H. Q., Beggs A. H., Kunkel L. M. (1998) Human skeletal muscle-specific alpha-actinin-2 and -3 isoforms form homodimers and heterodimers in vitro and in vivo. Biochem. Biophys. Res. Commun. 248, 134–139 [DOI] [PubMed] [Google Scholar]
- 6. Barstead R. J., Kleiman L., Waterston R. H. (1991) Cloning, sequencing, and mapping of an alpha-actinin gene from the nematode Caenorhabditis elegans. Cell Motil. Cytoskeleton 20, 69–78 [DOI] [PubMed] [Google Scholar]
- 7. Usmanova A., Astier C., Mejean C., Hubert F., Feinberg J., Benyamin Y., Roustan C. (1998) Coevolution of actin and associated proteins: an alpha-actinin-like protein in a cyanobacterium (Spirulina platensis). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 120, 693–700 [DOI] [PubMed] [Google Scholar]
- 8. Youssoufian H., McAfee M., Kwiatkowski D. J. (1990) Cloning and chromosomal localization of the human cytoskeletal alpha-actinin gene reveals linkage to the beta-spectrin gene. Am. J. Hum. Genet. 47, 62–71 [PMC free article] [PubMed] [Google Scholar]
- 9. Fyrberg E., Kelly M., Ball E., Fyrberg C., Reedy M. C. (1990) Molecular genetics of Drosophila alpha-actinin: mutant alleles disrupt Z disc integrity and muscle insertions. J. Cell Biol. 110, 1999–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beggs A. H., Byers T. J., Knoll J. H., Boyce F. M., Bruns G. A., Kunkel L. M. (1992) Cloning and characterization of two human skeletal muscle alpha-actinin genes located on chromosomes 1 and 11. J. Biol. Chem. 267, 9281–9288 [PubMed] [Google Scholar]
- 11. Burridge K., Feramisco J. R. (1981) Non-muscle alpha actinins are calcium-sensitive actin-binding proteins. Nature 294, 565–567 [DOI] [PubMed] [Google Scholar]
- 12. Kremerskothen J., Teber I., Wendholt D., Liedtke T., Bockers T. M., Barnekow A. (2002) Brain-specific splicing of alpha-actinin 1 (ACTN1) mRNA. Biochem. Biophys. Res. Commun. 295, 678–681 [DOI] [PubMed] [Google Scholar]
- 13. Berman Y., North K. N. (2010) A gene for speed: the emerging role of alpha-actinin-3 in muscle metabolism. Physiology (Bethesda) 25, 250–259 [DOI] [PubMed] [Google Scholar]
- 14. North K. N., Yang N., Wattanasirichaigoon D., Mills M., Easteal S., Beggs A. H. (1999) A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nat. Genet. 21, 353–354 [DOI] [PubMed] [Google Scholar]
- 15. MacArthur D. G., Seto J. T., Raftery J. M., Quinlan K. G., Huttley G. A., Hook J. W., Lemckert F. A., Kee A. J., Edwards M. R., Berman Y., Hardeman E. C., Gunning P. W., Easteal S., Yang N., North K. N. (2007) Loss of ACTN3 gene function alters mouse muscle metabolism and shows evidence of positive selection in humans. Nat. Genet. 39, 1261–1265 [DOI] [PubMed] [Google Scholar]
- 16. Schultheiss T., Choi J., Lin Z. X., DiLullo C., Cohen-Gould L., Fischman D., Holtzer H. (1992) A sarcomeric alpha-actinin truncated at the carboxyl end induces the breakdown of stress fibers in PtK2 cells and the formation of nemaline-like bodies and breakdown of myofibrils in myotubes. Proc. Natl. Acad. Sci. U. S. A. 89, 9282–9286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohapatra B., Jimenez S., Lin J. H., Bowles K. R., Coveler K. J., Marx J. G., Chrisco M. A., Murphy R. T., Lurie P. R., Schwartz R. J., Elliott P. M., Vatta M., McKenna W., Towbin J. A., Bowles N. E. (2003) Mutations in the muscle LIM protein and alpha-actinin-2 genes in dilated cardiomyopathy and endocardial fibroelastosis. Mol. Genet. Metab. 80, 207–215 [DOI] [PubMed] [Google Scholar]
- 18. Chiu C., Bagnall R. D., Ingles J., Yeates L., Kennerson M., Donald J. A., Jormakka M., Lind J. M., Semsarian C. (2010) Mutations in alpha-actinin-2 cause hypertrophic cardiomyopathy: a genome-wide analysis. J. Am. Coll. Cardiol. 55, 1127–1135 [DOI] [PubMed] [Google Scholar]
- 19. O'Sullivan M. L., O'Grady M. R., Pyle W. G., Dawson J. F. (2011) Evaluation of 10 genes encoding cardiac proteins in Doberman Pinschers with dilated cardiomyopathy. Am. J. Vet. Res. 72, 932–939 [DOI] [PubMed] [Google Scholar]
- 20. Guyon J. R., Steffen L. S., Howell M. H., Pusack T. J., Lawrence C., Kunkel L. M. (2007) Modeling human muscle disease in zebrafish. Biochim. Biophys. Acta 1772, 205–215 [DOI] [PubMed] [Google Scholar]
