Abstract
Ginkgo biloba extracts are currently used for a wide range of health-related conditions. Some of the medical benefits of these extracts are controversial, but their lack of toxicity in humans is not in doubt. These extracts are, however, highly toxic to insects. Their active components (ginkgolides and bilobalide) have structures similar to the convulsant picrotoxin, a GABAA receptor antagonist, so their lack of toxicity in mammals is puzzling. Here, we show that the different compositions of insect and vertebrate GABA receptor pores are responsible for the differing toxicities. Insect GABA receptors contain Ala at their 2′ position in the pore. Substitution with Val, which is the equivalent residue in vertebrate GABAA receptor α-subunits, decreases ginkgolide potency by up to 10,000-fold. The reverse mutation in vertebrate GABAA α1 subunits increased the sensitivity of α1β2 and α1β2γ2 receptors to ginkgolides. Mutant cycle analysis demonstrates a strong interaction between the ginkgolides and the 2′ residue, a result supported by in silico docking of compounds into a model of the pore. We conclude that the insecticidal activity of G. biloba extracts can be attributed to their effects at insect GABA receptors, and the presence of a Val at the 2′ position in vertebrate GABAA receptors explains why these compounds are not similarly toxic to humans.—Thompson, A. J., McGonigle, I., Duke, R., Johnston, G. A. R., Lummis, S. C. R. A single amino acid determines the toxicity of Ginkgo biloba extracts.
Keywords: Cys-loop receptor, picrotoxin, antagonist
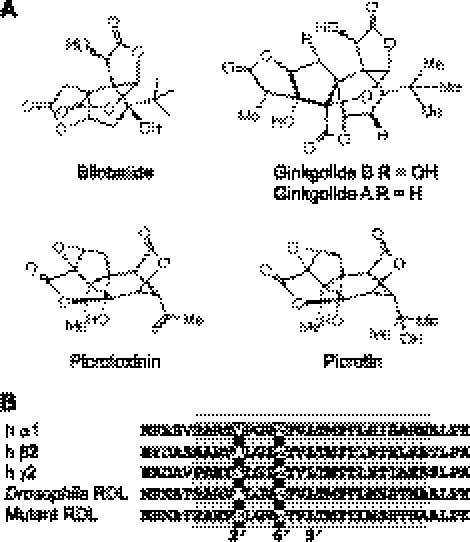
Ginkgo biloba has been used as a traditional medicine for >2500 yr, and its leaf extract (EGb761) is currently used for a range of health-related conditions (1–3). EGb761 contains a range of compounds, the best studied of which are bilobalide (BB) and ginkgolides A and B (GA and GB); hereafter collectively referred to as the ginkgolides. The structures of these compounds (Fig. 1A) are similar to that of picrotoxin (PTX), a classic γ-aminobutyric acid A (GABAA) receptor antagonist that is composed of 2 closely related compounds, picrotoxinin and picrotin. GABAA receptors are members of the Cys-loop family of ligand-gated ion channels, which also includes nicotinic acetylcholine (nACh), 5-hydroxytryptamine 3 (5-HT3), and glycine receptors (4–6). These important neuronal receptors are implicated in a range of neurological disorders, and they are also the site of action of some of the most widely used drugs, such as benzodiazepines and general anesthetics. All share a common structure, and binding of agonist to their extracellular domains opens an integral transmembrane channel that enables ions to cross the cell membrane. The channels of these receptors are lined by α-helices, and to simplify comparisons between receptors of this family, the amino acid residues of these α-helices are given an index number, with 0′ representing a conserved charged residue on the cytoplasmic side of the membrane, and a conserved Leu at 9′ forming the gate (Fig. 1B, Supplemental Fig. S1).
Figure 1.
Compounds (A) and amino acid alignment of the M2 region (shaded) of the subunits (B) used in this study. Residues important for binding channel blockers are highlighted. Subunit accession numbers: human GABA α1, P14867; human GABA β2, P47870; human GABA γ2, P18507; Drosophila RDL, P25123.
G. biloba also has a long history of use as an insecticide. Dry ginkgo leaves were traditionally used as bookmarks to protect against booklice and silverfish, ginkgo leaf extracts were used to control pests in paddy fields, and ginkgo wood was used for insect-proof cabinets in Japan hundreds of years ago (3). More recent work, seeking to understand these traditional uses, has shown that extracts have insecticidal activity on a range of insect species (7). Inhibition of GABA receptors provides a possible explanation for this property, as invertebrate GABA receptors are the target of a number of insecticides, including cyclodienes (such as dieldrin), lindane, and the leading pesticide, fipronil (8–10). These insecticides, however, are toxic to humans (fipronil, for example, has a U.S. Environmental Protection Agency ranking of moderately toxic; ref. 11), while G. biloba extracts are not, and instead may have neuroprotective, anxiolytic, and other beneficial properties (1–3).
To study the effects of gingkolides on insect GABA receptors, we used GABA-activated RDL (resistant to dieldrin) receptors. The RDL subunit (cloned from a dieldrin-resistant mutant; hence its name) is the best-studied insect GABA receptor subunit and is widely distributed throughout the Drosophila CNS (8, 9, 12, 13). By comparing the effects of the gingkolides at RDL with those at human GABAA receptors, we describe how the contrasting toxicities of these compounds in insects and humans can be explained by a single GABA receptor pore residue.
