Abstract
The plasma membrane dopamine (DA) transporter (DAT) is essential for reuptake of extracellular DA. DAT function in heterologous cells is regulated by subcellular targeting, endocytosis, and intracellular trafficking, but the mechanisms regulating neuronal DAT remain poorly understood. Hence, we generated a knock-in mouse expressing a hemagglutinin (HA)-epitope-tagged DAT to study endogenous transporter trafficking. Introduction of the HA tag into the second extracellular loop of mouse DAT did not perturb its expression level, distribution pattern, or substrate uptake kinetics. Live-cell fluorescence microscopy imaging using fluorescently labeled HA-specific antibody and a quantitative HA-antibody endocytosis assay demonstrated that in axons HA-DAT was primarily located in the plasma membrane and internalized mostly in growth cones and varicosities, where synaptic vesicle markers were also concentrated. Formation of varicosities was frequently preceded or accompanied by highly dynamic filopodia-like membrane protrusions. Remarkably, HA-DAT often concentrated at the tips of these filopodia. This pool of HA-DATs exhibited low lateral membrane mobility. Thus, DAT-containing filopodia may be involved in synaptogenesis in developing DA neurons. Treatment of neurons with amphetamine increased mobility of filopodial HA-DAT and accelerated HA-DAT endocytosis in axons, suggesting that chronic amphetamine may interfere with DA synapse development. Interestingly, phorbol esters did not accelerate endocytosis of axonal DAT.—Rao, A., Richards, T. L., Simmons, D., Zahniser, N. R., Sorkin, A. Epitope-tagged dopamine transporter knock-in mice reveal rapid endocytic trafficking and filopodia targeting of the transporter in dopaminergic axons.
Keywords: endocytosis, fluorescence imaging, amphetamine, endosome
The dopamine (DA) transporter (DAT) is responsible for removal of extracellular DA, an essential mechanism for terminating DA neurotransmission (1). DAT is not only important for normal DA signaling in the brain, but also for brain disorders, such as Parkinson's disease, schizophrenia, and attention-deficit hyperactivity disorder. In addition, interaction of amphetamines and cocaine with DAT results in increased extracellular DA levels that are closely linked with their addiction potential (2, 3). Despite DAT's importance, the molecular mechanisms regulating DAT function remain largely unknown.
DAT belongs to the SLC6 gene family of Na+/Cl−-dependent neurotransmitter transporters. These transporters have 12 transmembrane spanning domains (TMs), a large extracellular loop 2 (EL2), and cytoplasmic amino and carboxyl termini. Electron microscopic analysis in adult rat striatum demonstrated that axonal DAT is localized in the plasma membrane outside of the presynaptic active zones (4). In substantia nigra and ventral tegmental area, DAT was found in the plasma membrane of distal dendrites and in various intracellular compartments in the cell soma and proximal dendrites (5, 6). DAT functions only in the plasma membrane; therefore, the amount of DA uptake is regulated by endocytic trafficking of DAT (7, 8). The mechanisms of DAT endocytosis have been extensively studied in heterologous expression systems. Constitutive and protein kinase C (PKC)-dependent endocytosis of DAT was shown to occur via a clathrin-coated pit pathway (9, 10). Internalized DAT was found to accumulate in early recycling and late endosomes (10–13). More recently, fluorescent cocaine analogues were used to demonstrate the dynamin dependence of constitutive endocytosis of endogenous DAT and visualize DAT in various endosomal compartments of living cultured rat DA neurons (14). Interestingly, constitutively internalized DAT has been proposed to accumulate in late endosomes (15). Immunofluorescence microscopy and biochemical studies suggested that in rat axonal projection areas, DAT is mostly located in the plasma membrane and, to a small extent in recycling endosomes, whereas in somatodendritic compartments, DAT is present in all endosomal populations (16).
To generate an experimental model that would allow quantitative analysis of the regulation of endogenous DAT, we developed a knock-in mouse that expresses a DAT mutant containing a hemagglutinin (HA11) epitope in EL2 (HA-DAT). We have previously found that human HA-DAT, with or without bound HA11 antibody, is fully functional (17). In the knock-in mice, HA-DAT replaced endogenous DAT without detectable changes in striatal DA uptake kinetics or DAT expression level. This mouse model was used for quantitative analysis of the membrane mobility and endocytic trafficking of HA-DAT in living cultured DA neurons. These studies for the first time demonstrated endocytosis and filopodia targeting of DAT in axons, thus highlighting the usefulness of the HA-DAT mouse model to study regulation of endogenous DAT.
MATERIALS AND METHODS
Animals
Adult male and female C57/BL6 mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). All mice were housed in groups of 5 on a 14:10-h light-dark cycle with food and water available ad libitum. All experimental procedures involving the generation and use of laboratory mice were conducted in accordance with U.S. National Institutes of Health (NIH) guidelines (NIH Publication No. 80-23, revised 1996) as approved by the Institutional Animal Care and Use Committee at the University of Colorado–Anschutz Medical Campus.
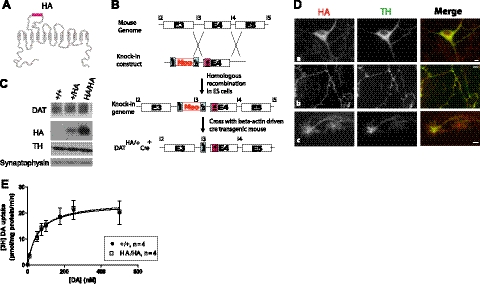
To generate the HA-DAT knock-in mice, the HA epitope-tag was cloned into exon 4 of the mouse DAT gene in the BAC clone RP23–34F24 by mutating the sequence corresponding to amino acid residues HSSNSSDGLGL to YPYDVPDYASL using a Stratagene Quick-change mutagenesis kit (Stratagene, La Jolla, CA, USA) according to the manufacture's protocol (see Fig. 1A). The knock-in construct consisted of HA-tagged exon 4 and a floxed neomycin cassette in intron 3 of the DAT gene, which was flanked by 5′ and 3′ (3 and 4.8 kb, respectively) homologous genomic fragments (see Fig. 1B). The HA-DAT knock-in mice were generated at the Transgenic and Gene Targeting Core Facility at the University of Colorado–Anschutz Medical Campus using standard procedures (18). Briefly, the targeting construct was electroporated in the EC7.1 embryonic stem cells (ESCs), and the positive clones were selected in the presence of G418 antibiotic. The ESC clones were screened by PCR. Karyotyping was performed on one positive ESC clone to confirm euploidy, and this clone was subsequently microinjected into C57/BL6 mouse blastocysts. The resulting chimeras were back-crossed with C57/BL6 mice. Heterozygous DATHA/+ mice were crossed with each other to generate the homozygous DATHA/HA line. Since these mice unexpectedly showed reduced DAT expression (see Results), they were crossed with β-actin-driven Cre transgenic mice (Jackson Laboratories; see Fig. 1B). Deletion of the neomycin cassette in the resulting DATHA/HACre+ mice was confirmed by PCR. DATHA/HACre+ mice were used in all experiments. The genotypes of the mice were confirmed by amplifying the regions flanking the HA-tag using primers HAf, 5′-GGTGATTCAACAAAGCTGTA-3′, and HAr, 5′-CGTACGGGCAAGGATAGGGA-3′. The resulting 810-bp PCR product has a unique restriction site for AatII. Therefore, the presence of an HA-epitope sequence resulted in two AatII digested products of size 609 and 201 bp. The presence of the Cre transgene was identified by PCR using the following primers: Cre1, 5′-GCTGGTTAGCACCGCAGGTGTAGAG-3′, and Cre3, 5′-CGCCATCTTCCAGCAGGCGCACC-3′ (courtesy of Dr. Trevor Williams, University of Colorado-Anschutz Medical Campus).
Figure 1.
