Abstract
Beneficial microbes and probiotics show promise for the treatment of pediatric gastrointestinal diseases. However, basic mechanisms of probiosis are not well understood, and most investigations have been performed in germ-free or microbiome-depleted animals. We sought to functionally characterize probiotic-host interactions in the context of normal early development. Outbred CD1 neonatal mice were orally gavaged with one of two strains of human-derived Lactobacillus reuteri or an equal volume of vehicle. Transcriptome analysis was performed on enterocyte RNA isolated by laser-capture microdissection. Enterocyte migration and proliferation were assessed by labeling cells with 5-bromo-2′-deoxyuridine, and fecal microbial community composition was determined by 16S metagenomic sequencing. Probiotic ingestion altered gene expression in multiple canonical pathways involving cell motility. L. reuteri strain DSM 17938 dramatically increased enterocyte migration (3-fold), proliferation (34%), and crypt height (29%) compared to vehicle-treated mice, whereas strain ATCC PTA 6475 increased cell migration (2-fold) without affecting crypt proliferative activity. In addition, both probiotic strains increased the phylogenetic diversity and evenness between taxa of the fecal microbiome 24 h after a single probiotic gavage. These experiments identify two targets of probiosis in early development, the intestinal epithelium and the gut microbiome, and suggest novel mechanisms for probiotic strain-specific effects.—Preidis, G. A., Saulnier, D. M., Blutt, S. E., Mistretta,T.-A., Riehle, K. P., Major, A. M., Venable, S. F., Finegold, M. J., Petrosino, J. F., Conner, M. E., Versalovic, J. Probiotics stimulate enterocyte migration and microbial diversity in the neonatal mouse intestine.
Keywords: animal models, cell movement, epithelium, metagenome
Probiotics are living microorganisms that, when ingested, confer health benefits by altering the balance of intestinal microbial communities (1, 2). The most striking evidence of probiotic efficacy is provided by pediatric gastroenterology (3). Multiple systematic reviews of controlled trials with thousands of patients conclude that probiotics reduce the duration of diarrhea in infants and children with acute gastroenteritis (4) and persistent diarrhea (5) and reduce the risk of both necrotizing enterocolitis and all-cause mortality in preterm, low-birth-weight neonates (6, 7). Despite this evidence, optimal therapeutic regimens are not yet known, which fuels the debate regarding whether or not probiotics are ready for integration into routine clinical practice (8, 9).
A better understanding of basic mechanisms underlying probiosis will facilitate optimal strain selection. Probiotics are believed to directly antagonize enteric pathogens, modulate innate or adaptive immunity, and strengthen mucosal barrier function (10–12). Most of the specific molecules and pathways mediating these effects have yet to be identified. Furthermore, most mechanisms have been proposed with studies delivering microbes to cultured cells, germ-free animals, or gnotobiotic mice with simple, “humanized” microbiota (13–16). Few probiotic effects on host physiology have been reported in more complex intestinal environments.
To address this knowledge gap, we used laser-capture microdissection to selectively harvest enterocyte RNA from conventionally reared outbred neonatal mice after ingesting one of two strains of Lactobacillus reuteri, a model probiotic organism with well-documented effects against acute gastroenteritis (17, 18) and infantile colic (19, 20). L. reuteri strains DSM 17938 and ATCC PTA 6475 were selected for these studies because they have been tested in humans and mice, and complete genome sequences for both strains are available (21). Enterocyte transcriptome profiling revealed multiple genes and canonical pathways altered by probiotics, most prominently those affecting cell motility. Using in vivo labeling to track the progression of enterocytes from the stem cell region to the villus tips, we reveal for the first time probiotic strain-specific increases in enterocyte migration and proliferation, which could help expel invasive enteric pathogens from the epithelium. Furthermore, 16S metagenomic sequencing of stool-derived bacteria indicated that administration of a single probiotic strain increases the phylogenetic diversity of the distal intestinal microbiome, which may confer resilience against pathogen-induced perturbations of commensal microbial communities. These data suggest that the intestinal epithelium and the gut microbiome are important targets of probiotic-based therapies and highlight novel mechanisms underlying probiosis and microbial strain-specificity.
MATERIALS AND METHODS
Probiotic strains
L. reuteri DSM 17938 and ATCC PTA 6475 (Biogaia AB, Stockholm, Sweden), originally isolated from breast milk of healthy Peruvian and Finnish women, respectively, were grown separately in anaerobic conditions to stationary phase in deMan, Rogosa, Sharpe (MRS) medium (Difco Laboratories, Detroit, MI, USA), washed 3 times with phosphate-buffered saline (PBS) to remove medium, and reconstituted in PBS at a concentration of 2 × 109 cfu/ml. This concentration provided the highest dose of bacteria that could be gavaged through fine polyethylene tubing at a volume of 50 μl, the maximum volume tolerable to neonatal mice. Preparations were made daily.
Mouse model
Male and female CD-1 neonatal mice (Charles River Laboratories, Kingston, NY, USA) from multiple litters were pooled, randomly redistributed to minimize between-group genetic variations, and housed in a traditional, specific pathogen-free, nonsterile environment. For transcriptome and metagenomic studies, pups received a single oral gavage of one probiotic strain (108 cfu/50 μl PBS) or vehicle (50 μl PBS) at 8 d of life. For functional characterizations, mice received daily gavages with probiotics or vehicle beginning at d 5. To label actively dividing cells, 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO, USA) was injected intraperitoneally (30 mg/kg body weight in 50 μl PBS; ref. 22) on d 8. All subsequent analyses were performed with the investigator blinded to treatment group. All protocols were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Fluorescence in situ hybridization (FISH)
Whole intestines were fixed in Carnoy solution, sectioned at 4 μm, treated for 1 h at 37°C with 1 mg/ml lysozyme (Sigma-Aldrich), hybridized for 45 min at 51°C with 50 ng/μl probe specific to a unique 16S rRNA sequence common to all L. reuteri (5′-GATCCATCGTCAATCAGGTGC-3′), and conjugated to Cy3 (Sigma-Aldrich; ref. 23). Slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) and imaged at ×200 with an Eclipse 90i fluorescent microscope (Nikon Instruments, Melville, NY, USA).