MATERIALS AND METHODS
Mutagenesis and preparation of mRNA and oocytes
GABAA (accession numbers α1 P14867, β2 P47870, and γ2 P18507) and RDL (P25123) receptor subunit cDNAs were subcloned into pGEMHE for oocyte expression, as described previously (14, 15). Site-directed mutagenesis was performed with the QuikChange mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA). cRNA was in vitro transcribed from linearized plasmid cDNA template using the mMessage mMachine T7 Transcription kit (Ambion, Austin, TX, USA). Stage V and VI oocytes from Xenopus laevis (NASCO, Fort Atkinson, WI, USA) were injected with 50 nl of 100 ng/μl cRNA (5 ng injected) and incubated at 16°C; currents were recorded at 1–3 d postinjection. Vertebrate GABAA receptor subunits were expressed in the ratio 1:1 (α1:β2) or 1:1:10 (α1:β2:γ2).
Characterization of receptors
Two-electrode voltage clamping of Xenopus oocytes was performed as described previously (15). Concentration–response data were measured at a holding potential of −60 mV, and responses for each oocyte were normalized to the maximum current for that oocyte. The mean response was iteratively fitted to the equation I = Imin + (Imax − Imin)/(1 + 10(log(EC50−[A])nH), where Imax is maximum response, Imin is minimum response, [A] is concentration of agonist, and nH is the Hill coefficient.
Homology modeling and docking
RDL was aligned with GLIC using FUGUE (16), and the RDL receptor pore homology model was generated using Modeller 9.8 (17), based on the crystal structure of GLIC [Protein Data Bank (PDB) ID: 3EAM]. The best model was selected after Ramachandran plot analysis of all the generated models. Docking of compounds into the homology model was performed using GOLD 3.0 (Cambridge Crystallographic Data Centre, Cambridge, UK). The binding site was defined as a 15-Å-radius docking sphere surrounding the Cα of the 6′ residues in chains A and C. Ten genetic algorithm runs were performed on each docking exercise using default parameters. The structures were visualized and hydrogen bonds were identified using PyMOL 1.3 (DeLano Scientific LLC, South San Francisco, CA, USA).
Fly rearing and coated vial bioassays
Lab strains of resistant (RdlMDRR) Drosophila were kindly provided by Brandeis University (Boston, MA, USA) and susceptible (CantonS) Drosophila by the University of Cambridge. Fly cultures were kept at 25°C with a 12-h light-dark cycle on corn meal, yeast, sucrose, and agar food. Flies had similar genetic backgrounds and were originally sourced from the Bloomington Stock Center (Bloomington, IN, USA); they are described in FlyBase (http://flybase.org/). Coated vial bioassays were developed from method 011 v3 as recommended by the Insecticide Resistance Action Committee (http://www.irac-online.org). BB, GA, GB, PTX, and dieldrin were diluted in ethanol at 25 mM and serially diluted with ethanol to working concentrations. These solutions (100 μl) were added to a 22-cm2 surface of a glass vial and rolled in a fume cupboard until the ethanol had evaporated. Flies were knocked down with CO2, and 10–15 individuals were added to each vial with a vented lid. Moist agar food (1 ml) was added to each vial. Flies were checked for recovery before being placed at 20°C under a 16:8-h light-dark regime. The endpoint scoring (dead/alive) was at 24, 48, and 72 h, and data were iteratively fitted to a 4-parameter logistic equation, as described above.
Statistical analysis
Significance was calculated using a Student's t test in Prism 4.03 (GraphPad Software, San Diego, CA, USA; http://www.graphpad.com); values of P < 0.05 were considered significant.
RESULTS
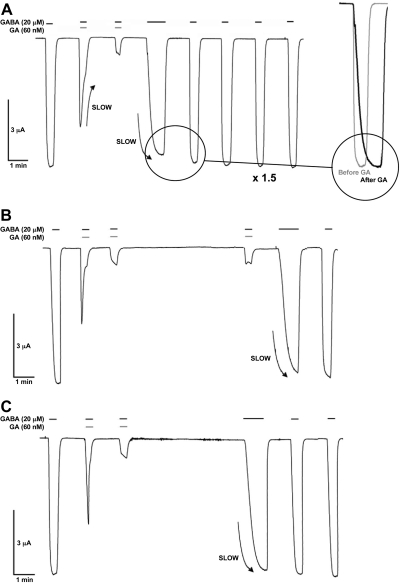
Application of GABA to RDL receptors expressed in Xenopus oocytes elicits concentration-dependent inward currents, with an EC50 of 19 μM and Hill slope of 1.8 (Table 1), similar to previously published results (13). These currents were inhibited by ginkgolides; inhibition of RDL receptors was slow to develop, and a subsequent application of agonist alone resulted in slowed receptor activation that was not dependent on the length of washout time, consistent with an inhibitor slowly leaving the open channel (Fig. 2). Ginkgolides had no effect when applied alone (data not shown).
Table 1.
Properties of wild-type and mutant RDL, α1β2, and α1β2γ2 receptors
| Receptor | pEC50 (M) | EC50 (μM) | nH | n |
|---|---|---|---|---|
| RDL | 4.72 ± 0.03 | 19.0 | 1.80 ± 0.20 | 5 |
| RDLA2'V | 4.70 ± 0.02 | 19.9 | 2.20 ± 0.02 | 5 |
| α1β2 | 5.16 ± 0.04 | 6.92 | 1.43 ± 0.18 | 4 |
| α1V2'Aβ2 | 6.85 ± 0.03* | 0.14 | 1.14 ± 0.12 | 3 |
| α1β2γ2 | 4.13 ± 0.03 | 74.1 | 1.64 ± 0.16 | 3 |
| α1V2'Aβ2γ2 | 5.51 ± 0.04* | 3.09 | 1.10 ± 0.11 | 3 |
P < 0.05 vs. wild type; Student's t test.