Design and characterization of the HA-DAT knock-in mice. A) HA tag (11 aa residues, YPYDVPDYASL; red rectangle) was introduced into EL2 of mouse DAT, as described previously (17). B) Schematic representation of the steps used to generate the HA-DAT knock-in mice. Mouse knock-in construct consisted of exon 4 (E4) with the HA tag (red rectangle), and a floxed neomycin cassette (Neo) in intron 3 and intron 4 (for further details, see Materials and Methods). C) Western blot analysis of striatal HA-DAT protein from 3 genotypes (+/+, WT; +/HA, heterozygote; HA/HA, homozygote). Striatal lysates were electrophoresed and probed by blotting with antibodies to DAT, HA, TH, and synaptophysin (loading control). There was no statistically significant difference between the signal intensity of DAT immunoreactivities (normalized to the amount of TH) in HA/HA as compared to WT striatal lysates (110±20% of WT; n=3). D) Localization of HA-DAT in cultured DA neurons. Embryonic DA neurons derived from E15 HA/HA embryos were costained with HA11 (red) and TH (green) antibodies. Images depict examples of soma with dendrites (a), axonal processes (b), and growth cones (c). Individual optical sections of z stack of images are shown. Scale bars = 5 μm. E) DAT-mediated uptake in striatal synaptosomes prepared from +/+ and HA/HA mice. Kinetic parameters (maximal velocity Vmax and affinity Km) derived from these curves are presented in Table 1. Values are means ± se for specific uptake of [3H]DA (n=4/group).
Immunohistochemistry in mouse brain slices
Animals were given an overdose of chloral hydrate and transcardially perfused with ice-cold PBS for 5 min, followed by ice-cold 4% paraformaldehyde in PBS for 10 min. Following perfusion, brains were removed and placed in 4% paraformaldehyde and stored overnight at 4°C. Brains were cryoprotected through incubation in 20% sucrose in PBS with shaking at 4°C overnight or until brains sank to the bottom of the tube, followed by 30% sucrose in PBS at 4°C until ready for cryosectioning. Brains were rinsed quickly with PBS and frozen with optimal cutting temperature (OCT) Compound (Tissue-Tek; Sakura, Torrance, CA, USA) to −20°C. Coronal sections (25 μm)were obtained and stored in PBS containing 0.01% sodium azide (Sigma-Aldrich, St. Louis, MO, USA). Coronal brain sections were treated with 3% hydrogen peroxide treatment in PBS and subsequently blocked/permeabilized in PBS containing 5% sucrose, 2% BSA, 10% normal donkey serum (NDS), and 0.03% Triton X-100 (primary solution). Tissue was subsequently labeled free-floating with mouse anti-HA11 (1:500; Covance, Princeton, NJ, USA) and rabbit anti-tyrosine hydroxylase (TH; 1:1000; Pel-Freez Biologicals, Rogers, AR, USA) at 4°C with shaking overnight followed by one wash in primary solution and two washes in secondary solution (5% sucrose, 2% BSA, 2.5% NDS). Tissue was labeled with Cy3-anti-mouse (HA-DAT) and FITC-anti-rabbit (TH) (Jackson Laboratories) overnight with shaking at 4°C. Tissue sections were washed in secondary solution, followed by 2 washes in PBS, and then mounted on clean frosted slides (Fisherbrand Superfrost slides; Thermo Fisher Scientific, Pittsburgh, PA, USA) and allowed to dry overnight. Slides were dipped in ddH2O, and a few drops of Invitrogen's Prolong Gold (with DAPI; Invitrogen, Carlsbad, CA, USA) were applied to preserve fluorescent labeling and stain nuclei of coronal sections; then they were coverslipped. Imaging was performed using a fluorescent microscope, as described below.
DA uptake assay
The synaptosomes were prepared and the specific [3H]DA/DA uptake kinetics assays were performed and analyzed, as described previously (19) with the following exceptions: tissue from 2 mice was pooled prior to homogenization, the P2 pellet (synaptosomes) was resuspended at 40 mg/ml (wet weight of tissue) in assay buffer, and the concentrations of unlabeled DA used for kinetic analysis were 0, 10, 50, 75, 175, 250, and 500 nM. (−)-Cocaine hydrochloride, used at 100 μM to define specific uptake, was obtained from National Institute on Drug Abuse (Research Triangle Institute International, Research Triangle Park, NC, USA); [3H]DA from Perkin Elmer Life Sciences (Boston, MA, USA); and scintillation cocktail from Research Products International Corp. (Mount Prospect, IL, USA). All other chemicals in all our studies were purchased from either Sigma-Aldrich or Thermo Fisher Scientific unless noted otherwise. Prism 5 (GraphPad Software, La Jolla, CA, USA) was used to derive the kinetic uptake parameters (maximal velocity Vmax and apparent affinity Km) from nonlinear curve-fitting. To compare the DA uptake rates in the absence and presence of HA11-Alexa Fluor 488, uptake assays were performed using a single, subsaturating concentration of [3H]DA (1 nM) in the absence or presence of 100 μM cocaine after synaptosomes were preincubated with 10 μg/ml HA11-Alexa Fluor 488 for 1 h at 37°C.
Immunoblotting
Striatal lysates were prepared from HA/HA mice, as described previously (17). In short, striatal tissue was homogenized, and proteins were solubilized in Triton X-100/glycerol/HEPES lysis buffer and subsequently denatured in sample buffer at 75°C for 10 min. Lysates were then electrophoresed and blotted with HA11, rat monoclonal DAT (Chemicon, Temecula, CA, USA), TH, and synaptophysin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) antibodies. Quantitative analysis of band intensity was performed using densitometry and ImageJ software (NIH, Bethesda, MD, USA).
DA neuronal cultures
Primary ventral mesencephalic cultures were prepared as described previously (16). Experiments were performed on neurons at days in vitro (DIV) 10–21, unless specified otherwise.
Fluorescence microscopy imaging
Postnatal mesencephalic neurons were plated on 35-mm glass-bottom dishes (MatTek, Ashland, MA, USA) for the purpose of live-cell imaging. On the day of the experiment, neurons were incubated with the HA11 antibody conjugated to Alexa Fluor 488 (HA11-Alexa Fluor 488, final concentration 10 μg/ml; Covance). The incubation was carried out in preconditioned neurobasal medium (medium used to maintain the neurons) for 10 min to 4 h at 37°C. The relative intensity of HA11-A488 fluorescence (arbitrary units of fluorescence intensity per cubic micrometer of DA neuron) of cultured postnatal DA neurons was calculated using SlideBook software (Intelligent Imaging Innnovations, Inc., Denver, CO, USA). In control experiments, HA11-Alexa Fluor 488 localization and trafficking were analyzed after desalting this antibody preparation using Zeba spinning columns (Pierce, Rockford, IL, USA).
After the incubation with HA11-Alexa Fluor 488, neurons were washed twice with warm KRH buffer (130 mM NaCl; 1.2 mM MgSO4; 1.2 mM CaCl2; 5.4 mM KCl; 25 mM HEPES, pH 7.4;1 mM l-ascorbic acid; and 5 mM dextrose). For transferrin (TnF) and LysoTracker colocalization experiments, neurons were incubated with Texas Red-conjugated TnF (Invitrogen) for 1 h or with LysoTracker Red (1 nM; Invitrogen) for 15 min in KRH buffer. For Hoechst 33342 (1 μg/ml, Invitrogen) staining, a 15-min incubation was performed. For FM4–64 dye (Invitrogen) uptake experiments, a previously described protocol was followed (20). Subsequently, neurons were washed twice with KRH buffer, and live-cell imaging was carried out in KRH buffer at 32°C. Images were acquired by the ×63 objective through FITC, Cy3, and DAPI filter channels using a Marianas epifluorescence workstation and SlideBook 5.0 software (Intelligent Imaging Innovation), as described previously (17). The single particle tracking module of SlideBook 5.0 was used to calculate speeds of HA-DAT vesicles.