Laser-capture microdissection and microarray analysis
Terminal 4 cm of ileum was flushed and flash-frozen (24), sectioned at 7 μm on polyethylene naphthalate (PEN)-membrane glass slides, and dehydrated with xylene (Histogene Frozen Section Kit; Applied Biosystems/Arcturus, Foster City, CA, USA). Enterocytes from 24 consecutive sections/mouse were collected with ArcturusXT Laser-Capture Microdissection System in CapSure HS LCM caps (Applied Biosystems/Arcturus), and an average of 350 ng RNA/mouse was isolated (PicoPure RNA Isolation Kit, Applied Biosystems/Arcturus). Biotinylated cRNA (TotalPrep RNA Amplification Kit, Illumina, San Diego, CA, USA) was hybridized to MouseWG-6 v2.0 BeadChips (Illumina) according to the manufacturer's protocols. Data were normalized using GeneSpring GX 11.02 (Agilent Technologies, Santa Clara, CA, USA) after background correction (available at: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE26992). Filtered gene lists (≥1.5 fold-change; P<0.05) were generated by one-way ANOVA for each time point and were probed with Ingenuity Pathway Analysis (IPA; Ingenuity Systems, Redwood City, CA, USA) to discover the most significantly altered canonical pathways and interacting protein networks.
Immunohistochemistry, migration, and proliferation assays
Whole intestines fixed in 10% formalin were sectioned at 3 μm, labeled with rabbit polyclonal antibody to β-actin (1:200; Thermo Fisher Scientific, Waltham, MA, USA) after enzymatic digestion, and counterstained with CAT hematoxylin (Biocare Medical, Concord, CA, USA). Alternatively, sections from BrdU-injected mice were labeled with rat anti-BrdU (1:200; Accurate Chemical, Westbury, NY, USA). To assess enterocyte migration in intestines harvested between 4 h and 6 d after BrdU, locations of the foremost and least-progressed BrdU-labeled enterocytes were recorded as the percent of epithelial cell positions between the crypt-villus boundary (0%) and villus tip (100%) in 10 well-oriented crypt-villus units per intestinal segment. Crypt and villus height were recorded using NIS-Elements (Nikon). To assess enterocyte proliferation in intestines harvested 4 h after BrdU, the proportion of crypt cells that had incorporated BrdU in 10 well-oriented crypts was determined.
16S rRNA sequence-based survey of the distal gut microbiome
In neonatal mice, stool is not available in adequate amounts for individual sampling until the third week of life (25); thus, samples were pooled from 20 8-d-old pups/group. Stool character did not differ between treatment groups or day of collection, and an average of 2.5 μg genomic DNA/group was isolated according to the manufacturer's protocol (QIAamp DNA Stool Mini Kit, Qiagen, Germantown, MD, USA), quantified using the Quant-iT PicoGreen double-stranded DNA assay (Invitrogen, Carlsbad, CA, USA). High-fidelity PCR was carried out in duplicate in 96-well plates to amplify the V3–V5 region of the microbial 16S rRNA gene. Master mix, composed of 13.85 μl RNase/DNase free water, 2 μl 10× AccuPrime PCR Buffer II, and 0.15 μl AccuPrime TaqDNA Polymerase (Invitrogen) was added to individual wells, then centrifuged at 2000 rpm. For the initial reaction, 2 μl of sample DNA diluted 1:1 in water was added to the reaction wells, along with 2 μl of barcoded universal primers (Eurofins MWG Operon, Huntsville, AL, USA), which contained the A and B sequencing adaptors (Roche/454 Life Sciences, Branford, CT, USA). The sequence of forward primer B-357F for the V3-V5 region was 5′-cctatcccctgtgtgccttggcagtctcaGCCTACGGGAGGCAGCAG-3′; the sequence of reverse primer A-936R was 5′-ccatctcatccctgcgtgtctccgactcagNNNNNCCGTCAATTCMTTTRAGT-3′. The sequences of the A and B adaptors are shown in lowercase letters; N represents a bar code that is unique for each sample.
Two PCR reactions were run per primer set. The plate was sealed, vigorously vortexed, briefly centrifuged at 2000 rpm, then placed in a Veriti 96-well-plate thermocycler (Applied Biosystems). Cycling conditions were 95°C for 2 min, followed by 30 cycles of 94°C for 20 s, 56°C for 30 s, and 72°C for 5 min, with a final extension period of 20 min at 4°C. PCR products were cleaned with Agencourt AMPure XP 1.8× volume beads (Beckman Coulter Genomics, Danvers, MA, USA), which were eluted with 25 μl 1× low TE (pH 8.0) and transferred to a new 96-well plate. PCR products were quantified using Quant-iT ds DNA high-sensitivity assay (Invitrogen) and quality-controlled on an Agilent 2100 bioanalyzer. All samples were diluted to equal concentrations, based on the sample with the lowest concentration. Equal volumes of each sample (5–10 μl) were concentrated using a MinElute column (Qiagen). Amplicon libraries were generated according to the manufacturer's protocol (Roche/454 Life Sciences). Sequencing was performed from the B end using the 454/Roche B sequencing primer kit with a Roche Genome Sequencer GS-FLX Titanium system. DNA amplification and metagenomic sequencing were performed at the Human Genome Sequencing Center, Houston, TX, USA.
