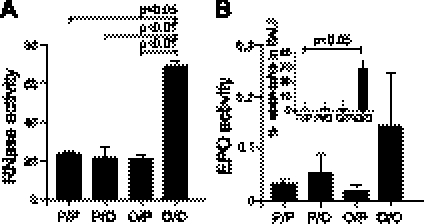Abstract
Rapid secretion of eosinophil-associated RNases (EARs), such as the human eosinophilic cationic protein (ECP), from intracellular granules is central to the role of eosinophils in allergic diseases and host immunity. Our knowledge regarding allergic inflammation has advanced based on mouse experimental models. However, unlike human eosinophils, capacities of mouse eosinophils to secrete granule proteins have been controversial. To study mechanisms of mouse eosinophil secretion and EAR release, we combined an RNase assay of mouse EARs with ultrastructural studies. In vitro, mouse eosinophils stimulated with the chemokine eotaxin-1 (CCL11) secreted enzymatically active EARs (EC50 5 nM) by piecemeal degranulation. In vivo, in a mouse model of allergic airway inflammation, increased airway eosinophil infiltration (24-fold) correlated with secretion of active RNases (3-fold). Moreover, we found that eosinophilic inflammation in mice can involve eosinophil cytolysis and release of cell-free granules. Cell-free mouse eosinophil granules expressed functional CCR3 receptors and secreted their granule proteins, including EAR and eosinophil peroxidase in response to CCL11. Collectively, these data demonstrate chemokine-dependent secretion of EARs from both intact mouse eosinophils and their cell-free granules, findings pertinent to understanding the pathogenesis of eosinophil-associated diseases, in which EARs are key factors.—Shamri, R., Melo, R. C. N., Young, K. M., B.-B, M., Xenakis, J. J., Spencer, L. A., Weller, P. F. CCL11 elicits secretion of RNases from mouse eosinophils and their cell-free granules.
Keywords: piecemeal degranulation, airway inflammation, cationic proteins, granulocytes
Eosinophils, both as end-stage effector cells and as collaborative interacting components of innate immune responses, rely on the secretion of varied proteins that are stored in their specific granules. Specific granules of human eosinophils contain cytotoxic, highly basic proteins, including major basic protein (MBP), eosinophil peroxidase (EPO), and the eosinophil-associated RNases (EARs): eosinophil-derived neurotoxin (EDN) and eosinophilic cationic protein (ECP). The principal process of secretion from intracellular granules is by means of piecemeal degranulation (PMD; ref. 1), in which intact eosinophils can mobilize and selectively secrete their preformed protein contents by transporting small packets within granule-derived secretory vesicles to the cell surface. In addition, in other eosinophil-associated diseases, cell-free eosinophil granules are found in tissues (2–4).
Over the years, EARs have been shown to have a major contribution to host defense against various pathogens, including helminths, fungi, viruses, and bacteria, as well as participating in tissue damage and remodeling in eosinophil-associated diseases and functioning as immunomodulators (5, 6). Among their activities is the antiviral capacity, which was proven to require their RNase activity (7). Both EDN and ECP can diminish the infectivity of respiratory syncytial virus (RSV) in vitro in an RNase activity-dependent manner (7, 8). Furthermore, infection of mice with pneumonia virus of mice or RSV can result in prominent expression of mouse EARs (9, 10). Their antiviral activity as well as their cytotoxic capacity persuaded us to study the mechanisms of EAR secretion from mouse eosinophils.
Mouse experimental models have contributed to our knowledge regarding asthma and allergic inflammation (11). However, unlike human eosinophils, the capacity of mouse eosinophils to secrete their granule contents, in vitro or in vivo in airway inflammation models, has been controversial (5, 12–18). One example of this controversy has been the capacity of eotaxin-1 (CCL11), the principal chemoattractant of human and mouse eosinophils (19), to elicit degranulation. It was shown previously that CCL11 stimulates eosinophil degranulation in human eosinophils (20–22) but failed to elicit granule protein secretion in mouse eosinophils (17). In addition, coexpression of IL-5 and CCL11 in the lungs of mice, an asthma model that mimics the airway environment in human asthma, failed to show eosinophil degranulation, as measured by the absence of MBP in bronchoalveolar lavage fluid (BALF; ref. 14). Moreover, while eosinophils from double transgenic mice coexpressing IL-5 and eotaxin-2 (CCL24) spontaneously degranulate in vivo, stimulation of wild-type mouse eosinophils with these two cytokines failed to induce mouse eosinophil degranulation (measured by EPO secretion; ref. 18). Therefore, the applicability of mouse models to eosinophil-associated diseases of humans in which eosinophil degranulation is often observed has been called into question (16). Understanding the conditions and environment required for mouse eosinophils to secrete granule protein contents is important in order to understand how mouse models mimic human and allergic inflammation. Our study aimed to detect the capacity and the mechanism of physiological stimuli, such as CCL11, to elicit EAR secretion from mouse eosinophils.
Studies of eosinophil secretory responses have utilized various techniques to demonstrate degranulation, such as activity assays of granule enzymes, electron microscopy, and ELISA assays, using specific antibodies against human eosinophil granule proteins. However, prior lack of antibodies specific to mouse eosinophil granule proteins, like mouse EARs (mEARs), EPO, or MBP, has impeded studies of secretory responses of mouse eosinophils (23). Antibodies against murine MBP and EPO, developed by Dr. James J. Lee and colleagues (Mayo Clinic Arizona, Scottsdale, AZ, USA), have been used to detect the presence of these granule proteins in BALF of mice that were primed and challenged with ovalbumin (OVA), using Western blotting (dot blot) methodology (17, 18). However, Western blotting is not as sensitive or quantitative as ELISA or enzyme activity assays and does not address the functionality of secreted products.
One way to circumvent this lack of specific antibodies is to use nonimmunological methods like enzyme activity. EPO activity assay is the most commonly used technique to examine degranulation. However, EPO activity assays, although popular, have caveats due to the basic nature of EPO, which tends to stick to surfaces after its release from granules (24). In addition, EPO activity autoinactivates the enzyme and decreases over time, resulting in a nonlinear readout. Thus, a more sensitive and reliable assay to quantitate secretory responses is needed in order to study degranulation responses of mouse eosinophils.
The mouse EAR family includes ≥13 members, with 70% identity among them (25, 26). At least 6 EARs are present in mouse eosinophil granules (27). The mEARs as well as their human orthologs, ECP and EDN, are members of the RNase A superfamily of polymeric RNA ribonucleases. ECP and EDN prefer single-stranded RNA (28), contributing to their antiviral capacity (7, 9, 26). To assay EARs secreted by mouse eosinophils, we utilized an assay to detect the RNase activity of secreted mEARs. With CCL11 as a stimulus, enzymatically active mEARs were secreted from both intact mouse eosinophils and isolated cell-free mouse eosinophil granules.