Figure 2.
Typical experiments showing that the inhibition of GABA responses in RDL receptors is slow to develop and wash out. A) Following an application of GABA alone, coapplication of GA with GABA shows that the response reaches 60% of maximal before it is slowly inhibited. A stable level of inhibition is only reached with a subsequent coapplication of GA and GABA. B) The level of inhibition is unchanged even after a long washout. C) Following inhibition, activation is slow but is independent of the wash time between agonist applications. This suggests that GA becomes trapped within the channel, and only slowly leaves the open channel on agonist application. Preapplication did not change ginkgolide potency, consistent with access also requiring an open channel. In this work, all concentration-inhibition curves and the derived parameters were calculated from measurements taken during a second, stable, level of inhibition. Ginkgolides were obtained as previously described (19).
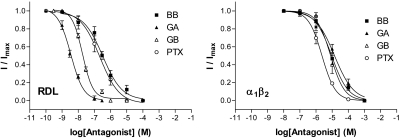
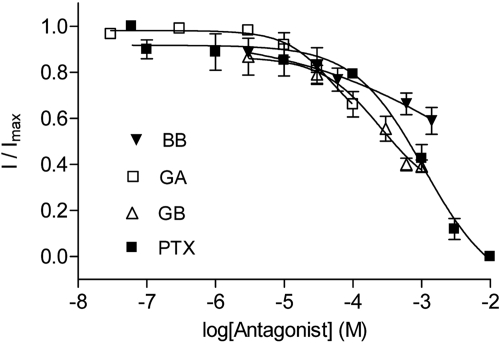
Potent inhibition of GABA-evoked RDL currents was seen for GA (IC50=3.1 nM) and GB (IC50=18 nM), while BB (IC50=320 nM) had a similar potency to PTX (IC50=220 nM) (Table 2 and Fig. 3A). Association rates (kon) determined at IC50 concentrations showed that those for GA and GB were higher than BB and PTX, while dissociation rates (koff) were similar for all the compounds (Supplemental Fig S1). These yielded Kd values (koff/kon) that were similar to IC50 values.
Table 2.
Effects of BB, GA, GB, and PTX on wild-type and mutant receptors
| Receptor | pIC50 (M) | IC50 (μM) | nH | n |
|---|---|---|---|---|
| BB | ||||
| RDL | 6.50 ± 0.09 | 0.32 | 0.89 ± 0.09 | 4 |
| RDLA2'V | 3.65 ± 0.03* | 224 | 1.85 ± 0.21 | 4 |
| α1β2 | 5.03 ± 0.07 | 9.33 | 0.85 ± 0.10 | 5 |
| α1V2'Aβ2 | 5.69 ± 0.04* | 2.04 | 1.06 ± 0.10 | 3 |
| α1β2γ2 | 4.64 ± 0.09 | 22.9 | 0.68 ± 0.10 | 5 |
| α1V2'Aβ2γ2 | 5.45 ± 0.07* | 3.55 | 1.16 ± 0.20 | 4 |
| GA | ||||
| RDL | 8.49 ± 0.07 | 0.003 | 1.13 ± 0.18 | 6 |
| RDLA2'V | 3.44 ± 0.04* | 360 | 1.48 ± 0.19 | 6 |
| α1β2 | 4.80 ± 0.07 | 15.8 | 0.65 ± 0.08 | 5 |
| α1V2'Aβ2 | 4.69 ± 0.09 | 20.4 | 0.87 ± 0.14 | 5 |
| α1β2γ2 | 4.41 ± 0.11 | 38.9 | 0.49 ± 0.07 | 3 |
| α1V2'Aβ2γ2 | 5.33 ± 0.08* | 4.68 | 0.86 ± 0.10 | 6 |
| GB | ||||
| RDL | 7.74 ± 0.03 | 0.018 | 1.43 ± 0.14 | 5 |
| RDLA2'V | 3.51 ± 0.07* | 310 | 1.02 ± 0.20 | 3 |
| α1β2 | 4.82 ± 0.04 | 15.1 | 0.94 ± 0.06 | 4 |
| α1V2'Aβ2 | 5.33 ± 0.04* | 4.68 | 1.13 ± 0.09 | 4 |
| α1β2γ2 | 3.94 ± 0.05 | 115 | 0.61 ± 0.06 | 4 |
| α1V2'Aβ2γ2 | 4.83 ± 0.05* | 14.8 | 1.43 ± 0.29 | 4 |
| PTX | ||||
| RDL | 6.66 ± 0.12 | 0.22 | 0.73 ± 0.13 | 6 |
| RDLA2'V | 3.75 ± 0.05* | 178 | 1.32 ± 0.23 | 4 |
| α1β2 | 5.65 ± 0.05 | 2.23 | 1.00 ± 0.09 | 5 |
| α1V2'Aβ2 | 4.61 ± 0.02* | 24.5 | 1.34 ± 0.06 | 5 |
| α1β2γ2 | 5.35 ± 0.06 | 4.47 | 0.80 ± 0.11 | 4 |
| α1V2'Aβ2γ2 | 5.45 ± 0.06 | 3.55 | 0.95 ± 0.09 | 3 |
P < 0.05 vs. wild type; Student's t test.