Individual samples were isolated and quality-filtered from standard flowgram format (SFF) files, using the following parameters: ≥200 nucleotides (nt); ≥20 average quality score; match exact bar code and exact proximal A primer; cut at first N or n; and cut at matched distal B primer. Up to 4 mismatches were allowed. After filtering, a mean of 65,628 sequences/treatment group (average read length 500 nt) were taxonomically binned using a local copy of RDP Classifier (Ribosomal Database Project, East Lansing, MI, USA; ref. 26) and normalized by the abundance of taxa present in each sample. Operational taxonomic unit (OTU) tables were produced from a local copy of QIIME 1.1.0 (27), using a chained multistep OTU-selecting algorithm. Deconvoluted sequences were concatenated into a single Fasta file, and a mapping file was created to contain the sample names and clinical metadata. The first step of the algorithm was to select OTUs from the sequence file using PrefixSuffix, a fast method. PrefixSuffix collapsed sequences that were identical in the first and last bases at the default length of 50 nt. Representative sequences were then chosen from the PrefixSuffix output, and a slow, more rigorous OTU-selecting algorithm, CD-HIT (28), was used, which applied a longest-sequence-first list removal algorithm to remove and cluster sequences above an identity threshold of 97%. The resultant OTU maps from the fast and slow methods were merged. The final OTU table was based on the relative abundance of each representative sequence in relation to the individual samples.
OTU tables were analyzed to study the relatedness of clinical metadata attributes for multiple diversity measurements. Shannon (29) and Chao1 diversity indices (30) were used to compute and visualize α diversity. Richness, evenness, rarefaction, and diversity indices were calculated using the Vegan package of R Statistical Programming (http://www.r-project.org). To minimize errors, decrease manual oversight, and increase repeatability, a local pipeline was created to submit the majority of these tasks to a cluster for processing.
Statistical analysis
Data were reported as means ± sd and compared by 1-way ANOVA. Tukey's multiple comparison tests were used to examine between-group differences when the global comparison was significant. Analyses were performed using GraphPad Prism 5.01 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Bacterial colonization studies by FISH
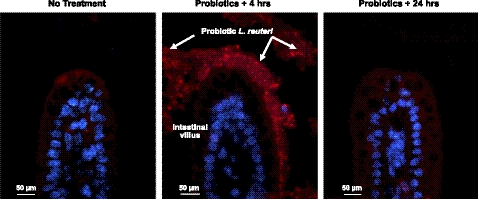
To gain insight into physiological effects of probiotic consumption during early development, we sought to explore intestinal epithelial cell gene expression under two separate conditions: when probiotics were in the distal small intestine, and shortly after excretion. To determine the timing of human-derived probiotic transit through the neonatal mouse gastrointestinal tract, we used L. reuteri species-specific FISH. Endogenous L. reuteri is present in the adult mouse intestine (data not shown), but had not yet colonized 8-d-old pups (Fig. 1). FISH identified L. reuteri in the distal ileum 3–4 h after gavage on d 8, but not 24 h later, indicating that human-derived probiotics were transiently present and rapidly excreted from the small bowel.
Figure 1.
Human-derived L. reuteri did not persist in the neonatal mouse small intestine. FISH using a Cy3-conjugated probe (red) to label L. reuteri (arrows); DAPI (blue) labeled host cell nuclei. Similar results were observed with both probiotic strains; L. reuteri 17938 shown in the ileum of 8-d-old mice, ×200 view.
Enterocyte transcriptome pathway analysis and cell motility
Based on these results, we harvested RNA from enterocytes of the distal ileum by laser-capture microdissection at 4 and 24 h postgavage, and identified probe sets that were differentially expressed between probiotic- and vehicle-treated pups using Illumina MouseWG-6 GeneChips. After 4 and 24 h, administration of L. reuteri strain 17938 led to differential expression of 29 and 97 enterocyte probe sets, whereas treatment with strain 6475 altered expression of 33 and 395 probe sets, respectively (data not shown). The two probiotic strains similarly up-regulated or down-regulated 4 probe sets at 4 h and 75 probe sets at 24 h. Differentially expressed genes were queried with IPA to identify whether these transcriptome changes might predict biological processes amenable to functional characterizations. When L. reuteri strain 17938 was present in the small intestine, the most significantly altered transcriptome pathways involved amine and carbohydrate oxidative metabolism, including downregulated genes encoding monoamine oxidase B, methyltransferase-like 7B, fructose bisphosphatase 1, and glucose-6-phosphatase. When strain 6475 was present in the intestine, the most significantly altered pathways predicted immune responses, such as increased expression of lymphocyte protein tyrosine kinase and tumor necrosis factor superfamily receptor member 13c (Supplemental Fig. S1).
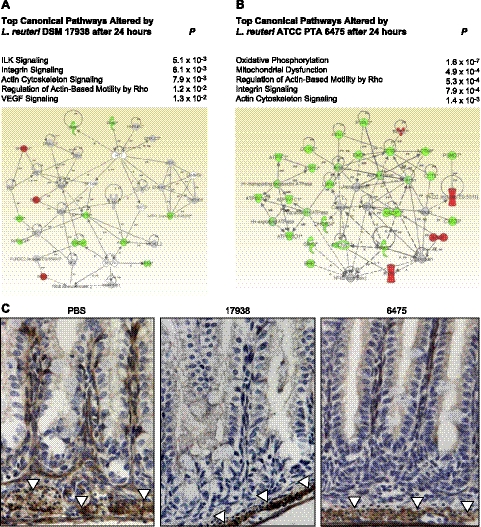
Unexpectedly, both L. reuteri strains altered multiple pathways mediating cell motility. The 4 canonical pathways most significantly modulated 24 h after administration of strain 17938 predicted changes in cell migration: integrin-linked kinase (ILK) signaling, integrin signaling, actin cytoskeleton signaling, and regulation of actin-based motility by rho (Fig. 2A). Three of these pathways were among the 5 most significantly altered pathways at 24 h after treatment with strain 6475. Cell migration pathway changes after treatment with either probiotic strain included down-regulation of multiple enterocyte genes, encoding β-actin, α-4-actinin, profilin-1, and the catalytic subunit of protein phosphatase-1, whose protein products contribute to cellular stabilization and suppression of cell motility.
Figure 2.