MATERIALS AND METHODS
Isolation of mouse eosinophils
IL-5 transgenic BALB/c mice (29), provided by Dr. Alison A. Humbles and Dr. Craig Gerard (Children's Hospital Medical, Boston, MA, USA), and BALB/c mice, purchased from Charles River Laboratories (Wilmington, MA, USA), were both housed in a pathogen-free facility. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center (Boston, MA, USA). Highly purified (>98%) mouse eosinophils were isolated from mechanically disrupted spleens of IL-5 transgenic mice as described previously (30). Purity and viability of >98% were determined by microscopic analysis after Hema 3 staining and trypan blue exclusion, respectively.
Stimulation of mouse eosinophils
Mouse eosinophils were stimulated at 37°C for 1 h with the indicated concentrations of phorbol myristate acetate (PMA; Sigma-Aldrich, St. Louis, MO, USA), recombinant mouse CCL11 (Peprotech, Rocky Hill, NJ, USA), or medium alone (RPMI medium 1640 without phenol red; BioWhittaker, Walkersville, MD, USA), supplemented with 0.1% OVA (Sigma-Aldrich) at a final concentration of 106 cells/0.25 ml. EC50 was calculated according to Alexander et al. (31). In some experiments, cells were pretreated 20 min before and during chemokine stimulation at 37°C with the transcription inhibitor actinomycin D (ActD; 1 and 10 μM; Sigma-Aldrich), or the protein synthesis blocker cycloheximide (CHX; 1 and 10 μM; Sigma-Aldrich). As controls, cells were pretreated with equivalent concentrations of vehicle. Cell viability after inhibition and stimulation, as detected by trypan blue exclusion or propidium iodide staining, was >93%. Supernatants were obtained by centrifugation at 300 g for 5 min at 4°C, followed by centrifugation at 20,000 g for 10 min at 4°C.
Eosinophil subcellular fractionation
Purified mouse eosinophils (10–30×106) were subjected to nitrogen cavitation (Parr, Moline, IL, USA), and postnuclear supernatants were ultracentrifuged (100,000 g, 1 h at 4°C) in linear isotonic gradients 0–45% (Optiprep; Axis-Shield PC, Norton, MA, USA), as described previously (32). Fractions of 0.5 ml were collected.
Granule purification and stimulation
Mouse eosinophil subcellular fractions that were shown to be enriched with the granule enzyme EPO were pooled. The eosinophil granules were spun down at 2500 g at 4°C and washed with Ca2+- and Mg2+-free HBSS. Granules were resuspended in RPMI, supplemented with 0.1% OVA (Sigma-Aldrich), and stimulated with 11.9 nM CCL11 for 1 h at 37°C. Supernatants were obtained by 2500 g centrifugation for 10 min at 4°C using a swinging bucket rotor, followed by further centrifugation at 20,000 g for 10 min at 4°C.
Flow cytometry
Mouse eosinophils or purified granules were incubated with Alexa-647-conjugated anti-CCR3, or FITC-conjugated anti-IFN-γR or isotype control monoclonal antibodies [1 μg/106 cells; Becton Dickinson (BD) Bioscience, San Jose, CA, USA] in FACS buffer (0.5% BSA/Ca2+- and Mg2+-free HBSS) on ice for 30 min for eosinophils and for 1 h for granules. Mouse eosinophils were preincubated for 10 min on ice with FcγR blocking mAbs prior to antibody staining. Data were acquired using the LSRII flow cytometer (BD Bioscience) and the analysis software, Flow Jo (Tree Star, Ashland, OR, USA).
Enzymatic activity assays
RNase activity assay
The human granule RNases ECP and EDN have a substrate preference for single-stranded RNA (28). Therefore, we measured the presence of mEARs in 1:8000 diluted supernatants of stimulated mouse eosinophils and stimulated granules or subcellular fractions by RNase activity assay using a fluorescent single-stranded RNA oligonucleotide (RNaseAlert QC system; Ambion, Austin, TX, USA), according to the manufacturer's instructions. Cleavage of the probe by RNases allows fluorescent emission that is detected by fluorometry. The total cellular content of mEARs was assessed by measuring RNase activity in eosinophils lysed in 0.2% Triton X-100. Data were acquired after 30 min (granules) or 50 min (eosinophils) by the 7300 thermocycler (Applied Biosystems, Austin, TX, USA) and were within the linear range. All reactions were performed in duplicate or triplicate wells. Relative fluorescence units (RFU; ×104) represent RNase activity levels from stimulated samples minus RNase activity levels from nonstimulated samples (except in Supplemental Fig. S1A, C, which shows the nonsubtracted values). Ten × 104 RFU represents enzyme activity that is equivalent to the activity of 4.0 ± 1.8 nU RNases (∼0.0398 pg), as calibrated with bovine pancreatic RNase A (Ambion). One unit is the amount of RNase equivalent to 0.1177 kunitz.
EPO activity assay
EPO activity was measured by a colorimetric assay [optical density (OD) was measured at 492 nm; ref. 24] in eosinophil and purified granule supernatants (30–50 μl) or subcellular fractions.
β-Hexosaminidase assay
β-Hexosaminidase activity was measured as described previously (32). Fluorescence (excitation, 360 nm; emission, 460 nm) was measured in a CytoFluor 2350 plate reader (Millipore, Billerica, MA, USA).
MBP dot blot
Supernatants of nonstimulated or CCL11-stimulated mouse eosinophils were serially diluted in 0.1% OVA/RPMI and applied to dry nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). Membranes were then bleached for endogenous peroxidase activity with 0.6% hydrogen peroxide in TBS solution (20 mM Tris, pH 7.5, and 150 mM NaCl) for 20 min, followed by blocking with TBST solution (1% BSA/TBS and 0.05% Tween 20) for 1 h. Membranes were incubated with a rat anti-mouse MBP antibody (a kind gift of Dr. James J. Lee, Mayo Clinic Arizona) in TBST solution (5 μg/ml) overnight at 4°C. Membranes were washed with TBST, incubated with horseradish peroxidase-conjugated goat anti-rat IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 45 min, and then developed with a Femto chemiluminescence kit (Pierce Biotechnology, Rockford, IL, USA).
Light microscopy
Samples were imaged by using a BX62 Olympus upright microscope (Olympus, Tokyo, Japan), coupled to a Hamamatsu Orca AG cooled CCD camera (Hamamatsu Photonics, Hamamatsu, Japan); images were acquired using iVision software (iVision, Atlanta, GA, USA).
Transmission electron microscopy (TEM)
Eosinophils stimulated with 11.9 nM CCL11 or medium alone were prepared for TEM as before (22). To quantify the total number of emptying granules within eosinophils, we randomly obtained electron micrographs of cell sections showing the entire cell profile and nucleus at ×12,000 and analyzed these at a final view of ×33,000. The percentages of altered granules among all granules were calculated for each cell. A total of 316 granules were counted.