To study HA-DAT localization relative to endosomal markers and clathrin, neurons were incubated with HA11-Alexa Fluor 488 in preconditioned medium for 4 h. Subsequently, neurons were fixed with 4% paraformaldehyde for 10 min, and permeabilized with 0.1% Triton X-100/0.5% BSA in PBS for 5 min. After blocking with 0.5% BSA, neurons were stained with rabbit primary antibodies for clathrin (Abcam, Cambridge, MA, USA), EEA.1 (Cell Signaling Technology, Danvers, MA, USA), Rab5 (Abcam) or Syntaxin13 (courtesy of Dr. Rytis Prekeris, University of Colorado–Anschutz Medical Campus). Subsequently, neurons were stained with anti-rabbit Cy3-conjugated secondary antibodies, washed, and mounted using ProLong antifade (Invitrogen). Images were acquired through FITC and Cy3 filter channels as described above.
Fluorescence recovery after photobleaching (FRAP)
Postnatal DA cultures and PAE cells expressing YFP-HA-DAT were grown on Mat-Tek dishes. The cells were incubated with 10 μg/ml HA11-Alexa Fluor 488 (DA neuronal cultures) or unlabeled HA11 (PAE) for 4 h and rinsed twice with KRH buffer. FRAP measurements were carried out in KRH buffer at room temperature. For d-amphetamine (AMPH; Sigma-Aldrich) treatment, neurons were incubated in KRH buffer containing 60 μM AMPH for 30 min at 37°C. Neurons were equilibrated to room temperature prior to imaging. FRAP experiments were performed using a spinning disk confocal microscope system equipped with Zeiss Axio Observer Z1, Yokogawa CSU-X1, Vector photomanipulation module, and Photometrics Evolve 16-bit EMCCD camera (Intelligent Imaging Innovation). The microscope is equipped with an environmental chamber ensuring a constant temperature and humidity 5% CO2 atmosphere throughout the duration of the neuronal imaging. Time-lapse imaging was conducted via a ×63 objective lens using a 488- or 515-nm laser line before and after photobleaching at 488 or 515 nm. Each experiment started with a minimum of 5 prebleach images, followed by photobleaching using 10-μm rectangles of different regions of the DA neuronal axons. Approximately 25% laser intensity was used to achieve 30–55% photobleaching. Experiments with >60% photobleaching were discarded. Images were obtained using a 150-ms exposure with 5-s intervals for a total of 150 s. Recovery curves were plotted, and mobility fractions (Mf) and diffusion half-life times (Td) were calculated by nonlinear regression using Prism 5 (21) or using SlideBook 5 FRAP module.
Endocytosis assay
The antibody feeding endocytosis assay was performed as described previously (17) with minor modifications. Briefly, postnatal DA neurons were grown on glass coverslips in 24-well plates. Coverslips were incubated with HA11 (1 μg/ml) in the preconditioned neurobasal medium for 3–4 h. After rinsing in KRH buffer, the cells were incubated with 1 μM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) or 60 μM AMPH in KRH for 1 h at 37°C. Subsequently, neurons were washed with ice-cold Ca2+, Mg2+-free PBS, and fixed with freshly prepared 4% paraformaldehyde for 10 min at room temperature. Neurons were then stained with anti-mouse secondary antibody conjugated to Cy5 (1:25; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h at room temperature to occupy surface HA-DATs. Neurons were then washed and postfixed with 4% paraformaldehyde for 5 min, followed by permeabilization with 0.1% Triton X-100/0.5% BSA in PBS for 5 min and blocking in 0.5% BSA in PBS for 30 min. To stain internalized HA-DATs, neurons were then incubated with the same anti-mouse secondary antibody conjugated to Cy3 (1:500), and rabbit polyclonal tyrosine hydroxylase (TH) antibody for 45 min at room temperature. After washing, neurons were incubated with anti-rabbit secondary conjugated with FITC (1:100) for 30 min. Coverslips were mounted in ProLong, and images were acquired through FITC, Cy3, and Cy5 filter channels using a Marianas epifluorescence workstation and SlideBook 5.0 software, as described previously (17). Z stacks were acquired of the axonal processes at an interval of 300 nm and deconvolved using the constrained iterative method (with Gaussian noise smoothing). The SlideBook mask feature was used to select voxels containing TH immunofluorescence in the background-subtracted images. The intensities of the Cy5 and Cy3 were calculated within the mask regions to obtain the ratios of internalized DAT/surface DAT (Cy3/Cy5). Statistical significance (P) between basal and drug-treated samples was calculated using unpaired Student's t tests (Prism 5); 15–20 images were obtained in each experimental variant.
RESULTS
HA-DAT knock-in mice display no apparent differences from wild-type (WT) mice
To develop an experimental model for studying trafficking of natively expressed DAT in DA neurons, we used ESC gene targeting to generate knock-in mice that have an HA11 epitope incorporated into the EL2 of DAT (HA-DAT; ref. 17 and Fig. 1A, B). This DAT allele in the endogenous mouse Slc6A locus consisted of an HA11 sequence cloned into exon 4 of mouse DAT, and a floxed neomycin cassette in the intron 3 of the DAT gene. Unexpectedly, mice homozygous for this DAT allele displayed ∼70% reduced protein levels of full-length striatal DAT, as compared to the WT mice. Instead, an increased amount of truncated DAT protein with an internal deletion (including the HA sequence) was present in mesencephalon (data not shown). We hypothesized that the presence of the neomycin cassette in intron 3 may have resulted in the expression of an aberrant form of DAT and low levels of full-length HA-DAT protein. To test this theory, we crossed HA-DAT knock-in mice with the β-actin-driven Cre transgenic mice. Neomycin deletion in the resulting DATHA/HACre+ mice (hereafter referred to as HA/HA mice) showed no apparent differences as compared with the WT mice with respect to fertility and body weight (data not shown). DAT protein levels in the striatum of HA/HA (homozygous for HA-DAT allele) and HA/+ (heterozygous for HA-DAT allele) mice were comparable to those levels in the striatum of WT (+/+) mice (Fig. 1C). As shown in Fig. 1C, these mice also displayed normal levels of TH, the rate-limiting enzyme in DA synthesis. Thus, HA/HA mice were further characterized.
In the primary postnatal mesencephalic cultures, HA-DAT localized to the cell body/dendrites (Fig. 1Da), axonal processes (Fig. 1Db), and growth cones (Fig. 1Dc) of the TH+ neurons. This demonstrates that HA-DAT mimics the expression pattern of the native DAT protein. It should be noted that in a small population of DA neurons, TH or DAT was exclusively expressed (data not shown).
In adult striatal synaptosomes prepared from HA/HA mice, HA-DAT was fully competent for DA uptake into striatal synaptosome preparations (Fig. 1E and Table 1). The values of kinetic uptake parameters Vmax and Km were similar in WT and HA/HA mice. Also, in adult mice, immunohistochemical staining of HA/HA mouse brain sections showed that HA-DAT is highly colocalized with TH in the striatum (Fig. 2A, B) and mesencephalon (Fig. 2C, D). Therefore, because expression, localization, and function of HA-DAT are not perturbed in the HA/HA mice, these mice can be used as an experimental model to study trafficking of endogenous mouse DAT.
Table 1.
Kinetic parameters derived from curves in Fig. 1E
| Parameter | Genotype |
|
|---|---|---|
| +/+ | HA/HA | |
| Vmax (pmol/mg protein/min) | 24 ± 1.1 | 25 ± 1.2 |
| Km (nM) | 55 ± 8.7 | 59 ± 11 |
Values are means ± se (n=4/group).
Figure 2.
Localization of HA-DAT in adult brain slices. HA/HA mouse brain sections were stained with HA11 (red) and TH (green) antibodies, and DAPI (blue). Images depict staining of the striatum (A, B) and the mesencephalon (C, D); panels B and D show magnified images of the boxed regions in A and C, respectively. Scale bars = 500 μm (A, C); 20 μm (B, D).