L. reuteri altered enterocyte RNA pathways involving cell motility. A, B) IPA of differentially expressed enterocyte genes showing the 5 most significantly altered canonical pathways and the highest-scoring protein interaction network for each probiotic strain vs. PBS (n=3 mice/group), 24 h after gavage with strain 17938 (A) or strain 6475 (B). C) Immunohistochemistry reveals diminished production of stabilizing cytoskeletal protein β-actin (product of ACTB gene in above networks) in enterocytes. Compared to PBS controls (left panel), decreased labeling at the basement membrane was found in mice treated with L. reuteri strains 17938 (center panel) or 6475 (right panel). However, smooth muscle cell β-actin (arrowheads) was labeled in all treatment groups. Red represents up-regulated genes; green represents down-regulated genes; gray represents genes not differentially expressed; white represents genes with no array data; solid lines/arrows represent direct interactions; dashed lines/arrows represent indirect interactions.
The most significantly altered host transcriptome pathway by L. reuteri was oxidative phosphorylation (P=1.57×10−7), based on differential expression of 10 related, down-regulated genes 24 h after treatment with strain 6475 (Fig. 2B). Thus, probiotics affect enterocyte transcriptomes and biological pathways involving known effects, namely, metabolism and immunomodulation, and target previously unidentified pathways governing cell motility.
To illustrate changes in cell motility pathways further, we used immunohistochemistry to label the cytoskeletal stabilizing protein β-actin, product of the enterocyte gene ACTB, which was down-regulated strongly 24 h after treatment with either probiotic strain. β-actin was similarly visible in smooth muscle layers surrounding the intestine in all mice (Fig. 2C). However, compared to vehicle-treated control mice, less labeled protein was apparent in the villi of mice treated 24 h previously with either strain of L. reuteri. Thus, probiotic treatment diminishes the production of stabilizing cytoskeletal and signaling proteins in enterocytes, which could consequently facilitate the ability of intestinal epithelial cells to migrate along the crypt-villus axis.
Strain-specific probiotic enhancement of enterocyte migration and proliferation
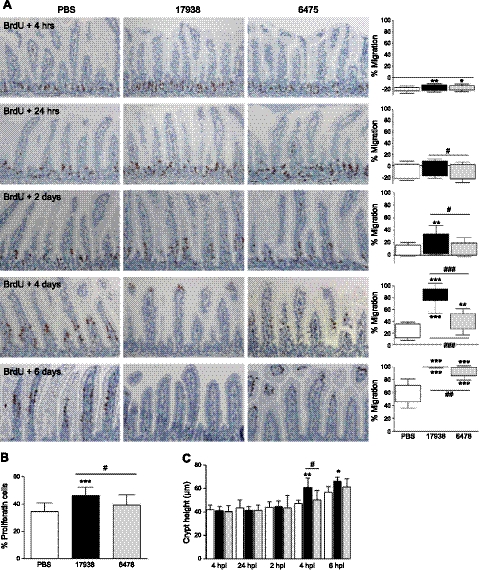
Based on ileal transcriptomic data, we quantified cell motility in vivo by labeling actively dividing cells with BrdU, a DNA-intercalating agent. BrdU was incorporated into enterocyte nuclei in the stem cell region and remained with cells during their transit to the villus tips (Fig. 3A). Just 4 h after injection, BrdU-labeled enterocytes had progressed further in probiotic-treated compared to vehicle-treated mice. Strain-specific differences were first observed after 24 h, with strain 17938 stimulating enterocytes to migrate faster than strain 6475 (P<0.05). After 4 d, BrdU-labeled cells from 17938-treated mice had migrated 3-fold further than those from PBS-treated mice (P<0.001); strain 6475 also increased migration (P<0.01), but to a lesser extent. At 6 d, BrdU-labeled enterocytes in control mice were located between 46 and 72% of the villus height, but only a few cells remained in the villus tips in mice receiving 6475. BrdU was no longer found in the epithelium of 17938-treated mice 6 d postinjection.
Figure 3.
Probiotics increased enterocyte migration and proliferation in the ileum in a strain-specific manner. A) Immunohistochemistry for BrdU at different times after injection. Graphs show mean ± sd position of foremost and least-progressed BrdU-labeled enterocytes. B) Crypt cell proliferation, determined 4 h post-BrdU. C) Crypt height at the same time points. Mice were gavaged daily with probiotics or vehicle. n = 7 mice/group for migration and crypt height analyses; n = 15 mice/group for proliferation analysis; ×200 view; hpi, hours postinjection; dpi, days postinjection. *P < 0.05, **P < 0.01, ***P < 0.001 vs. PBS controls; #P < 0.05, ##P < 0.01, ###P < 0.001 between strains.
To determine whether increased ileal enterocyte migration was associated with increased cell proliferation, we determined the percent of crypt cells that had incorporated BrdU within 4 h of injection. In PBS-treated mice and mice receiving L. reuteri strain 6475, 34.6 and 39.3% of ileum crypt cells were BrdU-positive, respectively. However, BrdU had incorporated in 46.2% of crypt cells after DSM 17938 ingestion (Fig. 3B; P<0.001 vs. PBS; P<0.05 between strains). Increased proliferative activity after 17938 was associated with increased crypt height (up to 23% greater than controls), which was significant at 4 and 6 d after BrdU injection (Fig. 3C; P<0.01 vs. PBS and P<0.05 vs. 6475). Villus height did not differ between treatment groups (data not shown). Similar probiotic effects on cell migration and proliferation were observed in the duodenum and jejunum (Supplemental Figs. S2 and S3). However, probiotics did not alter colonocyte migration, proliferation, or crypt height (Supplemental Fig. S4). These functional assays indicate that different strains of the same probiotic species differentially modulate neonatal small bowel architecture and epithelial cell physiology.