Figure 3.
Concentration-inhibition curves show that ginkgolides are more potent at insect RDL receptors than at human α1β2 GABAA receptors. Inhibition was measured at the respective GABA EC50 values for each receptor. Values are expressed as means ± se, with sample size and other parameters shown in Table 2.
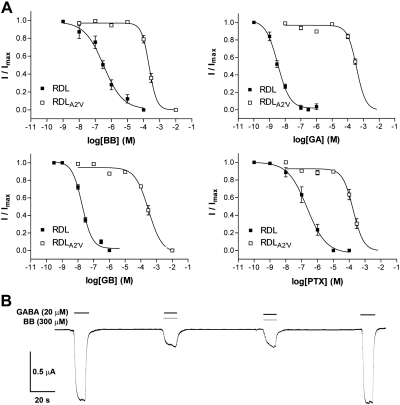
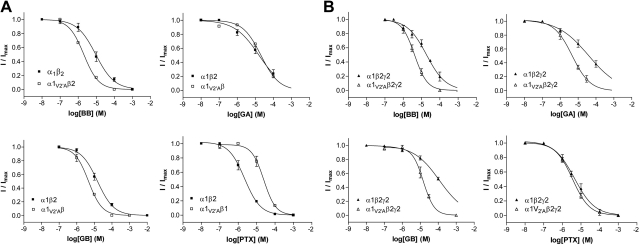
PTX is known to bind to the channel of GABAA receptors, and there is evidence from other receptors in the family that ginkgolide binding sites may be similarly located (18–21). Therefore, we focused on GABA receptor pore-lining residues as potential binding sites. The 2′ and 6′ residues are the most likely candidates, as these residues form the binding site for PTX in GABAA receptors (18, 22, 23), and the amino acids at these locations in insect GABA receptors confer sensitivity or resistance to insecticides that target GABA-activated channels, such as dieldrin (8, 9). An alignment of the transmembrane regions of insect and human GABA receptors shows that the 6′ residue is identical in RDL and GABAA receptor subunits, while the 2′ residue differs between subunits: RDL subunits have an Ala, while human GABAA receptor α subunits have Val, and β, γ, δ, ε Ala, Ser, or Thr (Fig. 1B and Supplemental Fig. S1). To probe the role of the 2′ residue in ginkgolide sensitivity, we substituted Val for Ala at the 2′ location in RDL subunits. This had a dramatic effect on ginkgolide potency (up to a 10,000-fold increase in IC50), while GABA sensitivity was unaltered (Fig. 4 and Tables 1 and 2). We also examined the reverse substitution: replacement of Val with Ala in the human α1 subunit. This caused decreases in almost all ginkgolide IC50 values in both α1V2′Aβ2 and α1V2′Aβ2γ2 mutant receptors (up to 8-fold), and the vertebrate mutant receptors also had increased GABA sensitivity (Fig. 5 and Tables 1 and 2). PTX behaved differently to the ginkgolides in the human receptor (its IC50 was increased and not decreased in α1V2′Aβ2 mutant receptors and unchanged in α1V2′Aβ2γ2 receptors; Table 2).
Figure 4.
Ginkgolide potency at RDLA2′V mutant receptors. A) Concentration-inhibition curves for RDLA2′V mutant receptors show that ginkgolide potency is significantly decreased compared to wild-type receptors. Values are expressed as means ± se, with sample size and other parameters shown in Table 2. B) Typical responses from an RDLA2′V mutant receptor show that onset and recovery from inhibition are faster in these receptors.
Figure 5.
Ginkgolide potency at wild-type and mutant α1β2 (A) and α1β2γ2 (B) GABAA receptors. Concentration-inhibition curves show that ginkgolide potency is mostly increased compared to wild-type receptors, although PTX potency is either decreased (α1V2′Aβ2 receptors; A) or unchanged (α1V2′Aβ2γ2 receptors; B). Values are expressed as means ± se, with sample size and other parameters shown in Table 2.
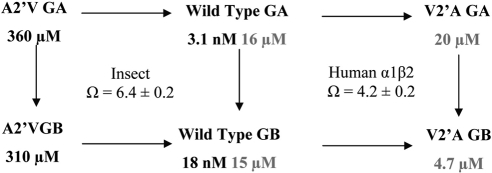
The subtle difference between GA and GB (which only differ by a single hydroxyl group at position 1; Fig. 1A) allowed us to use mutant cycle analysis to probe for direct interactions with the 2′ residue (24). This analysis identifies whether there is a specific interaction between a ligand atom and protein residue. A coupling parameter (Ω), calculated from IC50 values, will be 1 if the residue has no structural or energetic effect on the ligand binding group. However, if the residue and the group interact, the simultaneous change at both sites has an effect that is either greater or less than the product of their individual effects, producing a Ω value that differs from 1. Here the Ω values were significantly >1 (P<0.05) for both human and insect receptors, demonstrating an interaction between the ginkgolides and the 2′ residue (Fig. 6).
Figure 6.
Mutant cycle analysis of interactions between ginkgolides and the 2′ residue. IC50 values from RDL receptors are at left; those from human GABAA receptors are at right. A coupling parameter (Ω) was calculated from IC50 values (Table 2) using the standard equation Ω = IC50 wtL1 × IC50 mutL2/IC50 wtL2 × IC50 mutL1, where wt = wild type, mut = mutant, and L1 and L2 are the ligands GA and GB, respectively.