- 21. Gupta V., Kawahara G., Gundry S. R., Chen A. T., Lencer W. I., Zhou Y., Zon L. I., Kunkel L. M., Beggs A. H. (2011) The zebrafish dag1 mutant: a novel genetic model for dystroglycanopathies. Hum. Mol. Genet. 20, 1712–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steffen L. S., Guyon J. R., Vogel E. D., Beltre R., Pusack T. J., Zhou Y., Zon L. I., Kunkel L. M. (2007) Zebrafish orthologs of human muscular dystrophy genes. BMC Genomics 8, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holterhoff C. K., Saunders R. H., Brito E. E., Wagner D. S. (2009) Sequence and expression of the zebrafish alpha-actinin gene family reveals conservation and diversification among vertebrates. Dev. Dyn. 238, 2936–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Westerfield M. (2007) The Zebrafish Book, University of Oregon Press, Eugene, OR, USA [Google Scholar]
- 25. Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thisse C., Thisse B. (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 [DOI] [PubMed] [Google Scholar]
- 27. Hirata H., Watanabe T., Hatakeyama J., Sprague S. M., Saint-Amant L., Nagashima A., Cui W. W., Zhou W., Kuwada J. Y. (2007) Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease. Development 134, 2771–2781 [DOI] [PubMed] [Google Scholar]
- 28. Moncrief N. D., Kretsinger R. H., Goodman M. (1990) Evolution of EF-hand calcium-modulated proteins. I. Relationships based on amino acid sequences. J. Mol. Evol. 30, 522–562 [DOI] [PubMed] [Google Scholar]
- 29. Henry C. A., Poage C. T., McCarthy M. B., Campos-Ortega J., Cooper M. S. (2005) Regionally autonomous segmentation within zebrafish presomitic mesoderm. Zebrafish 2, 7–18 [DOI] [PubMed] [Google Scholar]
- 30. Arikawa K., Williams D. S. (1989) Organization of actin filaments and immunocolocalization of alpha-actinin in the connecting cilium of rat photoreceptors. J. Comp. Neurol. 288, 640–646 [DOI] [PubMed] [Google Scholar]
- 31. Cabello N., Remelli R., Canela L., Soriguera A., Mallol J., Canela E. I., Robbins M. J., Lluis C., Franco R., McIlhinney R. A., Ciruela F. (2007) Actin-binding protein alpha-actinin-1 interacts with the metabotropic glutamate receptor type 5b and modulates the cell surface expression and function of the receptor. J. Biol. Chem. 282, 12143–12153 [DOI] [PubMed] [Google Scholar]
- 32. Yao J., Le T. C., Kos C. H., Henderson J. M., Allen P. G., Denker B. M., Pollak M. R. (2004) Alpha-actinin-4-mediated FSGS: an inherited kidney disease caused by an aggregated and rapidly degraded cytoskeletal protein. PLoS Biol. 2, e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaplan J. M., Kim S. H., North K. N., Rennke H., Correia L. A., Tong H. Q., Mathis B. J., Rodriguez-Perez J. C., Allen P. G., Beggs A. H., Pollak M. R. (2000) Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 24, 251–256 [DOI] [PubMed] [Google Scholar]
- 34. Mills M., Yang N., Weinberger R., Vander Woude D. L., Beggs A. H., Easteal S., North K. (2001) Differential expression of the actin-binding proteins, alpha-actinin-2 and -3, in different species: implications for the evolution of functional redundancy. Hum. Mol. Genet. 10, 1335–1346 [DOI] [PubMed] [Google Scholar]
- 35. Wyszynski M., Kharazia V., Shanghvi R., Rao A., Beggs A. H., Craig A. M., Weinberg R., Sheng M. (1998) Differential regional expression and ultrastructural localization of alpha-actinin-2, a putative NMDA receptor-anchoring protein, in rat brain. J. Neurosci. 18, 1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanger J. W., Wang J., Holloway B., Du A., Sanger J. M. (2009) Myofibrillogenesis in skeletal muscle cells in zebrafish. Cell Motil. Cytoskeleton 66, 556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang W., Zhang R., Xu X. (2009) Myofibrillogenesis in the developing zebrafish heart: A functional study of tnnt2. Dev. Biol. 331, 237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanger J. M., Mittal B., Pochapin M. B., Sanger J. W. (1986) Myofibrillogenesis in living cells microinjected with fluorescently labeled alpha-actinin. J. Cell Biol. 102, 2053–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.