16S metagenomic survey of the distal intestinal microbiome
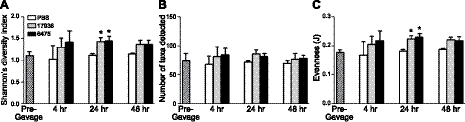
Probiotics are thought to act by improving the host's intestinal microbial balance (2), although little evidence supports these effects on the developing gut microbiome. To determine whether treatment with L. reuteri alters distal intestinal microbial community composition, we obtained serial stool samples from neonatal mice at 4 different time points in relation to treatment: prior to gavage (baseline microbiome for 8-d-old pups), and 4, 24, and 48 h after a single gastric gavage with either probiotic or sterile vehicle. For each fecal sample, we quantified Shannon's index of phylogenetic diversity, which takes into account both species richness, or the number of OTUs detectable, and the relative abundance of each OTU within the community. Stool obtained from mice 24 h after a single gavage with L. reuteri 17938 or L. reuteri 6475 demonstrated 27.7 and 29.5% greater microbial diversity, respectively, than stool obtained from mice 24 h after gavage with sterile vehicle (P<0.05 vs. PBS; Fig. 4A). This increase in microbial diversity was transient; neither strain significantly altered microbial diversity 48 h postgavage. Intriguingly, a trend showed increased phylogenetic diversity (P=0.097) just 4 h after gavage with probiotics.
Figure 4.
Probiotics increased phylogenetic diversity of the distal intestinal microbiome, as revealed by 16S rRNA V3-V5 sequences obtained by pyrosequencing. Microbial DNA was derived from pooled stool samples from 8-d-old pups, both before and 4 h, 24 h, and 48 h after a single gastric gavage. A) Shannon's diversity index. B) Species richness or α diversity, defined as the total number of unique OTUs per sample. C) Pielou's index of species evenness. Each data point represents the mean of 2 sequencing reactions/pooled sample. Values are means ± sd; n = 5 pools of 20 mice/treatment group, sampled at multiple time points. *P < 0.05 vs. PBS controls.
To determine whether probiotics modulate phylogenetic diversity within the microbiome by increasing microbial richness, community evenness, or both, we determined the number of taxa detectable in each fecal sample, and calculated Pielou's index of community evenness among taxa in a given sample. A single treatment with human-derived L. reuteri did not significantly change the number of OTUs detectable in fecal samples from mice obtained at 4, 24, or 48 h postgavage (Fig. 4B). However, L. reuteri strains 17938 and 6475 did increase community evenness (P<0.05), by 23.8 and 26.5%, respectively, 24 h after a single gavage. Thus, human-derived probiotics transiently increased phylogenetic diversity in the distal intestinal microbiome, not by increasing the number of species of bacteria but by enhancing the distribution of different OTUs within the microbial community.
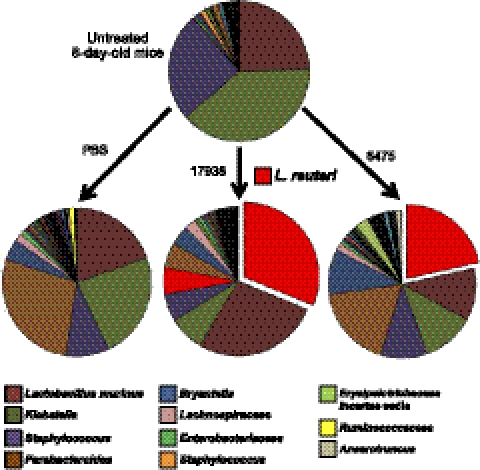
Finally, to identify which specific bacteria were involved in probiotic remodeling of the microbiome, we performed OTU-level alignment of 16S sequences for representative sets of stool samples. These analyses revealed a simple microbiome in untreated 8-d-old mice, with an average of just 74 detectable taxa, of which 3 OTUs, Lactobacillus murinus, Klebsiella, and Staphylococcus, comprised 89% of all bacterial sequences (Fig. 5). No endogenous L. reuteri was detected in vehicle-treated mice at 9 d of life, although L. reuteri accounted for 22–31% of OTUs 24 h after gavage with probiotic, when an average of 87 and 82 distinct taxa were detectable in mice having received L. reuteri strains 17938 and 6475, respectively. Enhanced microbial community evenness 24 h after treatment with either probiotic strain was mediated in part by reductions in the relative proportions of Klebsiella and Parabacteroides in the feces. Taken together, these metagenomic studies indicate that ingestion of a single probiotic strain can significantly remodel the microbiome by increasing the evenness of intestinal microbial communities in the neonatal mouse, in as little as 24 h after ingestion.
Figure 5.
Probiotics remodeled the distal intestinal microbiome. OTUs revealed by 16S rRNA V3-V5 sequences obtained by pyrosequencing. DNA was derived from stool pooled from 20 mice/group of 8-d-old pups before or 24 h after a single gastric gavage.
DISCUSSION
Mechanisms of probiosis relevant to neonatal development are poorly understood. We used a systems biology approach, including the first whole-genome transcriptome profiling of neonatal mouse enterocytes, to functionally characterize effects of probiotic ingestion in conventionally reared, outbred animals. Our results indicate that two strains of probiotic L. reuteri differentially increased the rate of enterocyte migration, while one strain increased crypt cell proliferation. Furthermore, probiotics increased the phylogenetic diversity and evenness of the developing intestinal microbial community. These data suggest that future clinical trials of targeted probiotic therapy should include consideration of effects on the intestinal epithelium and the gut microbiome.
L. reuteri down-regulated multiple enterocyte genes in the ILK, integrin, and actin signaling pathways; these pathways function to stabilize enterocytes against movement along the crypt-villus axis (31). These transcriptome changes were associated with decreased cytoskeletal β-actin along the basement membrane and an increased rate of cell migration along the crypt-villus axis. Rapid cell turnover is a host defense mechanism against invasive bacteria (32), parasites (33), and viruses (22), serving to extrude infected cells and pathogens from the epithelium. Recent studies revealed that Shigella flexneri secretes a virulence factor, OspE, that stabilizes ILK interactions and prevents detachment of infected cells from the basement membrane, enabling the pathogen to gain an infectious foothold to the host intestine (34). Intriguingly, OspE is highly conserved among enteric pathogens (35). Our data provide the first evidence that probiotics may destabilize these interactions with the basement membrane, and as a result, increase the rate of repopulation of intestinal epithelial cells. Restriction of these effects to the small intestine may reflect the larger proportional biomass of ingested probiotics in the small vs. large bowel, or the enhanced ability of microbes to functionally alter the proximal gastrointestinal tract (36). Additional studies are needed to delineate specific cell signaling interactions underlying this host response and to determine whether this mechanism contributes to resolution of enteric infections and disease by probiotics.