Trafficking of internalized HA-DAT in living cultured DA neurons
To analyze distribution of HA-DAT and monitor its movement, primary cultures of postnatal DA neurons were incubated at 37°C with HA11 antibody conjugated to Alexa Fluor 488 (HA11-Alexa Fluor 488; 10 μg/ml final concentration). The best signal-to-noise ratio was achieved after a 4-h incubation of neuronal cultures with HA11-Alexa Fluor 488 (Supplemental Fig. S1A). Therefore, we used this condition in all subsequent experiments. This approach allows specific labeling of HA-DAT present at the surface of neurons during the 4-h incubation time, whereas newly synthesized HA-DATs present in the endoplasmic reticulum and Golgi are not labeled. Incubation of cultured WT postnatal neurons with HA11-Alexa Fluor 488 for 4 h did not result in staining of any distinguishable cellular structures, TH+ cell bodies (Fig. 3A) or axonal processes (Fig. 3B), thus demonstrating the specificity of the HA-tag-mediated uptake of the antibody. Finally, preincubation of HA-DAT synaptosomes with 10 μg/ml HA11-Alexa Fluor 488 did not affect the DA uptake, suggesting that binding of this antibody does not alter the substrate uptake function of the transporter (Supplemental Fig. S1B).
Figure 3.
Localization of HA-DAT in somatic regions of DA neurons. A, B) Specificity of HA11-Alexa Fluor 488 binding to neurons. Live postnatal DA neurons derived from WT pups were incubated with HA11-Alexa Fluor 488 for 4 h at 37°C (green), fixed, and stained with TH antibody (red) to identify DA neurons. Images depict examples of soma (A) and axons (B). Individual optical section of z stack of images is shown. C) In the soma, a large pool of HA-DAT is in intracellular compartments. Living postnatal DA neurons from HA/HA (HA-DAT) pups were incubated with HA11-Alexa Fluor 488 for 4 h at 37°C to label HA-DAT (green); then they were fixed and stained with the TH antibody (red) under conditions identical to those in A and B. D) Living postnatal HA-DAT DA neurons were incubated with HA11-Alexa Fluor 488 (green; HA-DAT), washed, and stained with Hoechst 33342 (blue; labels nucleus). Time-lapse imaging was performed through FITC and DAPI filter channels at 33°C. Somatodendritic area of the neuron is shown. E, F) Magnified images of the boxed regions in D, corresponding to several time points of the time-lapse sequence. Image acquisition times (s) are indicated. Arrows indicate examples of mobile vesicular-tubular endosomes. G) Distal dendrites. Several time points of the time-lapse sequence are shown. Intracellular HA-DAT vesicles displayed rapid bidirectional movement. Red arrows indicate vesicles moving in the retrograde direction; green arrows indicate vesicles moving anterogradely. Time (s) is indicated. Scale bars = 5 μm (A–F); 10 μm (G).
In the somatodendritic compartment of DA (TH+) neurons, a large pool of HA11-Alexa Fluor 488:HA-DAT complexes were associated with the intracellular vesicles and appeared as heterogeneous puncta (Fig. 3C). Because HA-DAT can associate with HA11-Alexa Fluor 488 only at the cell surface, the labeled intracellular vesicles must have originated from the plasma membrane and are likely to be endosomes or lysosomes. Time-lapse imaging of living cells revealed that in the soma and proximal dendrites, large HA-DAT-containing compartments were typically stationary, whereas smaller compartments were often highly mobile (Fig. 3D–F). Apart from circular structures, some small compartments had elongated shapes suggestive of a tubule morphology characteristic of tubular membrane subcompartments of early endosomes and recycling carriers (Fig. 3E, F). Interestingly, the overall intensity of HA11-Alexa Fluor 488:HA-DAT fluorescence was considerably lower in cell bodies of DA neurons than in axons and distal dendrites, suggesting that the plasma membrane expression levels of HA-DAT are relatively lower in the soma and proximal dendrites, as compared to distal dendrites and axons. Practically, such dim fluorescence of the neuronal soma made detection and imaging of cell bodies of DA neurons difficult.
Time-lapse imaging of distal dendrites demonstrated rapid bidirectional, anterograde and retrograde, movement of endosomes containing HA11-Alexa Fluor 488 (Fig. 3G). In addition, flickering movement of vesicles was frequently observed. Similarly, we found several examples of bidirectional movement in axonal processes, although because of the complex morphology of the axonal network of cultured DA neurons, it was often difficult to determine the directionality (retrograde vs. anterograde) of the movement (Fig. 4A). However, in the proximity of some growth cones, in which the directionality was clear, movement of the vesicles was predominantly, or exclusively, retrograde (data not shown). Endosomes exhibited a wide range of speeds. While many endosomes were stationary or moved with slow speeds of 0.0005 to 0.1 μm/s, a very small population of endosomes (∼<5%) moved extremely fast, and reached speeds up to 10 μm/s. These data suggest that DAT can be relatively rapidly transported between axonal synaptic areas and the soma by means of vesicular transport.
Figure 4.
Localization of DAT in axons of DA neurons. A) Region of an axonal process. Several time points of the time-lapse sequence are shown. Intracellular HA-DAT vesicles displayed rapid bidirectional movement. Red arrows indicate vesicles moving in the retrograde direction; green arrows indicate vesicles moving anterograde. Time (s) is indicated. B–D) Live postnatal DA neurons were incubated with HA11-Alexa Fluor 488, washed, and imaged. Varicosities enriched with intracellular HA-DAT-containing vesicles (B), lacking intracellular HA-DAT (C), and decorated with highly motile filopodia (D) are shown. E) Cells incubated with HA11-Alexa Fluor 488 (green) were fixed and stained with synaptophysin antibody (red). Individual optical section of z stack of 2-dimensional images is shown. F) Cells incubated with HA11-Alexa Fluor 488 were washed and further incubated with FM 4–64 as described in Materials and Methods. Images were acquired through FITC (Alexa Fluor 488) and Cy3 (FM 4–64) filter channels. Insets: high-magnification images of boxed regions (E, F). Scale bars = 10 μm (A); 5 μm (B–F).
Similar to adult neurons (5), cultured DA neurons had long axonal processes with multiple varicosities when visualized by HA-DAT imaging (Fig. 4B–D). Most of the varicosities were small in size (up to 1–3-μm width), although larger axonal widenings were also observed that reached 10–20 μm in cross section (Fig. 4D). Even though the overall HA-DAT concentrations (most likely attributable to the surface pool of the transporter) were higher in axons than the cell bodies, the frequency of detection of vesicular HA11-Alexa Fluor 488:HA-DAT complexes in axons was relatively low (∼<30% of axons had intracellular vesicles, as seen by live-cell imaging). HA-DAT-containing vesicles in axons also appeared to be smaller in size, as compared to those in the soma. Internalized HA-DAT was enriched in the varicosities and widenings (Fig. 4B, D). Live imaging showed that typically, endosomes moved between varicosities with high speed but remained within varicosities for a much longer time than in the axonal shafts between varicosities (data not shown). Some varicosities, however, did not contain endosomal HA-DAT (Fig. 4C).
Internalized DAT is located mainly in early and recycling endosomes in axonal shafts and varicosities
To investigate the nature of DAT-containing varicosities in cultured DA neurons, the pattern of HA-DAT localization was compared to that of synaptophysin, a transmembrane protein present in synaptic vesicles, which is typically used as marker for neuronal presynaptic release sites. To this end, live DA neurons were incubated with HA11-Alexa Fluor 488, fixed, and stained with the synaptophysin antibody. Synaptophysin was found to be accumulated in HA-DAT-containing axonal varicosities, suggesting that these varicosities contain synapses (Fig. 4E). HA-DAT was also found to colocalize with the FM4–64 dye in multiple varicosities of DA neurons (Fig. 4F). FM4–64 binds to membranes, and its endocytosis results in a dramatic increase in fluorescence. Therefore, this dye was used to mark areas of intensive endocytosis, such as the presynaptic side of active synapses. Collectively, these data indicated that in postnatal DA neurons, DAT is present in or near the active zone of synapses in axonal varicosities. Within varicosities, HA-DAT was not colocalized with synaptophysin (Fig. 4E) but was partially colocalized with FM4–64 (Fig. 4F). These data suggest that HA-DAT can undergo endocytosis in presynaptic areas and accumulate in presynaptic endosomes but is not localized in synaptic vesicles.