Induction of allergic airway inflammation and isolation of BALF
BALB/c, 6–8 wk old female mice were sensitized i.p. 3 times with either 50 μg of OVA or phosphate-buffered saline (PBS; Sigma-Aldrich) plus a mixture of 0.4 mg Al(OH)3 and 0.4 mg Mg(OH)2 (Imject Alum Adjuvant; Thermo Scientific, South Logan, UT, USA) in 0.1 ml PBS on d 0, 7, and 14. On d 21–23, sensitized mice were anesthetized with a 50 mg/ml ketamine and 5 mg/ml xylazine mixture (Sigma-Aldrich), and challenged with either 20 μg OVA in 50 μl PBS or PBS alone by direct spray into the airways using an endotracheal intubing microsprayer apparatus (Penn-Century Inc., Wyndmoor, PA, USA; ref. 33). Each group included 3 mice. At 4 d after final challenge, lungs were lavaged, and BALF cells were labeled with anti-Siglec F monoclonal antibodies (BD Bioscience), an established mouse eosinophil marker. Eosinophil percentages [Siglec F+ cells in forward scatter (FSC)/side scatter (SSC) granulocyte gate] were calculated from total cells in BALF. Our gating strategy showed that 92–96% of the Siglec F+ cells in BALF of OVA primed- and challenged-mice were FSCint, where granulocytes and eosinophils reside, and only 2–4% of the Siglec F+ cells were FSChi, where alveolar macrophages usually reside. Conversely, 65–78% of the Siglec F+ cells in BALF of control mice (PBS-OVA, or PBS-PBS or OVA-PBS) were FSChi, representing resident alveolar macrophages, further confirming that the increase in Siglec F+ cells in BALF of OVA-OVA mice is due to the increase in eosinophils. Aliquots of BALF supernatants were stored at −80°C until assayed for RNase or EPO activity.
Statistical analysis
Levels of significance between groups were analyzed by 2-tailed paired Student's t tests. Values of P < 0.05 were considered statistically significant.
RESULTS
mEARs are secreted by PMA-stimulated mouse eosinophils
Initially, to test our hypothesis that mEARs are secreted in vitro by mouse eosinophils and can be detected by an RNase activity assay, we used the strong, nonphysiologic xenobiotic stimulator PMA (Supplemental Fig. S1A) to induce secretion. We measured the presence of mEARs in supernatants of stimulated mouse eosinophils by an RNase activity assay using a fluorescent single-stranded RNA oligonucleotide. Mouse eosinophils stimulated with 162 nM PMA for 1 h showed a significant increase in mEAR secretion of ∼70 × 104 RFU over unstimulated cells, as measured by RNase activity (Supplemental Fig. S1A). No significant difference was found between 16.2 and 162 nM PMA stimulations. Nonstimulated cells showed a baseline RNase activity of 68.5 ± 1.2 × 104 RFU above buffer-only values, which might reflect spontaneous degranulation or a degranulation mediated by autocrine cytokine stimulation, as previously reported for human eosinophils (34). This basal secretion was detectable due to the heightened sensitivity of the RNase assay; therefore, we depicted the values of PMA-mediated degranulation after subtraction of the nonstimulated values, as shown in Supplemental Fig. S1B. In addition, kinetic experiments showed that secretion of mEARs in response to PMA can be detected after 10 min, and mEARs increased linearly over time, reaching a maximum activity at 1 h stimulation (data not shown), with no further increase after 3 h stimulation.
Mouse EAR secretion is correlated with eosinophil infiltration into airways of OVA-challenged mice
To determine whether mEARs are secreted and can be detected in vivo, we examined the correlation between mouse eosinophil infiltration into airways and the secretion of mEARs in airways of OVA-primed and -challenged mice, a mouse model of allergic airway inflammation. At 4 d after final challenge, the RNase activity in BALFs from OVA-primed and -challenged (OVA/OVA) mice was significantly higher compared to the control groups, which were primed with PBS and challenged with PBS or OVA (PBS/PBS or PBS/OVA) or primed with OVA and challenged with PBS (OVA/PBS) (Fig. 1A). This increase in RNase activity was correlated with an increase in eosinophils found in BALF of OVA/OVA mice (Fig. 1B, inset). Control mice (PBS/PBS, PBS/OVA, and OVA/PBS), which had significantly lower levels of mEAR activity, had few eosinophils in their BALFs (Fig. 1B, inset). Along this line, EPO activity in BALFs correlated (Fig. 1B) with the RNase activity, further demonstrating mouse eosinophil degranulation. However, in some experiments, the EPO assay showed fluctuating values between mice in the same group. In those experiments, high OD492 nm values were correlated with the presence of red blood cells (RBCs) in the BALF. These high OD values are probably due to the peroxidase-like activity of hemoglobins (35), found in samples contaminated with RBCs. Conversely, RNase activity assay was not affected by the presence of RBCs in BALF samples, emphasizing the benefit of using RNase activity assay as an indicator for mouse eosinophil degranulation and EARs secretion in vivo.
Figure 1.
Mouse EAR secretion in vivo is correlated with eosinophil infiltration into airways of mouse OVA-challenge model of airway inflammation. RNase (A) or EPO (B) activity was measured in BALF supernatant isolated 4 d after the final challenge of OVA (O)- or PBS (P)-primed and -challenged mice. Mouse groups are depicted as priming/challenge with OVA or PBS (P/P, P/O, O/P, or O/O). Data are means ± sd of relative fluorescence (×104 RFU; A) or OD492 nm (B) acquired from triplicate wells of 3 mice/group. Inset: eosinophil percentage from total cells found in BALFs of OVA- or PBS-primed and -challenged mice (B). Data are representative of 2 independent experiments. P values of enzyme activity or eosinophil percentage in BALF are compared with OVA-primed and -challenged samples.
CCL11 stimulation elicits mEARs secretion from mouse eosinophils
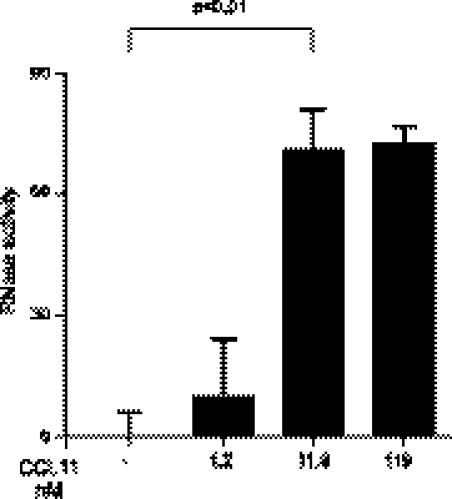
After confirming that the RNase activity assay can be utilized to examine EAR secretion from mouse eosinophils in vitro and in vivo, we investigated whether CCL11 can elicit mEARs secretion from mouse eosinophils. The chemokine CCL11 is primarily a chemoattractant and stimulator of eosinophils (19), which express its G-protein coupled receptor, CCR3. We evaluated whether this single physiological stimulus could induce secretion of mEARs from mouse eosinophils in vitro. We first documented the expression of CCR3 on mouse eosinophils purified from IL-5 transgenic mice (Supplemental Fig. S1C, inset). Mouse eosinophils stimulated with recombinant mouse CCL11 for 1 h showed significant increases in mEARs secretion in a dose-dependent manner, as measured by RNase activity assay (Fig. 2 and Supplemental Fig. S1C), with an EC50 value of 5 nM (∼33 ng/ml). A dose response of CCL11 (Supplemental Fig. S1C) revealed that RNase secretion could be detected at the lowest stimulus concentration of 0.12 nM with 1.34-fold over that of nonstimulated cells (dashed line). Further secretion of EARs was demonstrated with higher concentrations of CCL11 (1.2 and 11.9 nM, respectively) without a detrimental effect on cell viability (>93% viability). A greater concentration of CCL11 (119 nM) did not yield higher RNase activity in supernatants (Fig. 2 and Supplemental Fig. S1C). Kinetic experiments showed that secretion of mEARs in response to CCL11 can be detected after 10 min, and mEAR activity increased linearly over time, reaching a maximum activity at 2 h stimulation (Supplemental Fig. S1D), with a slight decrease after 6 h stimulation. Using total RNase activity of mouse eosinophil lysates as a standard, CCL11-induced RNase activity secreted in supernatants represented ∼12% of the total cellular mEARs (data not shown). This amount of secreted mEARs is equivalent to the activity of 2.3 ± 0.9 nU (or 0.0234±0.009 pg) of bovine pancreatic RNase. The percentages of secreted mEARs are similar to that previously found for the release of the human RNase ECP from human eosinophils stimulated with serum-opsonized Sephadex particles (36) and for the release of human EDN from human eosinophils stimulated with CCL11 (20).