We also observed increased crypt cell proliferation in mice receiving L. reuteri 17938, providing further evidence that luminal bacteria could contribute to maintenance of epithelial homeostasis following injury (37, 38). Previous studies showed that monoassociation of germ-free rats with Lactobacillus rhamnosus elicits a mitogenic effect (39), but our data provide the first evidence that specific probiotic strains increase enterocyte proliferation in the context of a naturally acquired microbiome. Others have suggested that microbes regulate intestinal stem cell activity, but proof of this interaction has been lacking due to limited tools with which to study the intestinal stem cell niche (40). Thus, we cannot exclude the possibility that L. reuteri mediates its proliferative effect by interacting with intestinal stem cells. Identification of the genomic basis of strain specificity for crypt cell proliferation will be an important avenue of further study, in addition to exploring the ability of probiotics to mediate intestinal regeneration and rehabilitation after injury.
L. reuteri enhanced the phylogenetic diversity of the distal intestinal microbiome of neonatal mice, specifically by increasing the evenness among taxa. Environmentally acquired microbes, including those in the birth canal and breast milk, shape the development of the infant intestinal microbiome from a sterile niche to a complex, adult-like community early in life (41). A highly diverse microbiome is considered optimal, given the strong correlations between decreased microbial diversity and recurrent diarrhea (42) or inflammatory bowel disease (43). Despite representing up to 31% of OTUs detectable in stool, L. reuteri increased phylogenetic evenness and overall community diversity. Furthermore, this remodeling occurred rapidly, within 24 h of probiotic ingestion. These data support recent studies in both gnotobiotic mice (25) and humans (44) that reveal measurable shifts in fecal microbial community composition within 1 d of modifying the diet. Our data further highlight the transient nature of these changes, given that fecal microbial diversity did not significantly differ between treatment groups 48 h after a single gavage with either human-derived probiotics or vehicle. Curiously, Parabacteroides was dramatically increased 24 h after 8-d-old mice received a single gavage of sterile vehicle; it is not yet known whether this finding represents the natural colonization process, an effect of the PBS gavage, or day-to-day variability of the neonatal intestinal microbiome. Together, these results indicate that distal intestinal microbial communities are incredibly dynamic and can be predictably altered with a single dose of a single probiotic species. The potential to rapidly increase microbiome diversity could prove useful in clinical settings, such as inducing remission in inflammatory bowel disease, where a loss of microbial diversity is linked to disease flares (45, 46), or correcting pathogenic microbial signatures in children with irritable bowel syndrome (47).
However, it may be possible to remodel the intestinal microbiome in a more sustained manner. Long-term changes could be effected by administering higher concentrations of bacteria, giving repeat doses of probiotics, or introducing a probiotic organism isolated from the same host species. Host specificity is a critical consideration for the selection of therapeutic microbes, given the extraordinary genetic diversity among bacterial strains, especially those that evolved in different mammalian hosts, as illustrated by recent studies with L. reuteri (48, 49). Among the mechanisms of bacterial-host specificity is the extraordinary strain-specific diversity of L. reuteri mucus-binding proteins, the structure and function of which evolved independently to mediate intestinal attachment to and colonization of specific mammalian host species (50). Furthermore, the dramatic probiotic-mediated functional effects illustrated in our studies, notably without evidence of colonization of the mouse epithelium, highlight a potentially critical role for secreted factors in the modulation of intestinal physiology (51).
Our metagenomics data are supported by recent observations that ingestion of lactobacilli promotes infant fecal microbial community evenness (52). Thus, we propose that probiotics can accelerate the natural development of the simple neonatal intestinal microbiome to a more complex, adult-like state. Specifically, L. reuteri and related bacteria could serve as keystone species for the ecological niche of the developing gut and could strengthen a neonate's ability to resist microbiome perturbations including infectious challenges. Our results should be confirmed in experimental models in which fecal sampling from individual animals is possible, but they highlight the potential for beneficial microbes to profoundly and rapidly alter microbial community composition at the beginning of life. Promoting microbiome development into a more diverse microbial community should be explored as a potential mechanism contributing to probiotic mortality reduction in preterm, low birth-weight infants (7, 53).
The data presented here highlight the functional diversity within probiotic species and the pangenome concept of bacteria in general. Although L. reuteri strains 17938 and 6475 are members of the same species, comparative genomic analyses reveal that they share just 70% of genes (21). This observation, together with probiotic remodeling of the gut microbiome and strain-specific functional effects on the intestinal epithelium, highlight the importance of mechanism-based strain selection among the vast array of microbes with therapeutic potential. Future studies should explore the biological relevance of host transcriptome changes that were common to both L. reuteri strains to identify physiological effects conferred by heterogeneous classes of beneficial microbes. Future analyses should also seek to identify the functional consequences of altered host metabolism by probiotics, especially amine metabolism and oxidative phosphorylation, which could be triggered by differential production of bacterial fermentation products such as butyrate (54).
If specific microbial genes, cell wall components, or secreted factors responsible for defined physiological effects on the intestinal epithelium, immune system, and microbiome can be identified, specific combinations of microbes may be strategically selected for specific clinical applications. This knowledge would provide gastroenterologists with a new array of tools to predictably modulate host biology, predisposition to disease, and overall health status.
