Abstract
Previous studies have demonstrated that bergamottin (BG), a component of grapefruit juice, is a mechanism-based inactivator of CYP3A4 and contributes, in part, to the grapefruit juice-drug interaction. Although the covalent binding of [14C]BG to the CYP3A4 apoprotein has been demonstrated by SDS-polyacrylamide gel electrophoresis, the identity of the modified amino acid residue and the reactive intermediate species of BG responsible for the inactivation have not been reported. In the present study, we show that inactivation of CYP3A4 by BG results in formation of a modified apoprotein-3A4 and a GSH conjugate, both exhibiting mass increases of 388 Da, which corresponds to the mass of 6′,7′-dihydroxybergamottin (DHBG), a metabolite of BG, plus one oxygen atom. To identify the adducted residue, BG-inactivated 3A4 was digested with trypsin, and the digests were then analyzed by liquid chromatography-tandem mass spectrometry (MS/MS). A mass shift of 388 Da was used for the SEQUEST database search, which revealed a mass increase of 388 Da for the peptide with the sequence 272LQLMIDSQNSK282, and MS/MS analysis of the adducted peptide demonstrated that Gln273 is the residue modified. Mutagenesis studies showed that the Gln273 to Val mutant was resistant to inactivation by BG and DHBG and did not generate two of the major metabolites of BG formed by 3A4 wild type. In conclusion, we have determined that the reactive intermediate, oxygenated DHBG, covalently binds to Gln273 and thereby contributes to the mechanism-based inactivation of CYP3A4 by BG.
Introduction
Adverse drug-drug interactions involving grapefruit juice were reported more than a decade ago. Grapefruit juice increases the oral availability of a variety of clinically used drugs that are metabolized primarily by CYP3A4 (Ducharme et al., 1995; Kupferschmidt et al., 1995; Benton et al., 1996; Bailey et al., 1998, 2000). Bergamottin (BG) and 6′,7′-dihydroxybergamottin (DHBG), the two most abundant furanocoumarins present in grapefruit, have been characterized as reversible inhibitors and as mechanism-based inactivators of CYP3A4 (Schmiedlin-Ren et al., 1997; He et al., 1998; Guo et al., 2000; Ohnishi et al., 2000; Tassaneeyakul et al., 2000). Subsequently, studies in primary cultured hepatocytes and more clinically related research with BG and DHBG were pursued (Wen et al., 2002; Goosen et al., 2004; Kakar et al., 2004; Paine et al., 2004, 2006). Accelerated degradation of CYP3A4 after covalent modification of the apoprotein by BG and DHBG was confirmed to play a role in the grapefruit juice effect by several in vivo studies (Goosen et al., 2004; Kakar et al., 2004; Paine et al., 2006).
We have previously demonstrated the covalent binding of a reactive intermediate of [14C]BG to the CYP3A4 apoprotein by SDS-polyacrylamide gel electrophoresis (PAGE) (Lin et al., 2005). However, in those studies, the mass increase of the adducted apoprotein (apo)-3A4 and the mass of the reactive intermediate species were not characterized. In the present study, CYP3A4 was reconstituted with NADPH-cytochrome P450 reductase (reductase) and cytochrome b5 and inactivated by [14C]BG as described previously (He et al., 1998; Lin et al., 2005). The [14C]BG-labeled apo-3A4 was separated from the reductase by SDS-PAGE and recovered by electroblotting to a nitrocellulose (NC) membrane or to a polyvinylidene difluoride (PVDF) membrane for digestion by lysyl endopeptidase (Lys C) or cyanogen bromide (CNBr), respectively (Roberts et al., 1993). All of the peptides that eluted from the PVDF membrane or the NC membrane were further separated by Tricine-SDS-PAGE and subjected to autoradiography (Roberts et al., 1993). These methods provided information about the stability of the BG-inactivated apoprotein to enzymatic digestion and the approximate size of BG-inactivated peptides.
Oxidation of the double bond of the furan ring to give the furanoepoxide or γ-ketoenal intermediate, resulting in modification of P450s, has been well documented during the irreversible inactivation of the rat liver microsomal P450s CYP2A6 and CYP2B1 by several furanocoumarins and the inactivation of CYP3A4 by furanopyridine (Mays et al., 1989; Sahali-Sahly et al., 1996; Koenigs and Trager 1998a,b; Lightning et al., 2000). These findings have led us to hypothesize that BG and DHBG, which both contain the intact furanocoumarin core structure, are activated via a similar mechanism to form a reactive intermediate capable of covalently binding with GSH or with amino acid residues in the 3A4 apoprotein (Lin et al., 2005; Kent et al., 2006).