Figure 2.
RNases are released from mouse eosinophils in response to CCL11. Mouse eosinophils were stimulated with the indicated concentrations of CCL11 at 37°C for 1 h. Supernatants were measured for RNase enzymatic activity. Data are means ± sd of relative fluorescence (×104 RFU) acquired from duplicate wells and are representative of 3 independent experiments. P values of CCL11-stimulated eosinophils are compared with nonstimulated eosinophils.
mEARs are secreted from preformed granule stores
Having established that CCL11 can elicit mEAR secretion from mouse eosinophils, we next investigated the source of those secreted mEARs. By using transcription and protein translation inhibitors, we demonstrated that mEARs were secreted from preformed stores and not de novo synthesized, since neither the transcription inhibitor ActD nor the protein translation inhibitor CHX had any effect on CCL11-elicited mEAR secretion (Supplemental Fig. S1E, F). Additional evidence for preformed mEARs is that CHX did not change the total amount of mEARs in the cell (found in supernatant and in cell pellet; Supplemental Fig. S1G).
Of the 13 mEARs, 6 to date have been shown to be localized in mouse eosinophil granules. Therefore, we sought to estimate the percentage of granule mEARs compared to nongranule mEARs in order to correlate EAR secretion and degranulation responses. We performed subcellular fractionation of mouse eosinophils and measured the activity of the granule enzymes in each fraction. Indeed, granule-enriched fractions (F7–F11), visualized by light microscopy and confirmed by EPO activity (Supplemental Fig. S2B), had the highest mEAR activity, with 48% of total RNase activity (Supplemental Fig. S2A). Lower levels (16%) of mEAR activity were detected in low-density fractions (F21–F24) that generally contain cytosolic proteins (37). This minor peak in the cytosolic fractions (F20–F21, lowest density fractions) was not decreased after further higher ultracentrifugation (Supplemental Fig. S3A), unlike the granules fractions and the in-between fractions (F7-F11), which likely contain secretory vesicles, thus suggesting that this minor peak of RNase activity was due to cytosolic and not vesicle-associated RNases. β-Hexosaminidase, an enzyme found in eosinophil granules (38), showed a dual modality peak pattern (Supplemental Fig. S2C), suggesting the localization of β-hexosaminidase enzymes in granules as well as in cytosol.
To further demonstrate that granules are the source of secreted mEARs, we performed subcellular fractionations of nonstimulated and CCL11-stimulated mouse eosinophils and measured the activity of mEARs in each fraction. Indeed, the amount of mEARs (measured by RNase activity) in granule-enriched fractions (F7-F11) was decreased by 26% after stimulation with CCL11 (Supplemental Fig. S3B) compared to the equivalent fractions from nonstimulated mouse eosinophils. The amount of mEARs in nongranule fractions (F1–F5) did not change. As expected, the amount of mEARs in the low-density fractions, usually containing secretory vesicles and the cytosol proteins, increased after CCL11 stimulation, suggesting that under CCL11 stimulation, mEARs are transferred from granules to secretory vesicles on their way out of the cells.
CCL11-mediated release of mEARs is correlated with the release of other granule-associated proteins, such as EPO, β-hexosaminidase, and MBP
In order to better correlate the release of mEARs with secretion of other granule proteins, we compared the secretion of mEARs to the secretion of the granule-stored enzymes EPO and β-hexosaminidase in response to CCL11. The secretion of mEARs from mouse eosinophils stimulated with CCL11 (11.9 nM; Fig. 3A) paralleled the secretion of the granule-associated enzymes EPO (Fig. 3B) and β-hexosaminidase (Fig. 3C). The RNase activity assay was very sensitive compared to β-hexosaminidase and EPO enzyme activity assays, since the amount of supernatant required for the detection of mEARs was 10,000-fold less compared to the amount required for the detection of β-hexosaminidase and EPO enzymes.
Figure 3.
mEAR release correlates with the release of granule proteins in response to CCL11 stimulation. A–C) Mouse eosinophils were stimulated with 11.9 nM of CCL11 for 1 h at 37°C, and supernatants were measured for RNase (A), EPO (B), and β-hexosaminidase (C) activity. Data are means ± sd of relative fluorescence (×104 RFU; A) or OD492 nm (B) or OD460 nm emission levels (C) acquired from duplicate wells. D) Left panel: dot blot detecting the presence of MBP in supernatants of mouse eosinophils stimulated with 11.9 nM of CCL11. Right panel: density values of the dot blot were calculated and depicted as arbitrary mean intensity units. Data are representative of 3 independent experiments. P values of stimulated eosinophils are compared with nonstimulated eosinophils.
To further ascertain degranulation, in an immunospecific assay, we measured the presence of mouse MBP in supernatants of CCL11-stimulated eosinophils using an antibody against mouse MBP (Fig. 3D). The dot blot (Fig. 3D, left panel) and the blot density values (Fig. 3D, right panel) of the nonstimulated and CCL11-stimulated eosinophil samples showed that mouse MBP is secreted (1.8-fold) in response to CCL11 stimulation (Fig. 3D) compared to nonstimulated eosinophils.