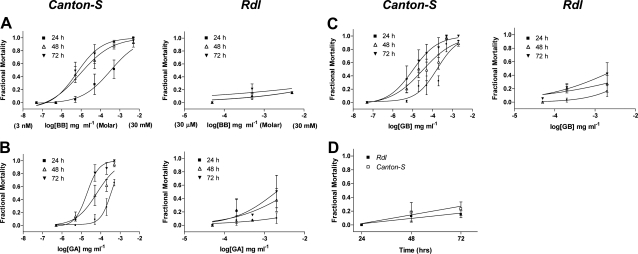
To determine whether the 2′ residue could be responsible for ginkgolide sensitivity in vivo, we used a naturally occurring A2′S mutant strain of dieldrin-resistant Drosophila. Dieldrin is a widely used insecticide whose mechanism of action is via insect GABA receptors. Our data demonstrate that it is highly effective in killing wild-type (susceptible) flies but has little effect in flies with an A2′S mutation (Fig. 7). To explore whether ginkgolide action in vivo was also mediated via GABA receptors, we tested gingkolide toxicity in wild-type and mutant flies. In coated vial bioassays, no mortality could be attributed to the compounds in flies containing the A2′S mutation (P>0.05 at all concentrations), even at maximal concentration (5000 ppm, limited by solubility). In contrast, the wild-type strain showed significant mortality at 24 h, with values of LD50 ∼ 100-fold lower than this maximal concentration at 48 h (Fig. 7 and Table 3).
Figure 7.
Insecticidal activities of ginkgolides in wild-type (CantonS) and dieldrin-resistant (Rdl) adult D. melanogaster in coated vial bioassays. A–C) Fractional mortality is plotted against ginkgolide concentration (mg/ml): BB (A), GA (B), GB (C). In panel A, molar equivalents are shown in parentheses. D) Mortality in the absence of compound. The resistant strain carries a target-site mutation (A2′S) in its Rdl gene that confers resistance (no significant mortality up to 72 h) to the cyclodiene insecticide dieldrin; in wild-type flies, LD50 was 2.0, 2.0 and 1.7 μg/ml at 24, 48, and 72 h, respectively. The resistant strain survived well at the highest concentrations of ginkgolides tested, showing that a mutation at the 2′ residue confers cross-resistance. For the wild-type strain, data are fitted with a 4-parameter logistic equation, yielding the LD50 values shown in Table 3. Comparable data could not be derived for Rdl, owing to its resistance to the compounds. Values are means ± se for the sample size shown. Similar experiments with PTX revealed no toxicity in either batch of flies, probably due to access problems, as in previous studies (28).
Table 3.
LD50 in Canton-S adult D. melanogaster
| Compound | LD50 (mg/ml) |
||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| BB | 0.331 | 0.009 | 0.006 |
| GA | 0.345 | 0.063 | 0.019 |
| GB | 0.171 | 0.031 | 0.010 |
The order of potency of the ginkgolides in the in vivo assay differed from that of the in vitro work in that GA was the least and not the most potent. We consider this is likely to be due to small variations between the mutant and wild-type fly populations, perhaps involving heterogeneous receptors or other proteins, such as those involved in metabolic or penetration mechanisms. In addition, as RDL subunits have been found to coexpress with GluCl subunits (25), a range of heteromeric receptors with different ginkgolide sensitivities may exist in the different flies. Overall, however, the in vivo data strongly support the in vitro studies in that in vivo mutations at the 2′ position, and in vitro mutations at the 2′ position, dramatically decrease organism sensitivity and receptor sensitivity, respectively, to ginkgolide effects.
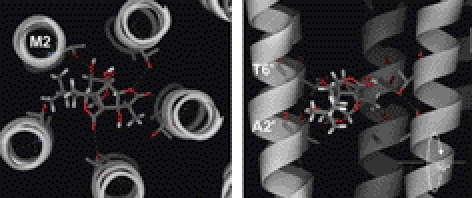
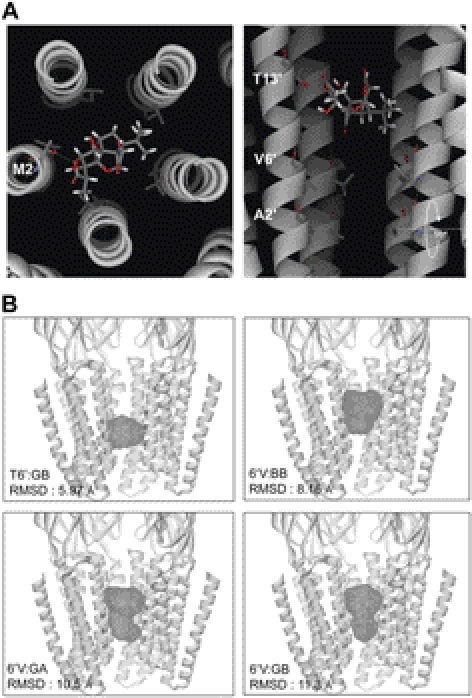
To further examine how the ginkgolides might bind in the pore, we generated a homology model of the RDL receptor channel. As our data suggested that ginkgolides act in the channel in the open state, we used a bacterial channel in the same conformation (Supplemental Fig S2). Docking of the ginkgolides into the channel revealed a binding location between the 2′ and 6′ residues (Fig. 8), supporting our functional data. The compounds were coordinated by hydrogen bonds with several of the hydroxyl side chains of 6′Thr residues and had hydrophobic interactions with the 2′Ala. To further probe the accuracy of our model, we removed the hydrogen bonding ability at the 6′ position by creating a T6′V mutant RDL receptor, and tested the consequences of this mutation both functionally and in silico. The substitution had a major effect on ginkgolide potency (>1000-fold increase in IC50; Table 4 and Fig. 9) at expressed receptors, and in silico docking revealed that the ginkgolides docked at a new location toward the extracellular end of the pore (Fig. 10). The docked poses generated from docking into this mutant also had a significantly increased RMSD variation compared to wild type, suggesting that the ginkgolides were located in a less energetically favorable position.