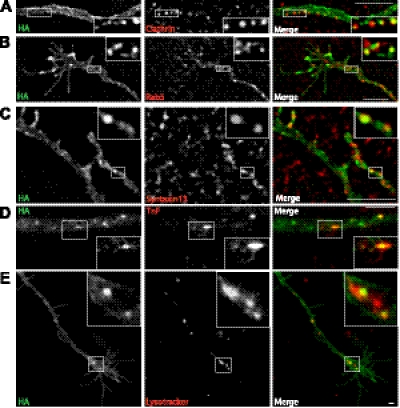
To characterize endocytic vesicles containing HA-DAT in axons of postnatal DA neurons, primary cultures were incubated with HA11-Alexa Fluor 488 at 37°C for 4 h, fixed, and immunostained for markers of the endocytic pathway. Microscopic analysis showed that HA-DAT was partially colocalized with clathrin puncta along axonal processes, suggesting that a pool of DAT is present in clathrin-coated pits (Fig. 5A). HA-DAT was partially colocalized with Rab5, an early endosome resident GTPase (Fig. 5B), and Syntaxin13, the v-SNARE present mainly in recycling endosomes (Fig. 5C). Interestingly, very little, or no, colocalization of DAT was observed with EEA.1, a Rab5-binding protein present in early/sorting endosomes (data not shown). To further confirm DAT localization in early and recycling endosomes, cultures preincubated with HA11-Alexa Fluor 488 were incubated with fluorescent transferrin (TnF-TxR) at 37°C. Time-lapse imaging of living neurons demonstrated occasional colocalization of HA-DAT in vesicular-tubular endosomes containing TnF-TxR in the axonal processes of DA neurons (Fig. 5D). To label late endosomes and lysosomes, LysoTrackerRed, known to accumulate in vesicles with low intralumenal pH, was used. LysoTracker-labeled compartments were typically enriched in varicosities (data not shown). Live-cell microscopy showed that a small amount of axonal HA-DAT was colocalized with LysoTracker (Fig. 5E). Altogether, these data indicate that in axonal processes and varicosities of DA neurons internalized HA-DAT is distributed among early endosomes, recycling compartments, and possibly, late endosomal compartments.
Figure 5.
Localization of HA-DAT in endocytic compartments. A–C) Postnatal DA neurons were incubated with HA11-Alexa Fluor 488, fixed, and stained with clathrin (A), Rab5 (B), or Syntaxin13 antibodies (C). Z stack of 2-dimensional images was acquired using FITC (Alexa Fluor 488) and Cy3 (antibodies) filter channels. Individual optical sections are shown. D, E) Cultures incubated with HA11-Alexa Fluor 488 were washed and further incubated with TnF-Texas Red (D) or LysoTracker (E). Images were acquired from living cells through FITC (Alexa Fluor 488, green) and Cy3 (TexasRed and LysoTracker, red) filter channels. Insets: high-magnification images of boxed regions. Scale bars = 5 μm.
Constitutive and induced endocytosis in DA neurons
Fluorescence microscopy demonstrated the presence of various endosomes containing internalized HA-DAT (Figs. 3–5); this indicates that HA-DAT is constitutively internalized under steady-state cell culture conditions. To directly measure the relative size of the internalized pool of HA-DAT and estimate the extent of constitutive endocytosis of DAT in DA neurons (Fig. 6), we carried out the antibody-feeding endocytosis assay previously described (17). DA neurons were incubated with HA11 antibody for 4 h to label the entire pool of transporters that resided at the cell surface or had been recycled through the plasma membrane during the incubation period. The cells were then fixed and stained with an excess of the Cy5 secondary antibody to label surface HA11:HA-DAT complexes. Cells were permeabilized and stained with the same secondary antibody conjugated with Cy3 to label internalized HA11:HA-DAT complexes. In addition, TH staining was used to identify DA neurons in these experiments. Examples of Cy3-labeled compartments (HA-DAT containing endosomes) that did not overlap with the Cy5 fluorescence (plasma membrane) are shown in Fig. 6A. However, in many cases the Cy3 and Cy5 fluorescence dots were observed to overlap considerably in thin axonal processes and small varicosities due to insufficient resolution of the fluorescence microcopy. Cy3-labeled endosomes were mostly concentrated in the varicosities. The ratio of the fluorescence intensities of internalized DAT/surface DAT (Cy3/Cy5, ∼0.35) in TH-positive axonal regions was calculated as a relative measure of DAT endocytosis (Fig. 6B).
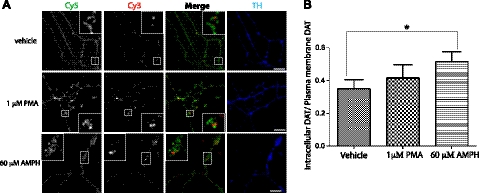
Figure 6.
Quantitative analysis of HA-DAT endocytosis in axons. A) HA11 antibody-feeding endocytosis assays were carried out as described in Materials and Methods. Postnatal cultures were incubated with HA11 antibody at 37°C for 4 h, washed, and then incubated with vehicle (DMSO), 1 μM PMA, or 60 μM AMPH at 37°C for 1 h. Cells were then fixed and stained with Cy5-conjugated secondary antibody to label the plasma membrane HA-DAT (green), followed by permeabilization and staining with Cy3-conjugated secondary to label internalized HA-DAT (red). Neurons were also stained with the TH antibody (blue). Scale bars = 10 μm. B) Quantification of the Cy3/Cy5 ratio from experiments exemplified in A was performed as described in Materials and Methods. Bars represent means ± se (n=14). Similar results were obtained in ≥4 independent experiments. *P < 0.05.
Endocytosis of DAT has been proposed to be accelerated when cells are treated with phorbol esters (PMA) or AMPH (22). Therefore, we used the HA11 uptake assay to investigate the effects of PMA and AMPH exposure on HA-DAT endocytosis in axonal processes of DA neurons. A treatment of primary cultures with either 1 μM PMA or 60 μM AMPH for up to 1 h did not result in any dramatic change in the subcellular distribution of HA11:HA-DAT complexes (Fig. 6A). However, quantification of the Cy3/Cy5 ratio revealed that AMPH treatment resulted in a significant increase in the Cy3/Cy5 ratio by ∼50% (Fig. 6B), indicative of accelerated endocytosis of axonal HA-DAT in neurons as a result of this treatment. The same effect was observed less consistently when DA neurons were treated with 20 μM AMPH (apparent Cy3/Cy5 ratio for controls=1.1±0.07; and 20 μM AMPH-treated DA neurons=1.6±0.2; n=18, P=0.045). Therefore, nonspecific effects of relatively high AMPH concentrations cannot be ruled out. On the other hand, PMA did not significantly alter DAT endocytosis in axons (Fig. 6B). We also performed the endocytosis assays using HA11-Alexa Fluor 488 instead of unlabeled HA11, and the results of PMA and AMPH treatments were identical to those obtained with unlabeled HA11 (data not shown). Furthermore, on visual inspection, PMA did not cause any detectable increase in HA-DAT endocytosis in cell bodies of postnatal DA neurons. Since, however, labeled soma were difficult to locate, we were unable to perform reliable measurements of endocytosis in the somatic regions of DA neurons. Although in some of our initial experiments with embryonic mesencephalic DA neurons cultured from HA/HA mice, we observed increased PMA-induced endocytosis increases, this increase was inconsistent and not statistically significant.