Ultrastructural changes within granules of CCL11-activated mouse eosinophils are consistent with PMD
Mouse EAR secretion from granules might take place by means of 3 well-described processes: classic exocytosis, in which granules fuse with the eosinophil plasma membrane in order to release their entire contents (39); cytolysis, characterized by plasma membrane rupture and the extracellular liberation of intact membrane-bound granules (4); and/or PMD. Electron microscopy is the most suitable method to distinguish the 3 processes (40). To characterize ultrastructural events within secretory granules that underlie agonist-elicited degranulation, mouse eosinophils that were stimulated with CCL11 or medium alone for 1 h were examined by TEM. In nonstimulated eosinophils, granules were seen as round or elliptical structures, full of electron-dense contents, with a well-organized core and an outer granule matrix surrounded by a delimiting trilaminar membrane (Fig. 4A). After stimulation, granules showed morphological evidence of degranulation associated with PMD (Fig. 4Bi) such as enlarged granules, reduced electron density, matrix coarsening associated with reduced electron density, disassembled cores, or loss of the matrix or cores (Fig. 4Bii). Most changes were localized to the granule matrices, while core changes were less pronounced. These altered granules were always intermingled, in the same cell section, with resting, nonmobilized granules. However, granule-granule or granule-plasma membrane fusion events were not seen in any cell section, suggesting that mouse eosinophils do not undergo classic or compound exocytosis under stimulation with CCL11 and thus respond analogously to what was previously observed for human eosinophils stimulated with this agonist (41). To quantify the number of granules undergoing PMD, eosinophil sections showing the entire cell profile were randomly analyzed, and a total of 316 granules were counted. Eosinophil activation induced significant increases (5-fold) in the numbers of emptying granules (Fig. 4C). These ultrastructural changes in CCL11-stimulated mouse eosinophils indicate that mouse eosinophils undergo PMD after brief stimulation with CCL11.
Figure 4.
CCL11-mediated activation of mouse eosinophils induced significant granule emptying. A–Bii) Mouse eosinophils were incubated with medium alone (A) or 11.9 nM CCL11 (Bi, Bii) at 37°C and prepared for transmission electron microscopy. After 1 h of stimulation with CCL11, granules exhibited emptying of their contents indicative of PMD. Bii corresponds to the boxed area in Bi. Altered granules are shown in higher magnification (arrows). Not all granules exhibited signs of content loss. Note that granules did not show fusion events. C) Percentage of emptying granules per cell section. Data are means ± se, n = 20 cells. Gr, granule; N, nucleus. Scale bars = 1.9 μm (A, Bi); 0.5 μm (Bii).
Cell-free, purified granules of mouse eosinophils express functional CCR3 receptors and secrete mEARs in response to CCL11 stimulation
In addition to secretion of granule proteins from eosinophils by PMD, eosinophils have the capacity to release intact granules on cytolysis (2–4). Cell-free granules released from eosinophils were found in human inflamed tissues obtained from patients with hypereosinophilic syndromes or other pathological conditions (3, 42–44). Those cell-free granules from human eosinophils were recently shown to have the capacity to secrete their content in response to cytokine stimulation, such as IFN-γ or CCL11, or lipid mediators, such as cysteinyl leukotrienes, ligands for receptors expressed on the surface membranes of granules (43, 45). However, the capacity of mouse eosinophils to release granules and the capacity of these cell-free eosinophil granules to secrete mEARs are not known. Therefore, we looked for evidence of eosinophil cytolysis and cell-free granules in mouse models of inflammation and investigated the capability of these free granules to secrete their content following CCL11 stimulation.
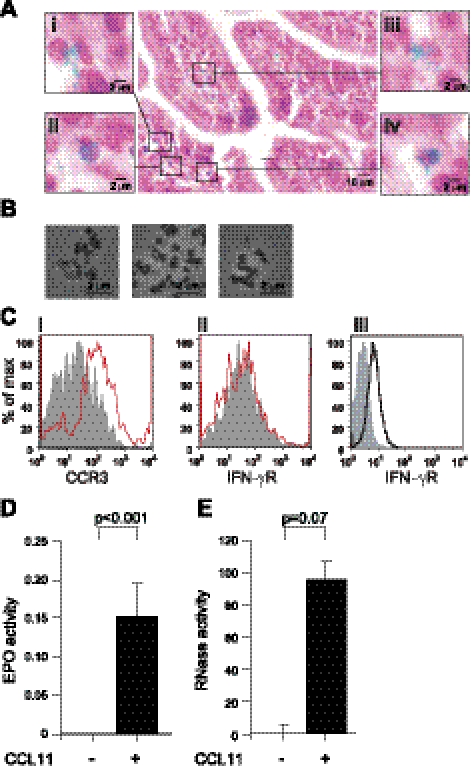
Evidence for a cytolysis mechanism in mouse eosinophils and the presence of cell-free granules were found within intestinal tissue following OVA-induced inflammation (Fig. 5A). Intact eosinophils are depicted by fast green granule staining and neutral red nuclear staining (Fig. 5Aiv). In addition, some eosinophils showed signs of cytolysis (Fig. 5Aii, iii) where the fast green-stained granules were scattered around the cells. Moreover, cell-free granules were observed in the tissue as well (Fig. 5Ai).
Figure 5.
Cell-free purified granules of mouse eosinophils express functional CCR3 receptors and can secrete EPO and mEARs in response to CCL11 stimulation. A) Cell-free eosinophil granules and intact eosinophils were identified in intestinal tissue section in OVA-sensitized and challenged mice. Samples were stained with fast green and neutral red and imaged using a BX62 Olympus upright microscope fitted with a ×40 1.3 UPlanApo objective. Insets i–iv depict either intact cells (iv) or cells undergoing cytolysis and cell free granules (i–iii). B) Purified mouse eosinophil granules were imaged using ×60 1.42 UPlanApo objective. C) Immunostaining of isolated eosinophil granules with Alexa-647 conjugated anti-CCR3 antibody (i) or FITC conjugated anti-IFN-γR antibody (ii; red traces) or isotype controls (gray), or immunostaining of nonpermeabilized mouse eosinophils for surface plasma expression of IFN-γR (solid black trace; iii) or isotype control (gray). Results are representative of 4 independent experiments. D, E) Purified granules from mouse eosinophils were stimulated with 11.9 nM of CCL11, and supernatants were assayed for EPO (D) or RNase (E) activity. Data are means ± sd of OD492 (D) or relative fluorescence (×104 RFU; E), respectively, acquired from duplicate wells. Data are representative of ≥6 independent experiments. P values are compared with nonstimulated granules.
To investigate the functionality of these released intact granules, we isolated granules from mouse eosinophils (Fig. 5B) and examined their ability to secrete their content in a cell-free context. Cell-free granules were found to express CCR3 (Fig. 5Ci), but not IFN-γR (Fig. 5Cii), on their surface. The fact that granules, unlike intact eosinophils (Fig. 5Ciii), do not express IFN-γR suggests that the purified granules are free from intact cells or residual cell membranes. Stimulation of the cell-free eosinophil granules with 11.9 nM CCL11 induced EPO secretion (Fig. 5D), as well as secretion of mEARs (Fig. 5E), as measured by EPO or RNase A activity assays in granule supernatants. Thus, cell-free granules express functional receptors that can elicit secretion of granule-contained proteins, such as EPO and EARs, in response to CCL11.