Figure 8.
Docking of ginkgolides to wild-type RDL receptors. All ginkgolides and PTX docked at a similar location; here, GB is shown as a typical example. From above, it can be seen to fit snugly in the pore between the 5 M2 helices; from the side, it is located between the 2′ and 6′ residues, where it forms hydrogen bonds with 6′Thr residues.
Table 4.
Properties of RDLT6'V
| Compound | pEC50/pIC50 | EC50/IC50 (μM) | nH | n |
|---|---|---|---|---|
| GABA | 5.13 ± 0.06 | 7.41 | 1.44 ± 0.29 | 9 |
| BB | NI | – | – | 4 |
| GA | NI | – | – | 3 |
| GB | 3.59 ± 0.36* | 257 | 1.00 ± 0.70 | 4 |
| PTX | 2.96 ± 0.19* | 1096 | 0.85 ± 0.18 | 5 |
NI, not inhibited (50% inhibition was not reached at 1 mM).
P < 0.05 vs. wild type; Student's t test.
Figure 9.
Ginkgolide concentration-inhibition curves at RDLT6′V mutant receptors. Gingkolides inhibited GABA-activated responses only at high concentrations, with values of IC50 > 100 μM. Values are means ± se, with sample size and other parameters shown in Table 4.
Figure 10.
Docking at RDLT6′V mutant receptors. A) Docked pose for GB using the same template as in Fig. 8. B) Locations of 10 docked poses are superimposed to show the distributions of ginkgolides in the channel. These data show that all ligands docked ∼10 Å higher in the channel compared with wild-type receptors. In addition, the poses of GB in the wild-type (T6′) receptor are closely clustered, while in RDLT6′V (6′V) receptors, they are more widely distributed. RMSD for clusters is shown at bottom left in each panel.
DISCUSSION
Ginkgolides have structures similar to the well-studied GABAA receptor antagonist picrotoxin and have a similar mechanism of action at both this and a range of other Cys-loop receptors. Picrotoxin, however, is a potent and toxic convulsant in humans, whereas ginkgolides are widely used as herbal medicines (1–5). Nevertheless, ginkgolides are toxic in insects, where they are highly effective insecticidal agents (7). Here, we reveal a possible explanation for this discrepancy: ginkgolides potently inhibit insect but not human GABA receptors because there is no 2′Val in insect receptor pores.
Many GABAA receptor channel blockers act at the 2′ and/or 6′ channel lining residues. An amino acid alignment of insect and human GABAA receptor subunits shows that the 6′ residue of all these subunits is a conserved Thr, while the 2′ residue varies between subunits; this residue is, however, conserved as Val in all GABAA α subunits (Fig. 1 and Supplemental Fig. S1). Our results demonstrate the importance of this residue, as substitution of Val at the 2′ position into the RDL receptor pore caused dramatic (up to 10,000-fold) decreases of ginkgolide potency. GABAA receptors with the reverse mutation (V2′A) in the α1 subunit had predominantly the opposite effect, although the changes in potencies were less pronounced (up to 8-fold) and were accompanied by an increase in GABA sensitivity (EC50: α1β2, 6.9 μM; αV2′A1β2, 0.14 μM; α1β2γ2, 74 μM; α1V2′Aβ2γ2, 3.1 μM; Table 1). All RDL receptors identified to date have an Ala at the 2′ position, unless they are dieldrin resistant (Fig. 1 and Supplemental Fig. S1), emphasizing the importance of this position.
Our data also show the importance of the 6′ residues: ginkgolide inhibition was eliminated in the RDL T6′V mutant, showing the hydrogen bonding ability of this residue is critical, as indicated in our models of ginkgolide binding in the RDL receptor pore (Fig. 8). Introduction of a 6′ residue that cannot hydrogen bond results in ginkgolides binding at quite different locations, toward the extracellular end of the pore (Fig. 10). Taken together, these data show both 2′ and 6′ residues are critical in defining ginkgolide potency.
The 2′ position acts as a secondary binding site for PTX in GABAA receptors, with the 6′ residue being the main interacting residue (22). Our results show that the 6′ residue is also a major determinant of PTX binding in insect GABA receptors. Inhibition curves in T6′V mutant RDL receptors reveal an IC50 for PTX of 1.1 mM, which is 5000-fold higher than in wild-type receptors (0.22 μM). This change in PTX IC50 was more pronounced than for the human GABAA receptor, where studies have shown substitution causes only a 48-fold shift (23). In insect GABA receptors, however, the 2′ position plays a more important role in PTX inhibition (an ∼1000-fold increase in IC50 in RDLV2′A receptors compares to small or no change in αV2′A1β2 or α1V2′Aβ2γ2 receptors), suggesting that the structures of the vertebrate and insect pore regions may be subtly different.