Localization of an immobile pool of HA-DAT at the tip of axonal filopodia and its possible role in dopaminergic synaptogenesis
Visual examination of many DA neurons from HA/HA mice preincubated with HA11-Alexa Fluor 488 revealed localization of HA-DAT in membrane protrusions with filopodia-like morphology (Figs. 7A and 4D). Numerous HA-DAT-containing filopodia were present in growth cones, axonal varicosities, and shafts, and these DAT-labeled filopodia exhibited rapid movement in various directions. Interestingly, HA11-Alexa Fluor 488:HA-DAT complexes were often concentrated at the very tip of the filopodia (Fig. 7A). To exclude the possibility that HA11-Alexa Fluor 488 may affect HA-DAT trafficking and lead to aberrant localization in filopodia tips and other structures, several control experiments were performed. First, low-molecular-weight components, including sodium azide, were removed from the HA11-Alexa Fluor 488 preparation. The cultures were then incubated with desalted HA11-Alexa Fluor 488 for 4 h at 37°C. Live-cell imaging revealed essentially the same pattern of HA-DAT localization in filopodia, intracellular vesicles, and plasma membrane in cells treated with the original and desalted preparation of HA11-Alexa Fluor 11 (Supplemental Fig. S1C). Second, we used HEK 293 cells stably expressing CFP-HA-DAT and demonstrated that HA11-Alexa Fluor 488 does not interfere with PMA-induced endocytosis of CFP-HA-DAT (Supplemental Fig. S1D). Third, one of the caveats of using bivalent IgG1 antibodies like HA11 is the possibility of antibody-induced dimerization and clustering of HA-DAT. Therefore, monovalent Fab fragments were prepared from HA11-Alexa Fluor 488 and incubated with neuronal cultures for 4 h at 37°C. Unfortunately, the resulting Fab fragments were not fluorescent, presumably because Alexa Fluor 488 was conjugated to the Fc portion of the antibody. Therefore, the cells were fixed and further stained with the Fab-specific secondary antibody conjugated to Cy3. Subsequently, staining with IgG1 HA11-Alexa Fluor 488 was performed. As shown in Supplemental Fig. S1E, HA-DAT staining with Fab and IgG well overlapped in filopodial tips, varicosities, and axonal processes. Altogether, these control experiments demonstrated that the commercial preparation of HA11-Alexa Fluor 488 can be used to study filopodial HA-DAT and HA-DAT endocytosis.
Figure 7.
Filopodia motility and formation of varicosities in dopaminergic axons. Postnatal cultures were incubated with HA11-Alexa Fluor 488 for 4 h at 37°C, and live-cell imaging was conducted. A) Filopodia-like protrusions along the axonal processes with HA-DAT concentrated at the tips (arrows); at right, a varicosity with filopodia containing filopodia-like tips enriched in HA-DAT. B, C) Time-lapse imaging of HA11-Alexa Fluor 488-labeled neurons demonstrates formation of an axonal varicosity and the filopodia that form and disappear during varicosity formation. B) Selected time frames from the time-lapse sequence (Supplemental Movie S1). C) Magnified images of boxed regions in B. Time points (s) are indicated. D–F) Examples of time-lapse imaging demonstrating formation of varicosities accompanied by filopodia motility. F) Arrow indicates position of HA-DAT cluster at the tip of filopodia. Time points (s) are indicated. G) Example of time-lapse imaging demonstrates retrograde movement of the HA-DAT-cluster from the tip of filopodia (arrow). Scale bars = 2 μm (A–F); 5 μm (G).
To monitor the dynamics of HA-DAT-containing filopodia, time-lapse imaging of DA neurons preincubated with HA11-Alexa Fluor 488 was performed (Fig. 7B). Several filopodia can be seen on the indicated growth cone. In the example presented in Fig. 7B, C and Supplemental Movie S1, the filopodia on the axonal shaft displayed highly dynamic motility (time points 0–600 s), subsequently decreasing in length (time points 700–1100 s), while the axonal shaft near the filopodia became wider and eventually began to resemble an axonal varicosity. In another example (Fig. 7D and Supplemental Movie S2), a mobile filopodia fused with an adjacent axon shaft, forming a varicosity-like structure. In another instance (Fig. 7E and Supplemental Movie S3), axonal shafts first became wider (time point 185 s), and later the filopodia-like extensions (230–355 s) retracted and became shorter over a period of time. Although in these examples (Fig. 7D, E), the accumulation of DAT at the filopodial tips was not pronounced, examples of an apparent interaction and fusion of the HA-DAT containing tip of filopodia with the membrane of the axonal shaft, leading to formation of a varicosity, were also observed (Fig. 7F). In fact, in several time-lapse sequences, the HA-DAT cluster initially located at the filopodia tip was seen moving along the filopodia (Fig. 7G). Thus, numerous observations during time-lapse imaging of HA11-Alexa Fluor 488 (examples shown in Fig. 7) suggested that HA-DAT-containing filopodia may be involved in the formation, as well as subsequent pruning or stabilization, of axonal varicosities, which on the basis of our studies (Fig. 4E, F), contain synapses in developing mesencephalic DA neurons.
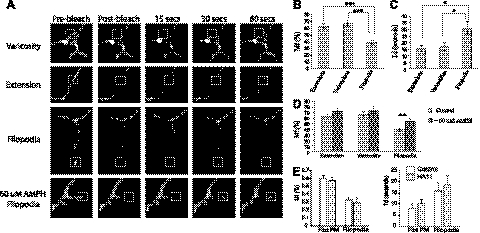
To further characterize HA-DAT in filopodia, we compared the lateral mobility of HA-DAT in the plane of the plasma membrane at the tips of filopodia with that in axonal shafts and varicosities using FRAP. To this end, neurons were incubated with HA11-Alexa Fluor 488 for 4 h, and small regions of axonal shafts between varicosities, edges of varicosities, or tips of filopodia in DA neurons were rapidly photobleached by high-intensity excitation at 488 nm (Fig. 8A). Subsequent time-lapse imaging allowed quantification of the mobile fraction Mf of HA-DAT and the diffusion rates (half-time of recovery, Td). It has been proposed that the plasma membrane DATs associated with presynaptic sites (varicosities) and axonal shafts have slightly distinct membrane mobility rates (14). Our FRAP experiments revealed that a large fraction of HA-DAT located at the tip of filopodia (Mf∼40%) was immobile, whereas HA-DAT associated with both shafts and varicosities was highly mobile, with Mf = 65–70% (Fig. 8B). The rate of fluorescence recovery of filopodial HA-DAT (Td∼30 s) was lower than those rates in other regions of axon (Td=15–17 s; Fig. 8B, C). The lower mobility of DAT in the tips of filopodia is consistent with our previous observations of a large immobile fraction of filopodia-localized human DAT expressed in PAE cells (23). The latter study also suggested that AMPH reduced the amount of DAT located in filopodia. Treatment of cultured DA neurons from the HA/HA mice with 60 μM AMPH for 30 min did not lead to visible retrograde movement of HA-DAT from filopodia and did not change Td values (data not shown) but significantly increased Mf of HA-DAT in filopodia from ∼38 to ∼55% (Fig. 8D).
Figure 8.
Lateral mobility of plasma membrane HA-DAT mobility measured by FRAP. Postnatal DA neurons were incubated with HA11-Alexa Fluor 488 as in Fig. 3, and then treated with vehicle only (control) or with 60 μM AMPH for 30 min. FRAP measurements were performed on the axonal processes. A) Examples of imaging during FRAP experiments. Boxed regions were photobleached at 488 nm. Images were acquired before, immediately after, and at 15, 30 and 60 s after photobleaching by time-lapse imaging. B, C) Fluorescence recovery curves for different axonal regions were fitted using nonlinear regression to obtain Mf (B) and Td values (C) for varicosities, shafts, and filopodia, as described in Materials and Methods. D) FRAP measurements were carried in DA neurons treated with vehicle only or with 60 μM AMPH. Bars in B–D represent mean ± se values (n=15–20). Essentially similar results were obtained in ≥4 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. E) FRAP measurements were performed in PAE cells stably expressing YFP-HA-DAT with and without (control) prebound HA11 antibody. Bars represent Mf (left) and Td values (right) obtained from flat plasma membrane (PM) regions and filopodia. Values are means ± se (n=10).
To test whether binding of HA11 antibodies influences the mobility of HA-DAT, we performed FRAP experiments in PAE cells expressing YFP-tagged HA-DAT with and without prebound HA11. As shown in Fig. 8E, HA11 did not have any effect on the Mf and Td values in flat plasma membrane regions and filopodia (Fig. 8E), suggesting that the mobility of YFP-HA-DAT is not affected by HA11.