DISCUSSION
We aimed to study the capacities and mechanisms whereby mouse eosinophils might secrete their granule-derived RNase proteins. We evaluated whether mouse eosinophils can respond to specific stimuli, including the eosinophil active chemokine CCL11, to secrete granule-derived proteins and whether mouse eosinophils and their isolated cell-free granules can secrete EARs. As cells of the innate immune system, it is notable that eosinophils of many species contain within their granules prominent proteins with RNase A activities. Yet within eosinophils of vertebrates, evolution has endowed eosinophils with a considerably varied repertoire of granule-associated EARs (26). In human eosinophils, two predominant cationic granule proteins, ECP and EDN, have homologies to RNases. Levels of ECP and EDN, as assessed by immunoassays of secreted proteins, both in vivo in humans and in vitro, are widely used as measures of eosinophil degranulation and as markers of eosinophil associated disease activities (46). Even within primates, eosinophil granule RNases are different. Old World primate eosinophils, like human eosinophils, contain both ECP and EDN, whereas New World primates lack EDN (47). ECP exhibits relatively weak RNase activity, whereas EDN has RNase activities 1000-fold greater than ECP and approximate to pancreatic RNase activities (27).
In rodents, several EARs have low homologies to EDN or ECP. To date, mEARs include 11 genes (EAR 1–11) and 2 pseudogenes (25) and are suggested to represent several events of gene duplication occurring after the separation of primates from rodents (25). In mice, mEARs are estimated to constitute ∼50% of the protein content within eosinophil granules and at least 6–8 mEARs are granule proteins (27). The facts that both the human EARs and several mEARs are stored as preformed enzymes in granules and ready to be released indicates that regulated secretion of these eosinophil granule-derived RNase-related proteins may contribute to the roles of eosinophils in host-defense and/or immunopathogenesis.
Activities of human EARs include functions not related to their RNase capacities (48, 49). In addition, based on their RNase activities, EARs have been implicated in antiviral responses in humans and in mouse models (7–9, 50). Human and mouse EARs can also directly activate dendritic cells and enhance Th2 immune responses (51). Conversely, single-stranded RNA can activate degranulation of eosinophils via TLR-7, and eosinophil RNases might limit eosinophil degranulation as well as functioning in antiviral host responses (10).
To date, assays of the secretion of human EARs have relied solely on immunoassays of human ECP and EDN, two granule-derived proteins, without assessing their secreted RNase activity. Moreover in many mouse models, the capacity of mouse eosinophils to secrete granule proteins, their apparently higher thresholds for degranulation, and their nominally impaired abilities to degranulate in vitro with physiological stimuli (17) and not with PMA (18) have been of concern. To better assess the capacities and mechanisms whereby mouse eosinophils might secrete their mEARs, we utilized a functional enzymatic RNase assay of secreted mEARs. By this approach, we were not limited by lack of antibodies to assay the multiple mEARs, and we could directly measure the collective activity of all secreted mEARs based not on their protein levels, but rather on their biologically active roles as functional RNases.
By utilizing RNase activity assays, we found that mouse eosinophils secrete preformed and enzymatically active EARs in vitro in response to the xenobiotic PMA, in vivo in the BALF of a mouse model of allergic inflammation, and in vitro in response to CCL11. Responses of mouse eosinophils to secrete their mEARs paralleled secretory responses of other granule-derived enzymes (EPO, β-hexosaminidase) and MBP. Although the RNase activity assay itself cannot distinguish whether the secreted RNases originated from granules, the finding that 48% of RNase activity in resting eosinophils was found to be localized in granule-enriched subcellular fractions, together with our results showing that the RNase content of granule fractions, but not cytosolic or vesicle-enriched fractions, was reduced following CCL11 stimulation, indicates that the main source of released mEARs was mouse eosinophil granules. Moreover, isolated granules secreted their EARs content in response to CCL11. Therefore, RNase activity assays can serve as measures of secretory degranulation in mouse eosinophils. In addition, other granule proteins, such as EPO, β-hexosaminidase, and MBP, were also found to be released in response to CCL11, accompanied by granule emptying.
As noted above, 3 processes of granule protein secretion are recognized for human eosinophils: classic exocytosis, PMD, and cytolysis. PMD and cytolysis are the major mechanisms of human eosinophil secretion. Our in vitro studies showed that secretion of granule proteins from mouse eosinophils in response to CCL11 was accompanied by ultrastructural changes, including reduced electron density, granule matrix coarsening, disassembled cores, or loss of the matrix or cores within granules, all of which are characteristics of PMD (13, 17). We found no evidence in vitro for other secretion mechanisms, such as exocytosis or cytolysis, in response to CCL11. Our results, confirming previous in vivo studies, identify PMD as the principal mechanism of mouse eosinophil secretion (17). To our knowledge, this work represents the first demonstration of CCL11-mediated PMD by mouse eosinophils.
In addition to our in vitro studies of secretory responses on intact mouse eosinophils, we also evaluated the capacities of isolated, cell-free murine eosinophil granules to secrete their mEARs in response to CCL11. The presence of eosinophil cell-free granules in tissues and fluids in association with eosinophil-mediated inflammation has previously been well documented, notably in humans as well as guinea pigs (43, 52). Recently, Verjan-Garcia et al. (53) have shown the presence of MBP+, CD63+, DAPI− extracellular eosinophil granules in small intestinal sections of mice expressing a mutation in the inhibitory receptor SIRPα/CD172α. We now also demonstrate evidence of released cell-free eosinophil granules in a murine model of OVA sensitization and challenge. We have demonstrated that isolated human eosinophil granules can secrete their contents in a cell-free context (43, 45). These cell-free human eosinophil granules were shown to express ligand-responsive cytokine and leukotriene receptors and secrete their contents, including ECP, in agonist-receptor-dependent mechanisms. Here we show for the first time that cell-free granules isolated from mouse eosinophils express functional CCR3 receptors and can secrete EPO and mEARs in response to CCL11 stimulation. These findings support the import primacy of mouse eosinophils as a source of secreted mEARs, both from within intact eosinophils and from their cytolytically released granules. These unique and novel abilities of eosinophil cell-free granules to remain secretory competent extracellularly are evolutionarily common to both humans and mice.
Supplementary Material
Acknowledgments
The authors thank Dr. James J. Lee (Mayo Clinic Arizona, Scottsdale, AZ, USA) for providing anti-mouse MBP. The authors also thank Dr. Ionita Ghiran and Dr. Roi Gazit for editorial assistance.
This study was funded by grants from the U.S. National Institutes of Health to P.F.W. (R01/R37 AI020241 and R01 AI051645) and L.A.S. (R01 HL095699), and from Conselho Nacional de Pesquisa Cinetífica e Tecnológica (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) to R.C.N.M.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ActD
- actinomycin D
- BALF
- bronchoalveolar lavage fluid
- CCL11
- eotaxin-1
- CCL24
- eotaxin-2
- CHX
- cycloheximide
- EAR
- eosinophil-associated RNase
- ECP
- eosinophilic cationic protein
- EDN
- eosinophil-derived neurotoxin
- EPO
- eosinophil peroxidase
- FSC
- forward scatter
- MBP
- major basic protein
- mEAR
- mouse eosinophil-associated RNase
- OVA
- ovalbumin
- PMA
- phorbol myristate acetate
- PBS
- phosphate-buffered saline
- PMD
- piecemeal degranulation
- RBC
- red blood cell
- RFU
- relative fluorescence unit
- RSV
- respiratory syncytial virus
- SSC
- side scatter
- TEM
- transmission electron microscopy.