An important question is whether we can explain the toxicity of ginkgolides in insects, and the lack of toxicity in humans. In insects, GA and GB are highly effective at inhibiting GABA receptors at nanomolar concentrations, providing an explanation for their insecticidal activity. In humans, plasma concentrations of ginkgolides are between 4 and 70 nM, depending on the treatment, with values as high as 0.5 μM being reported (26, 27). At these concentrations, ginkgolides would not significantly inhibit human GABAA receptors, and they would therefore not cause GABA-related toxic effects. There is the potential for such effects if there is a naturally occurring GABAA α1 A2′V mutation, but we consider this unlikely, as the resulting increase in GABA sensitivity (20–50-fold) would have dramatic effects on GABA-activated responses in vivo that would probably render the fetus nonviable.
In summary, we have found the major active ingredients of G. biloba extract (BB, GA, and GB) to be potent antagonists of insect RDL receptors, but only weak inhibitors of human GABAA receptors. Both 2′ and 6′ residues affect their potency at RDL receptors, but only the 2′ residue differs between insects and humans. The introduction of the human α1 2′Val residue into RDL subunits hugely decreases the potency of the compounds, and Drosophila carrying a 2′ mutation are resistant to ginkgolides, while the introduction of the insect 2′Ala residue into the human α1 subunit results in increased susceptibility. These data can explain why the ginkgolides display potent insecticidal activity but do not have similar GABA-related toxicities in humans. As such, these natural and nontoxic compounds could provide a suitable pest-management approach for the control of range of species, such as bedbugs, where the likelihood of human contact is high. Given the wide range of insect GABA receptor subunits and the variety of ginkgolides, there is also the exciting possibility of discovering ginkgolides that have actions that target specific insect species. This is potentially a route to solve serious agricultural problems, such as bee colony collapse disorder; ginkgolides could provide an insecticide that is harmless to bees, but highly toxic to the pests that may contribute to this disorder.
Supplementary Material
Acknowledgments
The authors thank The Wellcome Trust (81925; S.C.R.L., A.J.T.; S.C.R.L. is a Wellcome Trust Senior Research Fellow in Basic Biomedical Science), the UK Medical Research Council (I.M. held a MRC studentship), the National Health and Medical Research Council of Australia (R.D.; G.A.R.J.) and the University of Sydney (a travel grant to S.C.R.L.). A.J.T. and I.M. conducted the experiments; R.D. and G.A.R.J. contributed new reagents or analytic tools; A.J.T. and S.C.R.L. performed data analysis; A.J.T., S.C.R.L., I.M., R.D., and G.A.R.J. wrote or contributed to the writing of the manuscript; A.J.T. and S.C.R.L. participated in research design; S.C.R.L. and G.A.R.J. acquired funding for the research. The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BB
- bilobalide
- GA
- ginkgolide A
- GABA
- γ-aminobutyric acid
- GB
- ginkgolide B
- nACh
- nicotinic acetylcholine
- PTX
- picrotoxin
- RDL
- resistant to dieldrin.
REFERENCES
- 1. Ahlemeyer B., Krieglstein J. (2003) Neuroprotective effects of Ginkgo biloba extract. Cell. Mol. Life Sci. 60, 1779–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drieu K., Vranckx R., Benassayad C., Haourigi M., Hassid J., Yoa R. G., Rapin J. R., Nunez E. A. (2000) Effect of the extract of Ginkgo biloba (EGb 761) on the circulating and cellular profiles of polyunsaturated fatty acids: correlation with the anti-oxidant properties of the extract. Prostaglandins Leukot. Essent. Fatty Acids 63, 293–300 [DOI] [PubMed] [Google Scholar]
- 3. Hori T., Ridge R. W., Tulecke W., Del Tredici P., Tremouillaux-Guiller J., Tobe H., eds. (1997) Ginkgo biloba—A Global Treasure: From Biology to Medicine, Springer-Verlag, Tokyo [Google Scholar]
- 4. Olsen R. W. (1982) Drug interactions at the GABA receptor-ionophore complex. Ann. Rev. Pharmacol. Toxicol. 22, 245–277 [DOI] [PubMed] [Google Scholar]
- 5. Sieghart W. (2006) Structure, pharmacology, and function of GABAA receptor subtypes. Adv. Pharmacol. 54, 231–263 [DOI] [PubMed] [Google Scholar]
- 6. Thompson A. J., Lester H. A., Lummis S. C. (2010) The structural basis of function in Cys-loop receptors. Q. Rev. Biophys. 43, 449–499 [DOI] [PubMed] [Google Scholar]
- 7. Ahn Y. J., Kwon M., Park H. M., Han C. K. (1997) Potent insecticidal activity of Ginkgo biloba-derived trilactone terpenes against Nilaparvata lugens. In: Phytochemical Pest Control Agents (Hedin P. A., Hollingworth R., Miyamoto J., Masler E., Thompson D., eds) pp. 90–105 ACS Symposium Series, Vol. 658, ACS Publications, Washington, DC [Google Scholar]