DISCUSSION
We have generated an HA-epitope-tagged DAT knock-in mouse to facilitate trafficking and biochemical studies of DAT regulation in DA neurons (Fig. 1). In these mice, the endogenous DAT locus was genetically targeted to insert the HA11 epitope; therefore, regulation of HA-DAT is under the influence of all endogenous gene regulatory elements. The decision to generate this mouse model was prompted by an early observation of the full functionality of the human DAT mutant bearing an HA11 tag in EL2 (17). Similarly, in our HA/HA mouse model, we found normal DA uptake in adult striatal synaptosomes (Fig. 1E, Table 1, and Supplemental S1A). In addition, biochemical analysis revealed endogenous levels of mature, full-length HA-DAT protein in striatum (Fig. 1C), suggesting that HA-DAT efficiently exits the endoplasmic reticulum, is processed in the Golgi, and is delivered to the striatal axons. Therefore, the HA-DAT mouse is a novel physiological model system allowing direct visualization of the dynamics of native DAT in live DA neurons.
Our general experimental approach was to expose DA neurons to HA11-Alexa Fluor 488 (or unlabeled HA11) antibody for extended periods of time (3–4 h), so that the majority of surface HA-DATs and endosomal HA-DATs, which have been internalized from the cell surface, form complexes with HA11. Because these complexes are not pH-sensitive, they are unlikely to dissociate in endosomes or lysosomes. As a result, localization of HA11 immunoreactivity reflects a steady-state distribution of mature, full-length HA-DAT protein in DA neurons. Intracellular vesicular and tubular compartments containing internalized HA-DAT were detected throughout the neurons, suggesting that HA-DAT endocytosis occurs in both somatodendritic and axonal regions. However, unlike axonal DAT, the soma-associated transporter was mostly found in endosomes (Fig. 3). Interestingly, DA neuronal soma were often difficult to locate because of their low levels of HA11 signal (Fig. 3D), as compared to the axons (Fig. 4). In contrast, previous studies efficiently visualized endogenous DAT in the soma with fluorescent cocaine analogs (14). This difference could reflect the shorter incubation times used for cocaine analog labeling, as opposed to the longer HA11 antibody incubation period required to achieve equilibrium labeling of HA-DAT in our experiments. Under steady-state conditions, the relatively lower HA11 fluorescence intensities and/or lower surface HA-DAT levels in the soma could be indicative of a faster rate of somal, as compared to axonal, HA-DAT internalization. In addition, it is possible that, as proposed previously, a pool of internalized somal DAT is rapidly delivered to late endosomes/lysosomes and, therefore, escapes recycling (15).
The major physiological function of DAT is reuptake of extracellular DA along the axons; therefore, we focused in the present study on analyzing DAT trafficking in axons. Live-cell imaging revealed that the majority of axonal DAT molecules were uniformly distributed at the plasma membrane of axonal varicosities and shafts (Fig. 4B–D). Intriguingly, intracellular DATs were often enriched in varicosities (Fig. 4B, D), which contained synapses, as evidenced by accumulation of markers for synaptic vesicles. On the basis of these observations, we hypothesize that DAT is more readily regulated via endocytosis near its site of action, i.e., DA synapses, rather than along the axonal shafts that constitute the extrasynaptic regions. In axonal shafts, very rapid saltatory-like bidirectional movement of HA-DAT-containing vesicles, characteristic of microtubular dependent vesicular motility, was observed (Fig. 4A). Partial colocalization of HA-DAT with clathrin dots (Fig. 5A) suggests that HA-DAT, at least in part, is internalized via clathrin-coated pits. Clathrin-dependent endocytosis of DAT has been demonstrated in non-neuronal cells and DA neurons (10, 14), although internalization through a clathrin-independent, flotillin-dependent pathway has also recently been shown (24). On investigation of the fate of the small pool of internalized axonal DAT, we found that the transporter was mainly distributed between early and recycling endosomes (Fig. 5). Surprisingly, early endosome fusion events that are frequently observed in non-neuronal cells were not observed in axons or distal dendrites of DA neurons, suggesting that most vesicles containing HA-DAT have a carrier function, such as transport of DAT between somatodendritic and axonal or intra-axonal regions. Colocalization of HA-DAT with transferrin in tubular compartments (Fig. 5D) is consistent with their proposed function as recycling cargo carriers. A small pool of HA-DAT was found colocalized with LysoTracker that accumulates in compartments with intralumenal pH <6, such as intermediate, late endosomes, and lysosomes (Fig. 5E). However, we have been unable to obtain reliable immunostaining for endogenous markers of late endosomes in DA neurons, and the extent of DAT sorting to the late endosome/lysosome pathway in our mouse model remains uncertain.
The HA-DAT mouse model allowed us for the first time to perform quantitative HA11 antibody-feeding assays to analyze endogenous DAT endocytosis. These assays demonstrated constitutive internalization of DAT in DA neuronal axons and also showed that PMA treatment of mouse postnatal neurons did not accelerate HA-DAT endocytosis in any neuronal region (Fig. 5). The lack of the visible effect of PKC activation on DAT distribution in somatodendritic regions of DA neurons has been reported previously by us and others (14, 16, 17). Nonetheless, a recent study demonstrated increased DAT endocytosis in DA neurons treated with PMA (24). It is quite possible that PKC affects DAT endocytosis in a subpopulation of DA neurons and/or under specific cell culture conditions. In contrast, we found that AMPH, albeit at relatively high concentrations, significantly accelerated HA-DAT endocytosis in neurons (Fig. 6). This increase in endocytosis is similar to that reported in non-neuronal cells (23, 25).
Live-cell imaging revealed that axonal processes (shafts and varicosities) and growth cones of postnatal DA neurons are decorated with abundant HA-DAT-containing dynamic filopodia-like protrusions (Fig. 7), characteristic of immature neurons. A remarkable feature of these filopodia was the frequent concentration of DAT at the very tip of these processes (Fig. 7A). FRAP measurements revealed a large fraction of the immobile DAT in the filopodial tips, as compared to the other regions of the axons (Fig. 8). It has been proposed that the plasma membrane DATs associated with presynaptic sites (varicosities) and axonal shafts have slightly distinct membrane mobility rates (14). However, in our experiments, lateral mobility rates of DAT in these regions were similar and comparable with rates of freely diffusing transmembrane proteins. Furthermore, AMPH increased the relative amount of the mobile HA-DAT in filopodia, which was accompanied by increases in mobility rates (Fig. 8B). Although the effect of AMPH on lateral mobility of human DAT in non-neuronal systems has not been measured, AMPH decreased accumulation of DAT in filopodia in PAE cells, which is consistent with the increased mobility of DAT molecules (23). It can be hypothesized that a pool of DAT is retained in filopodia through interactions with proteins or lipids, and AMPH-mediated phosphorylation of the amino terminus of DAT (26) releases these interactions, thereby changing membrane mobility of DAT. In agreement with this view, deletion of the DAT amino terminus or mutations leading to outward-facing DAT conformation prevents accumulation of the transporter in filopodia (23). On the other hand, no significant differences in values of Mf or Td for HA-DAT located in presynaptic sites (varicosities) or axonal shafts were found (Fig. 8B), and AMPH did not affect these parameters.
Multiple time-lapse imaging sequences demonstrated that along the axons, filopodia rapidly elongate and retract and, interestingly, some are eventually replaced by varicosities (Fig. 7B–E). Past studies have shown that filopodia found along axons, dendrites and growth cones are crucial for formation of presynaptic specializations and axonal branching (27, 34), dendritic arborization and formation of dendritic spines (28, 32, 33), and axonal pathfinding (29), respectively. Axonal filopodia have also been implicated in the formation of the neuromuscular junction (30) and may contain clusters of synaptic vesicles that govern the formation of mature presynaptic sites (31). In the brain, expression of DAT is one of the key events during development of DA neurons, and it is possible that DAT accumulation in filopodia is important for synaptogenesis and/or pruning of presynaptic dopaminergic terminals in the striatum and mesencephalon. The precise role of DAT at the tip of filopodia remains to be elucidated. It is possible that by increasing mobility and accelerating endocytosis of DAT in axons, chronic AMPH treatment may interfere with DA synapse development and axonal branching.