Supplementary Material
Acknowledgments
This work was supported by the U.S. National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) F30 DK081269 (G.A.P.), R01 DK065075 (J.V.), UH3 DK083990 (J.V.), and P30 DK56338 (M.J.F. and J.V.); by the National Institute of Allergy and Infectious Diseases (NIAID) RO1 AI24998 (M.E.C.); and by the National Center for Complementary and Alternative Medicine (NCCAM) R01 AT004326 (J.V.). J.V. received an unrestricted grant from Biogaia AB; he acts as an advisor and consultant to Danone. The other authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BrdU
- 5-bromo-2′-deoxyuridine
- DAPI
- 4′,6-diamidino-2-phenylindole
- FISH
- fluorescence in situ hybridization
- ILK
- integrin-linked kinase
- IPA
- Ingenuity Pathway Analysis
- OTU
- operational taxonomic unit
- PBS
- phosphate-buffered saline.
REFERENCES
- 1. Food and Agricultural Organization of the United Nations (FAO)/World Health Organization (WHO) (2001) Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, FAO/WHO, Geneva, Switzerland [Google Scholar]
- 2. Fuller R. (1989) Probiotics in man and animals. J. Appl. Bacteriol. 66, 365–378 [PubMed] [Google Scholar]
- 3. Thomas D. W., Greer F. R. (2010) Probiotics and prebiotics in pediatrics. Pediatrics 126, 1217–1231 [DOI] [PubMed] [Google Scholar]
- 4. Allen S.J., Martinez E.G., Gregorio G. V., Dans L. F. (2010) Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst. Rev. CD003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernaola Aponte G., Bada Mancilla C.A., Carreazo Pariasca N. Y., Rojas Galarza R. A. (2010) Probiotics for treating persistent diarrhoea in children. Cochrane Database Syst. Rev. CD007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alfaleh K., Anabrees J., Bassler D., Al-Kharfi T. (2011) Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. CD005496. [DOI] [PubMed] [Google Scholar]
- 7. Deshpande G., Rao S., Patole S., Bulsara M. (2010) Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 125, 921–930 [DOI] [PubMed] [Google Scholar]
- 8. Tarnow-Mordi W. O., Wilkinson D., Trivedi A., Brok J. (2010) Probiotics reduce all cause mortality and necrotizing enterocolitis: it is time to change practice. Pediatrics 125, 1068–1070 [DOI] [PubMed] [Google Scholar]
- 9. Soll R. F. (2010) Probiotics: are we ready for routine use? Pediatrics 125, 1071–1072 [DOI] [PubMed] [Google Scholar]
- 10. Preidis G. A., Versalovic J. (2009) Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology 136, 2015–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shanahan F. (2010) Probiotics in perspective. Gastroenterology 139, 1808–1812 [DOI] [PubMed] [Google Scholar]
- 12. Preidis G. A., Hill C., Guerrant R. L., Ramakrishna B. S., Tannock G. W., Versalovic J. (2011) Probiotics, enteric and diarrheal diseases, and global health. Gastroenterology 140, 8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sonnenburg J. L., Chen C. T., Gordon J. I. (2006) Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 4, e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peterson D. A., McNulty N. P., Guruge J. L., Gordon J. I. (2007) IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2, 328–339 [DOI] [PubMed] [Google Scholar]
- 15. Zhang W., Azevedo M. S., Wen K., Gonzalez A., Saif L. J., Li G., Yousef A. E., Yuan L. (2008) Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine 26, 3655–3661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin F. P., Wang Y., Sprenger N., Yap I. K., Lundstedt T., Lek P., Rezzi S., Ramadan Z., van Bladeren P., Fay L. B., Kochhar S., Lindon J. C., Holmes E., Nicholson J. K. (2008) Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol. Syst. Biol. 4, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shornikova A. V., Casas I. A., Isolauri E., Mykkanen H., Vesikari T. (1997) Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. J. Pediatr. Gastroenterol. Nutr. 24, 399–404 [DOI] [PubMed] [Google Scholar]
- 18. Shornikova A. V., Casas I. A., Mykkanen H., Salo E., Vesikari T. (1997) Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. Pediatr. Infect. Dis. J. 16, 1103–1107 [DOI] [PubMed] [Google Scholar]
- 19. Savino F., Pelle E., Palumeri E., Oggero R., Miniero R. (2007) Lactobacillus reuteri (American Type Culture Collection strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics 119, e124–130 [DOI] [PubMed] [Google Scholar]
- 20. Savino F., Cordisco L., Tarasco V., Palumeri E., Calabrese R., Oggero R., Roos S., Matteuzzi D. (2010) Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, doubleblind, placebo-controlled trial. Pediatrics 126, e526–533 [DOI] [PubMed] [Google Scholar]
- 21. Saulnier D. M., Santos F., Roos S., Mistretta T. A., Spinler J. K., Molenaar D., Teusink B., Versalovic J. (2011) Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS One 6, e18783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boshuizen J.A., Reimerink J.H., Korteland-van Male A.M., van Ham V.J., Koopmans M.P., Büller H.A., Dekker J., Einerhand A.W. (2003) Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. J. Virol. 77, 13005–13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swidsinski A., Ung V., Sydora B. C., Loening-Baucke V., Doerffel Y., Verstraelen H., Fedorak R. N. (2009) Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm. Bowel Dis. 15, 359–364 [DOI] [PubMed] [Google Scholar]
- 24. Stappenbeck T. S., Hooper L. V., Manchester J. K., Wong M. H., Gordon J. I. (2002) Laser capture microdissection of mouse intestine: characterizing mRNA and protein expression, and profiling intermediary metabolism in specified cell populations. Methods Enzymol. 356, 167–196 [DOI] [PubMed] [Google Scholar]
- 25. Turnbaugh P.J., Ridaura V.K., Faith J.J., Rey F.E., Knight R., Gordon J. I. (2009) The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 1, 6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cole J. R., Wang Q., Cardenas E., Fish J., Chai B., Farris R. J., Kulam-Syed-Mohideen A. S., McGarrell D. M., Marsh T., Garrity G. M., Tiedje J. M. (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37, D141–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., Huttley G. A., Kelley S. T., Knights D., Koenig J. E., Ley R. E., Lozupone C. A., McDonald D., Muegge B. D., Pirrung M., Reeder J., Sevinsky J. R., Turnbaugh P. J., Walters W. A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. (2010) QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li W., Godzik A. (2006) Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659 [DOI] [PubMed] [Google Scholar]