- 8. Le Goff G., Hamon A., Berge J. B., Amichot M. (2005) Resistance to fipronil in Drosophila simulans: influence of two point mutations in the RDL GABA receptor subunit. J. Neurochem. 92, 1295–1305 [DOI] [PubMed] [Google Scholar]
- 9. Ffrench-Constant R. H., Rocheleau T. A., Steichen J. C., Chalmers A. E. (1993) A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature 363, 449–451 [DOI] [PubMed] [Google Scholar]
- 10. Zhao X., Salgado V. L., Yeh J. Z., Narahashi T. (2003) Differential actions of fipronil and dieldrin insecticides on GABA-gated chloride channels in cockroach neurons. J. Pharmacol. Exp. Ther. 306, 914–924 [DOI] [PubMed] [Google Scholar]
- 11. Tomlin C. D., ed. (2006) The Pesticide Manual: A World Compendium, 14th Ed. pp. 462–464, British Crop Protection Council, Hampshire, UK [Google Scholar]
- 12. Harrison J. B., Chen H. H., Sattelle E., Barker P. J., Huskisson N. S., Rauh J. J., Bai D., Sattelle D. B. (1996) Immunocytochemical mapping of a C-terminus anti-peptide antibody to the GABA receptor subunit, RDL in the nervous system in Drosophila melanogaster. Cell Tissue Res. 284, 269–278 [DOI] [PubMed] [Google Scholar]
- 13. Buckingham S. D., Biggin P. C., Sattelle B. M., Brown L. A., Sattelle D. B. (2005) Insect GABA receptors: splicing, editing, and targeting by antiparasitics and insecticides. Mol. Pharmacol. 68, 942–951 [DOI] [PubMed] [Google Scholar]
- 14. Millar N. S., Buckingham S. D., Sattelle D. B. (1994) Stable expression of a functional homo-oligomeric Drosophila GABA receptor in a Drosophila cell line. Proc. Biol. Sci. 258, 307–314 [DOI] [PubMed] [Google Scholar]
- 15. Thompson A. J., Price K. L., Lummis S. C. (2011) Cysteine modification reveals which subunits form the ligand binding site in human heteromeric 5-HT3AB receptors. J. Physiol. 589, 4243–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi J., Blundell T. L., Mizuguchi K. (2001) FUGUE: sequence-structure homology recognition using environment-specific substitution tables and structure-dependent gap penalties. J. Mol. Biol. 310, 243–257 [DOI] [PubMed] [Google Scholar]
- 17. Sali A., Blundell T. L. (1993) Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 [DOI] [PubMed] [Google Scholar]
- 18. Sedelnikova A., Erkkila B. E., Harris H., Zakharkin S. O., Weiss D. S. (2006) Stoichiometry of a pore mutation that abolishes picrotoxin-mediated antagonism of the GABAA receptor. J. Physiol. 577, 569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thompson A. J., Duke R. K., Lummis S. C. (2011) Binding sites for bilobalide, diltiazem, ginkgolide, and picrotoxinin at the 5-HT3 receptor. Mol. Pharmacol. 80, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heads J. A., Hawthorne R. L., Lynagh T., Lynch J. W. (2008) Structure-activity analysis of ginkgolide binding in the glycine receptor pore. J. Neurochem. 105, 1418–1427 [DOI] [PubMed] [Google Scholar]
- 21. Jensen A. A., Bergmann M. L., Sander T., Balle T. (2010) Ginkgolide X is a potent antagonist of anionic Cys-loop receptors with a unique selectivity profile at glycine receptors. J. Biol. Chem. 285, 10141–10153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen L., Durkin K. A., Casida J. E. (2006) Structural model for gamma-aminobutyric acid receptor noncompetitive antagonist binding: widely diverse structures fit the same site. Proc. Natl. Acad. Sci. U. S. A. 103, 5185–5190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Erkkila B. E., Sedelnikova A. V., Weiss D. S. (2008) Stoichiometric pore mutations of the GABAAR reveal a pattern of hydrogen bonding with picrotoxin. Biophys. J. 94, 4299–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horovitz A. (1996) Double-mutant cycles: a powerful tool for analyzing protein structure and function. Folding Design 1, R121–R126 [DOI] [PubMed] [Google Scholar]
- 25. Ludmerer S. W., Warren V. A., Williams B. S., Zheng Y., Hunt D. C., Ayer M. B., Wallace M. A., Chaudhary A. G., Egan M. A., Meinke P. T., Dean D. C., Garcia M. L., Cully D. F., Smith M. M. (2002) Ivermectin and nodulisporic acid receptors in Drosophila melanogaster contain both gamma-aminobutyric acid-gated Rdl and glutamate-gated GluCl alpha chloride channel subunits. Biochemistry 41, 6548–6560 [DOI] [PubMed] [Google Scholar]
- 26. Woelkart K., Feizlmayr E., Dittrich P., Beubler E., Pinl F., Suter A., Bauer R. (2009) Pharmacokinetics of bilobalide, ginkgolide A and B after administration of three different Ginkgo biloba L. preparations in humans. Phytother. Res. 24, 445–450 [DOI] [PubMed] [Google Scholar]
- 27. Xie J., Ding C., Ge Q., Zhou Z., Zhi X. (2008) Simultaneous determination of ginkgolides A, B, C and bilobalide in plasma by LC-MS/MS and its application to the pharmacokinetic study of Ginkgo biloba extract in rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 864, 87–94 [DOI] [PubMed] [Google Scholar]
- 28. Miller T.A., Maynard M., Kennedy J. M. (1979) Structure and insecticidal activity of picrotoxinin analogs, Pestic. Biochem. Physiol. 10, 128–136 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.