REFERENCES
- 1. Dvorak A. M., Weller P. F. (2000) Ultrastructural analysis of human eosinophils. Chem. Immunol. 76, 1–28 [DOI] [PubMed] [Google Scholar]
- 2. Leiferman K. M., Ackerman S. J., Sampson H. A., Haugen H. S., Venencie P. Y., Gleich G. J. (1985) Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. Comparison with onchocerciasis. N. Engl. J. Med. 313, 282–285 [DOI] [PubMed] [Google Scholar]
- 3. Cheng J. F., Ott N. L., Peterson E. A., George T. J., Hukee M. J., Gleich G. J., Leiferman K. M. (1997) Dermal eosinophils in atopic dermatitis undergo cytolytic degeneration. J. Allergy. Clin. Immunol. 99, 683–692 [DOI] [PubMed] [Google Scholar]
- 4. Erjefalt J. S., Greiff L., Andersson M., Matsson E., Petersen H., Linden M., Ansari T., Jeffery P. K., Persson C. G. (1999) Allergen-induced eosinophil cytolysis is a primary mechanism for granule protein release in human upper airways. Am. J. Respir. Crit. Care Med. 160, 304–312 [DOI] [PubMed] [Google Scholar]
- 5. Hogan S. P., Rosenberg H. F., Moqbel R., Phipps S., Foster P. S., Lacy P., Kay A. B., Rothenberg M. E. (2008) Eosinophils: biological properties and role in health and disease. Clin. Exp. Allergy 38, 709–750 [DOI] [PubMed] [Google Scholar]
- 6. Shamri R., Xenakis J. J., Spencer L. A. (2011) Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 343, 57–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Domachowske J. B., Dyer K. D., Bonville C. A., Rosenberg H. F. (1998) Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J. Infect. Dis. 177, 1458–1464 [DOI] [PubMed] [Google Scholar]
- 8. Domachowske J. B., Dyer K. D., Adams A. G., Leto T. L., Rosenberg H. F. (1998) Eosinophil cationic protein/RNase 3 is another RNase A-family ribonuclease with direct antiviral activity. Nucleic Acids Res. 26, 3358–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garvey T. L., Dyer K. D., Ellis J. A., Bonville C. A., Foster B., Prussin C., Easton A. J., Domachowske J. B., Rosenberg H. F. (2005) Inflammatory responses to pneumovirus infection in IFN-alpha beta R gene-deleted mice. J. Immunol. 175, 4735–4744 [DOI] [PubMed] [Google Scholar]
- 10. Phipps S., Lam C. E., Mahalingam S., Newhouse M., Ramirez R., Rosenberg H. F., Foster P. S., Matthaei K. I. (2007) Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 110, 1578–1586 [DOI] [PubMed] [Google Scholar]
- 11. Kumar R. K., Foster P. S. (2002) Modeling allergic asthma in mice: pitfalls and opportunities. Am. J. Respir. Cell Mol. Biol. 27, 267–272 [DOI] [PubMed] [Google Scholar]
- 12. Stelts D., Egan R. W., Falcone A., Garlisi C. G., Gleich G. J., Kreutner W., Kung T. T., Nahrebne D. K., Chapman R. W., Minnicozzi M. (1998) Eosinophils retain their granule major basic protein in a murine model of allergic pulmonary inflammation. Am. J. Respir. Cell Mol. Biol. 18, 463–470 [DOI] [PubMed] [Google Scholar]
- 13. Hamelmann E., Takeda K., Schwarze J., Vella A. T., Irvin C. G., Gelfand E. W. (1999) Development of eosinophilic airway inflammation and airway hyperresponsiveness requires interleukin-5 but not immunoglobulin E or B lymphocytes. Am. J. Respir. Cell Mol. Biol. 21, 480–489 [DOI] [PubMed] [Google Scholar]
- 14. Mould A. W., Ramsay A. J., Matthaei K. I., Young I. G., Rothenberg M. E., Foster P. S. (2000) The effect of IL-5 and eotaxin expression in the lung on eosinophil trafficking and degranulation and the induction of bronchial hyperreactivity. J. Immunol. 164, 2142–2150 [DOI] [PubMed] [Google Scholar]
- 15. Denzler K. L., Borchers M. T., Crosby J. R., Cieslewicz G., Hines E. M., Justice J. P., Cormier S. A., Lindenberger K. A., Song W., Wu W., Hazen S. L., Gleich G. J., Lee J. J., Lee N. A. (2001) Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J. Immunol. 167, 1672–1682 [DOI] [PubMed] [Google Scholar]
- 16. Malm-Erjefalt M., Persson C. G., Erjefalt J. S. (2001) Degranulation status of airway tissue eosinophils in mouse models of allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 24, 352–359 [DOI] [PubMed] [Google Scholar]
- 17. Clark K., Simson L., Newcombe N., Koskinen A. M., Mattes J., Lee N. A., Lee J. J., Dent L. A., Matthaei K. I., Foster P. S. (2004) Eosinophil degranulation in the allergic lung of mice primarily occurs in the airway lumen. J. Leukoc. Biol. 75, 1001–1009 [DOI] [PubMed] [Google Scholar]
- 18. Ochkur S. I., Jacobsen E. A., Protheroe C. A., Biechele T. L., Pero R. S., McGarry M. P., Wang H., O'Neill K. R., Colbert D. C., Colby T. V., Shen H., Blackburn M. R., Irvin C. C., Lee J. J., Lee N. A. (2007) Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J. Immunol. 178, 7879–7889 [DOI] [PubMed] [Google Scholar]
- 19. Bandeira-Melo C., Herbst A., Weller P. F. (2001) Eotaxins. Contributing to the diversity of eosinophil recruitment and activation. Am. J. Respir. Cell Mol. Biol. 24, 653–657 [DOI] [PubMed] [Google Scholar]
- 20. El-Shazly A., Masuyama K., Nakano K., Eura M., Samejima Y., Ishikawa T. (1998) Human eotaxin induces eosinophil-derived neurotoxin release from normal human eosinophils. Int. Arch. Allergy. Immunol. 117(Suppl. 1), 55–58 [DOI] [PubMed] [Google Scholar]
- 21. Kampen G. T., Stafford S., Adachi T., Jinquan T., Quan S., Grant J. A., Skov P. S., Poulsen L. K., Alam R. (2000) Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood 95, 1911–1917 [PubMed] [Google Scholar]
- 22. Melo R. C., Spencer L. A., Perez S. A., Ghiran I., Dvorak A. M., Weller P. F. (2005) Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic 6, 1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ochkur S. I., Kim J. D., Protheroe C. A., Colbert D., Moqbel R., Lacy P., Lee J. J., Lee N. A. (2012) The development of a sensitive and specific ELISA for mouse eosinophil peroxidase: Assessment of eosinophil degranulation ex vivo and in models of human disease. J. Immunol. Methods 375, 138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adamko D. J., Wu Y., Gleich G. J., Lacy P., Moqbel R. (2004) The induction of eosinophil peroxidase release: improved methods of measurement and stimulation. J. Immunol. Methods 291, 101–108 [DOI] [PubMed] [Google Scholar]
- 25. Cormier S. A., Larson K. A., Yuan S., Mitchell T. L., Lindenberger K., Carrigan P., Lee N. A., Lee J. J. (2001) Mouse eosinophil-associated ribonucleases: a unique subfamily expressed during hematopoiesis. Mamm. Genome 12, 352–361 [DOI] [PubMed] [Google Scholar]