- 29. Shannon C.E., Weaver W. (1963) The Mathematical Theory of Communication, University of Illinois Press, Chicago [Google Scholar]
- 30. Chao A. (1984) Nonparametric estimation of the number of classes in a population. Scand. J. Statist. 11, 265–270 [Google Scholar]
- 31. McDonald P. C., Fielding A. B., Dedhar S. (2008) Integrin-linked kinase–essential roles in physiology and cancer biology. J. Cell Sci. 121, 3121–3132 [DOI] [PubMed] [Google Scholar]
- 32. Mulvey M. A., Schilling J. D., Martinez J. J., Hultgren S. J. (2000) Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. U. S. A. 97, 8829–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cliffe L. J., Humphreys N. E., Lane T. E., Potten C. S., Booth C., Grencis R. K. (2005) Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 308, 1463–1465 [DOI] [PubMed] [Google Scholar]
- 34. Kim M., Ogawa M., Fujita Y., Yoshikawa Y., Nagai T., Koyama T., Nagai S., Lange A., Fässler R., Sasakawa C. (2009) Bacteria hijack integrin-linked kinase to stabilize focal adhesions and block cell detachment. Nature 459, 578–582 [DOI] [PubMed] [Google Scholar]
- 35. Tobe T., Beatson S. A., Taniguchi H., Abe H., Bailey C. M., Fivian A., Younis R., Matthews S., Marches O., Frankel G., Hayashi T., Pallen M. J. (2006) An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc. Natl. Acad. Sci. U. S. A. 103, 14941–14946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Baarlen P., Troost F. J., van Hemert S., van der Meer C., de Vos W. M., de Groot P. J., Hooiveld G. J., Brummer R. J., Kleerebezem M. (2009) Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc. Natl. Acad. Sci. U. S. A. 106, 2371–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pull S. L., Doherty J. M., Mills J. C., Gordon J. I., Stappenbeck T. S. (2005) Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. U. S. A. 102, 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. (2004) Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 [DOI] [PubMed] [Google Scholar]
- 39. Banasaz M., Norin E., Holma R., Midtvedt T. (2002) Increased enterocyte production in gnotobiotic rats mono-associated with Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 68, 3031–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walker M. R., Patel K. K., Stappenbeck T. S. (2009) The stem cell niche. J. Pathol. 217, 169–180 [DOI] [PubMed] [Google Scholar]
- 41. Palmer C., Bik E. M., DiGiulio D. B., Relman D. A., Brown P. O. (2007) Development of the human infant intestinal microbiota. PLoS Biol. 5, e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chang J. Y., Antonopoulos D. A., Kalra A., Tonelli A., Khalife W. T., Schmidt T. M., Young V. B. (2008) Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J. Infect. Dis. 197, 435–438 [DOI] [PubMed] [Google Scholar]
- 43. Willing B.P., Dicksved J., Halfvarson J., Andersson A.F., Lucio M., Zheng Z., Järnerot G., Tysk C., Jansson J. K., Engstrand L. (2010) A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139, 1844–1854 e1841 [DOI] [PubMed] [Google Scholar]
- 44. Wu G. D., Chen J., Hoffmann C., Bittinger K., Chen Y. Y., Keilbaugh S. A., Bewtra M., Knights D., Walters W. A., Knight R., Sinha R., Gilroy E., Gupta K., Baldassano R., Nessel L., Li H., Bushman F. D., Lewis J. D. (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Manichanh C., Rigottier-Gois L., Bonnaud E., Gloux K., Pelletier E., Frangeul L., Nalin R., Jarrin C., Chardon P., Marteau P., Roca J., Dore J. (2006) Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 55, 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Swidsinski A., Loening-Baucke V., Vaneechoutte M., Doerffel Y. (2008) Active Crohn's disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm. Bowel Dis. 14, 147–161 [DOI] [PubMed] [Google Scholar]
- 47. Saulnier D. M., Riehle K., Mistretta T. A., Diaz M. A., Mandal D., Raza S., Weidler E. M., Qin X., Coarfa C., Milosavljevic A., Petrosino J. F., Highlander S., Gibbs R., Lynch S. V., Shulman R. J., Versalovic J. (2011) Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 141, 1782–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walter J., Britton R. A., Roos S. (2011) Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc. Natl. Acad. Sci. U. S. A. 108(Suppl. 1), 4645–4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frese S.A., Benson A.K., Tannock G.W., Loach D.M., Kim J., Zhang M., Oh P.L., Heng N.C., Patil P.B., Juge N., Mackenzie D.A., Pearson B.M., Lapidus A., Dalin E., Tice H., Goltsman E., Land M., Hauser L., Ivanova N., Kyrpides N. C., Walter J. (2011) The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri. PLoS Genet. 7, e1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mackenzie D. A., Jeffers F., Parker M. L., Vibert-Vallet A., Bongaerts R. J., Roos S., Walter J., Juge N. (2010) Strain-specific diversity of mucus-binding proteins in the adhesion and aggregation properties of Lactobacillus reuteri. Microbiology 156, 3368–3378 [DOI] [PubMed] [Google Scholar]
- 51. Yan F., Cao H., Cover T. L., Whitehead R., Washington M. K., Polk D. B. (2007) Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132, 562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cox M. J., Huang Y. J., Fujimura K. E., Liu J. T., McKean M., Boushey H. A., Segal M. R., Brodie E. L., Cabana M. D., Lynch S. V. (2010) Lactobacillus casei abundance is associated with profound shifts in the infant gut microbiome. PLoS One 5, e8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alfaleh K., Bassler D. (2008) Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. CD005496. [DOI] [PubMed] [Google Scholar]
- 54. Gallis J. L., Tissier P., Gin H., Beauvieux M. C. (2007) Decrease in oxidative phosphorylation yield in presence of butyrate in perfused liver isolated from fed rats. BMC Physiol. 7, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.