In summary, optical imaging analysis using this new HA-DAT mouse model demonstrated the dynamic and differential regulation of DAT within specific regions of the DA neuron and highlighted the utility of this experimental model for future analysis of the cellular mechanisms involved in rapid regulation of DATs and other neurotransmitter transporters in the brain.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health grants R01 DA014204 (A.S., N.R.Z.), F32 DA029357 (A.R.), K05 DA015050 (N.R.Z.), and T32 AA007464 (D.S.), and the Transgenic Vectors Core of Rocky Mountain Neurological Disorders Core Center [University of Colorado–Anschutz Medical Campus (UC-AMC)], supported by P30 NS048154. The authors also thank Wallace Chick for help with making the HA-DAT mice, Dr. Rytis Prekeris (UC-AMC) for the Syntaxin13 antibody, and Dr. Trevor Williams (UC-AMC) for the β-actin cre mice.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AMPH
- d-amphetamine
- DA
- dopamine
- DAT
- dopamine transporter
- EL2
- extracellular loop 2
- ESC
- embryonic stem cell
- FRAP
- fluorescence recovery after photobleaching
- HA
- hemagglutinin
- PKC
- protein kinase C
- PMA
- phorbol 12-myristate 13-acetate
- TnF
- transferrin
- TH
- tyrosine hydroxylase.
REFERENCES
- 1. Giros B., Jaber M., Jones S. R., Wightman R. M., Caron M. G. (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612 [DOI] [PubMed] [Google Scholar]
- 2. Riddle E. L., Fleckenstein A. E., Hanson G. R. (2005) Role of monoamine transporters in mediating psychostimulant effects. AAPS J. 7, E847–E851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sulzer D. (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69, 628–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nirenberg M. J., Chan J., Pohorille A., Vaughan R. A., Uhl G. R., Kuhar M. J., Pickel V. M. (1997) The dopamine transporter: comparative ultrastructure of dopaminergic axons in limbic and motor compartments of the nucleus accumbens. J. Neurosci. 17, 6899–6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nirenberg M. J., Vaughan R. A., Uhl G. R., Kuhar M. J., Pickel V. M. (1996) The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J. Neurosci. 16, 436–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nirenberg M. J., Chan J., Vaughan R. A., Uhl G. R., Kuhar M. J., Pickel V. M. (1997) Immunogold localization of the dopamine transporter: an ultrastructural study of the rat ventral tegmental area. J. Neurosci. 17, 5255–5262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pristupa Z. B., McConkey F., Liu F., Man H. Y., Lee F. J., Wang Y. T., Niznik H. B. (1998) Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse 30, 79–87 [DOI] [PubMed] [Google Scholar]
- 8. Melikian H. E. (2004) Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacol. Ther. 104, 17–27 [DOI] [PubMed] [Google Scholar]
- 9. Daniels G. M., Amara S. G. (1999) Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J. Biol. Chem. 274, 35794–35801 [DOI] [PubMed] [Google Scholar]
- 10. Sorkina T., Hoover B. R., Zahniser N. R., Sorkin A. (2005) Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic 6, 157–170 [DOI] [PubMed] [Google Scholar]
- 11. Loder M. K., Melikian H. E. (2003) The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J. Biol. Chem. 278, 22168–22174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melikian H. E., Buckley K. M. (1999) Membrane trafficking regulates the activity of the human dopamine transporter. J. Neurosci. 19, 7699–7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miranda M., Sorkin A. (2007) Regulation of receptors and transporters by ubiquitination: new insights into surprisingly similar mechanisms. Mol. Interv. 7, 157–167 [DOI] [PubMed] [Google Scholar]
- 14. Eriksen J., Rasmussen S. G., Rasmussen T. N., Vaegter C. B., Cha J. H., Zou M. F., Newman A. H., Gether U. (2009) Visualization of dopamine transporter trafficking in live neurons by use of fluorescent cocaine analogs. J. Neurosci. 29, 6794–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eriksen J., Bjorn-Yoshimoto W. E., Jorgensen T. N., Newman A. H., Gether U. (2010) Postendocytic sorting of constitutively internalized dopamine transporter in cell lines and dopaminergic neurons. J. Biol. Chem. 285, 27289–27301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rao A., Simmons D., Sorkin A. (2011) Differential subcellular distribution of endosomal compartments and the dopamine transporter in dopaminergic neurons. Mol. Cell. Neurosci. 46, 148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sorkina T., Miranda M., Dionne K. R., Hoover B. R., Zahniser N. R., Sorkin A. (2006) RNA interference screen reveals an essential role of Nedd4–2 in dopamine transporter ubiquitination and endocytosis. J. Neurosci. 26, 8195–8205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramirez-Solis R., Davis A. C., Bradley A. (1993) Gene targeting in embryonic stem cells. Methods Enzymol. 225, 855–878 [DOI] [PubMed] [Google Scholar]
- 19. Richards T. L., Zahniser N. R. (2009) Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. J. Neurochem. 108, 1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daniel J. A., Galbraith S., Iacovitti L., Abdipranoto A., Vissel B. (2009) Functional heterogeneity at dopamine release sites. J. Neurosci. 29, 14670–14680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yguerabide J., Schmidt J. A., Yguerabide E. E. (1982) Lateral mobility in membranes as detected by fluorescence recovery after photobleaching. Biophys. J. 40, 69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zahniser N. R., Sorkin A. (2009) Trafficking of dopamine transporters in psychostimulant actions. Semin. Cell. Dev. Biol. 20, 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sorkina T., Richards T. L., Rao A., Zahniser N. R., Sorkin A. (2009) Negative regulation of dopamine transporter endocytosis by membrane-proximal N-terminal residues. J. Neurosci. 29, 1361–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cremona M. L., Matthies H. J., Pau K., Bowton E., Speed N., Lute B. J., Anderson M., Sen N., Robertson S. D., Vaughan R. A., Rothman J. E., Galli A., Javitch J. A., Yamamoto A. (2011) Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci 14, 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saunders C., Ferrer J. V., Shi L., Chen J., Merrill G., Lamb M. E., Leeb-Lundberg L. M., Carvelli L., Javitch J. A., Galli A. (2000) Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc. Natl. Acad. Sci. U. S. A. 97, 6850–6855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fog J. U., Khoshbouei H., Holy M., Owens W. A., Vaegter C. B., Sen N., Nikandrova Y., Bowton E., McMahon D. G., Colbran R. J., Daws L. C., Sitte H. H., Javitch J. A., Galli A., Gether U. (2006) Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron 51, 417–429 [DOI] [PubMed] [Google Scholar]
- 27. Matteoli M., Coco S., Schenk U., Verderio C. (2004) Vesicle turnover in developing neurons: how to build a presynaptic terminal. Trends Cell Biol. 14, 133–140 [DOI] [PubMed] [Google Scholar]
- 28. Yoshihara Y., De Roo M., Muller D. (2009) Dendritic spine formation and stabilization. Curr. Opin. Neurobiol. 19, 146–153 [DOI] [PubMed] [Google Scholar]
- 29. Drees F., Gertler F. B. (2008) Ena/VASP: proteins at the tip of the nervous system. Curr. Opin. Neurobiol. 18, 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li P. P., Chen C., Lee C. W., Madhavan R., Peng H. B. (2011) Axonal filopodial asymmetry induced by synaptic target. Mol. Biol. Cell 22, 2480–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kraszewski K., Mundigl O., Daniell L., Verderio C., Matteoli M., De Camilli P. (1995) Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J. Neurosci. 15, 4328–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chiu S. L., Cline H. T. (2010) Insulin receptor signaling in the development of neuronal structure and function. Neural Dev. 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niell C. M., Meyer M. P., Smith S. J. (2004) In vivo imaging of synapse formation on a growing dendritic arbor. Nat. Neurosci. 7, 254–260 [DOI] [PubMed] [Google Scholar]
- 34. Gallo G. (2011) The cytoskeletal and signaling mechanisms of axon collateral branching. Dev. Neurobiol. 71, 201–220 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.