- 26. Rosenberg H. F., Domachowske J. B. (2001) Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J. Leukoc. Biol. 70, 691–698 [PubMed] [Google Scholar]
- 27. Lee J. J., Lee N. A. (2005) Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin. Exp. Allergy 35, 986–994 [DOI] [PubMed] [Google Scholar]
- 28. Boix E. (2001) Eosinophil cationic protein. Methods Enzymol. 341, 287–305 [DOI] [PubMed] [Google Scholar]
- 29. Dent L. A., Strath M., Mellor A. L., Sanderson C. J. (1990) Eosinophilia in transgenic mice expressing interleukin 5. J. Exp. Med. 172, 1425–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang H. B., Ghiran I., Matthaei K., Weller P. F. (2007) Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J. Immunol. 179, 7585–7592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alexander B., Browse D. J., Reading S. J., Benjamin I. S. (1999) A simple and accurate mathematical method for calculation of the EC50. J. Pharmacol. Toxicol. Methods 41, 55–58 [DOI] [PubMed] [Google Scholar]
- 32. Neves J. S., Perez S. A., Spencer L. A., Melo R. C., Weller P. F. (2009) Subcellular fractionation of human eosinophils: isolation of functional specific granules on isoosmotic density gradients. J. Immunol. Methods 344, 64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bivas-Benita M., Zwier R., Junginger H. E., Borchard G. (2005) Non-invasive pulmonary aerosol delivery in mice by the endotracheal route. Eur. J. Pharm. Biopharm. 61, 214–218 [DOI] [PubMed] [Google Scholar]
- 34. Reimert C. M., Poulsen L. K., Bindslev-Jensen C., Kharazmi A., Bendtzen K. (1993) Measurement of eosinophil cationic protein (ECP) and eosinophil protein X/eosinophil derived neurotoxin (EPX/EDN). Time and temperature dependent spontaneous release in vitro demands standardized sample processing. J. Immunol. Methods 166, 183–190 [DOI] [PubMed] [Google Scholar]
- 35. De Marinis E., Casella L., Ciaccio C., Coletta M., Visca P., Ascenzi P. (2009) Catalytic peroxidation of nitrogen monoxide and peroxynitrite by globins. IUBMB Life 61, 62–73 [DOI] [PubMed] [Google Scholar]
- 36. Xu X., Hakansson L. (2000) Regulation of the release of eosinophil cationic protein by eosinophil adhesion. Clin. Exp. Allergy 30, 794–806 [DOI] [PubMed] [Google Scholar]
- 37. Spencer L. A., Szela C. T., Perez S. A., Kirchhoffer C. L., Neves J. S., Radke A. L., Weller P. F. (2009) Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J. Leukoc. Biol. 85, 117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nusse O., Lindau M., Cromwell O., Kay A. B., Gomperts B. D. (1990) Intracellular application of guanosine-5′-O-(3-thiotriphosphate) induces exocytotic granule fusion in guinea pig eosinophils. J. Exp. Med. 171, 775–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moqbel R., Lacy P. (1999) Exocytotic events in eosinophils and mast cells. Clin. Exp. Allergy 29, 1017–1022 [DOI] [PubMed] [Google Scholar]
- 40. Melo R. C., Dvorak A. M., Weller P. F. (2010) Contributions of electron microscopy to understand secretion of immune mediators by human eosinophils. Microsc. Microanal. 16, 653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Melo R. C., Perez S. A., Spencer L. A., Dvorak A. M., Weller P. F. (2005) Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic 6, 866–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toyoda M., Maruyama T., Morohashi M., Bhawan J. (1996) Free eosinophil granules in urticaria: a correlation with the duration of wheals. Am. J. Dermatopathol. 18, 49–57 [DOI] [PubMed] [Google Scholar]
- 43. Neves J. S., Perez S. A., Spencer L. A., Melo R. C., Reynolds L., Ghiran I., Mahmudi-Azer S., Odemuyiwa S. O., Dvorak A. M., Moqbel R., Weller P. F. (2008) Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc. Natl. Acad. Sci. U. S. A. 105, 18478–18483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Neves J. S., Weller P. F. (2009) Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology. Curr. Opin. Immunol. 21, 694–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neves J. S., Radke A. L., Weller P. F. (2010) Cysteinyl leukotrienes acting via granule membrane expressed receptors elicit secretion from within cell-free human eosinophil granules. J. Allergy Clin. Immunol. 125, 477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bystrom J., Amin K., Bishop-Bailey D. (2011) Analysing the eosinophil cationic protein-a clue to the function of the eosinophil granulocyte. Respir. Res. 12, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosenberg H. F. (2008) Eosinophil-derived neurotoxin / RNase 2: connecting the past, the present and the future. Curr. Pharm. Biotechnol. 9, 135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Molina H. A., Kierszenbaum F., Hamann K. J., Gleich G. J. (1988) Toxic effects produced or mediated by human eosinophil granule components on Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 38, 327–334 [DOI] [PubMed] [Google Scholar]
- 49. Rosenberg H. F. (1995) Recombinant human eosinophil cationic protein. Ribonuclease activity is not essential for cytotoxicity. J. Biol. Chem. 270, 7876–7881 [DOI] [PubMed] [Google Scholar]
- 50. Garofalo R., Kimpen J. L., Welliver R. C., Ogra P. L. (1992) Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J. Pediatr. 120, 28–32 [DOI] [PubMed] [Google Scholar]
- 51. Yang D., Chen Q., Su S. B., Zhang P., Kurosaka K., Caspi R. R., Michalek S. M., Rosenberg H. F., Zhang N., Oppenheim J. J. (2008) Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2 MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J. Exp. Med. 205, 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Persson C. G., Erjefalt J. S. (1997) Eosinophil lysis and free granules: an in vivo paradigm for cell activation and drug development. Trends Pharmacol. Sci. 18, 117–123 [DOI] [PubMed] [Google Scholar]
- 53. Verjan Garcia N., Umemoto E., Saito Y., Yamasaki M., Hata E., Matozaki T., Murakami M., Jung Y. J., Woo S. Y., Seoh J. Y., Jang M. H., Aozasa K., Miyasaka M. (2011) SIRPalpha/CD172a regulates eosinophil homeostasis. J. Immunol. 187, 2268–2277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.