To unambiguously identify the amino acid residue in CYP3A4 that is covalently adducted by BG using liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, the precise mass of the reactive intermediate of BG must be first determined. Three GSH conjugates, GSH that reacted with oxygenated BG (mass of 354 Da), oxygenated monohydroxy BG (mass of 370 Da), and oxygenated DHBG (mass of 388 Da), were identified as being formed during the metabolism of BG by CYP3A4. In most cases, although more than one reactive intermediate can be trapped by reaction with GSH, only one of the reactive intermediate species covalently binds to the apoprotein of P450, leading to mechanism-based inactivation (Baer et al., 2007; Pearson et al., 2007; Lin et al., 2009). Thus, it is necessary to determine the mass increase for the apo-3A4 inactivated by the formation of an adduct with BG. Electrospray ionization-liquid chromatography-mass spectrometry (ESI-LC-MS) analysis of an incubation mixture after inactivation revealed two deconvoluted spectra for an unmodified and a modified apo-3A4 that differed in mass by 388 ± 2 Da. The mass increase of 388 Da for the GSH conjugate with MH+ at m/z 696 is in good agreement with the mass increase for the formation of an adduct of DHBG plus one oxygen atom with the apoprotein of 3A4 (Bateman et al., 2004).
This report describes trypsin digestion of BG-inactivated 3A4 followed by LC-MS/MS analysis. The mass shift of 388 Da was used to identify the covalently modified peptide in the tryptic digest by using the SEQUEST database search (Lin et al., 2010). The modified peptide was then analyzed by LC-MS/MS to determine the identity of the DHBG-modified residue. Mutagenesis studies of the modified residue were performed to test whether the residue identified by the SEQUEST search is the residue responsible for the inactivation of CYP3A4 by BG. The position of the adducted residue in the CYP3A4 crystal structure was investigated to better understand the experimental results.
Materials and Methods
Chemicals.
NADPH, catalase, GSH, testosterone, l-α-dilauroyl-phosphocholine, l-α-dioleyl-sn-glycero-3-phosphocholine, l-α-phosphatidylserine, and His-Select Nickel affinity gel were purchased from Sigma-Aldrich (St. Louis, MO). BG and bergaptol were purchased from Indofine Chemicals (Hillsborough, NJ). [14C]BG (55 mCi/mmol) with 99% purity was obtained from American Radiolabeled Chemicals (St. Louis, MO). DHBG was a gift from the Florida Department of Citrus (Lakeland, FL). All other chemicals and solvents were of the highest purity available from commercial sources.
Purification of Enzymes.
The human CYP3A4 plasmid was a generous gift from Dr. J. R. Halpert (University of California at San Diego, La Jolla, CA) and was used as the template to construct a mutant in which Gln at position 273 was replaced with Val (Q273V). Mutagenesis was carried out using the in vitro QuickChange site-directed mutagenesis kit (Stratagene products; Agilent Technologies, Santa Clara, CA). The primer 5′-CGAGTGGATTTCCTTGTGCTGATGATTGACTCTC-3′ was used for the mutation of Gln273 to Val. The mutation was confirmed by DNA sequencing carried out at the University of Michigan Biomedical Core Facility (Ann Arbor, MI). The plasmids for the wild-type (WT) CYP3A4 and the Q273V mutant were expressed as His-tagged proteins in Escherichia coli TOPP3 cells as reported previously (Lin et al., 2005). The purification procedures for both P450s, cytochrome b5, and reductase have been described previously (Lin et al., 2005).
Enzyme Assays and Inactivations.
The primary reaction mixture for inactivation contained 60 μg of a mixture of l-α-dilauroyl-phosphocholine, l-α-dioleyl-sn-glycero-3-phosphocholine, and l-α-phosphatidylserine (1:1:1), 1 nmol of P450, 2 nmol of reductase, 1 nmol of b5, 100 units of catalase, and 2 mM GSH in a final volume of 1 ml of 100 mM HEPES buffer, pH 7.4, containing 20% glycerol, 30 mM MgCl2, and 0.5 mM EDTA. The reconstituted system was incubated at 37°C for 30 min and kept on ice until it was used for the experiments. For the studies on the concentration- and time-dependent inactivation of the P450s by BG, the reactions were initiated by adding 1 mΜ ΝADPH to the primary reaction mixture containing various concentrations of BG at 37°C. At the time points indicated, 50-μl aliquots were transferred into 950 μl of a secondary reaction mixture containing 100 μM testosterone in 100 mM potassium phosphate buffer, pH 7.7, with the addition of 200 μM NADPH. Incubations of the secondary reaction mixtures were carried out at 37°C for 15 min, and the reactions were then terminated by the addition of 2 ml of ethyl acetate. The formation of the 6β-hydroxytestosterone product was determined by high-pressure liquid chromatography (HPLC) analysis as described previously (He et al., 1998).
Digestion of [14C]BG-Labeled Apo-3A4 by CNBr on PVDF Membranes.
After inactivation by incubation with 20 μM [14C]BG at 37°C for 30 min, 1 nmol of P450 in the primary reaction mixture was subjected to 10% SDS-PAGE to separate the reductase, CYP3A4, and b5, and the proteins were then transferred to a PVDF membrane by electroblotting. The membrane was stained with 0.1% Ponceau S in 5% acetic acid and destained with excess water. The band corresponding to CYP3A4 was chopped into 1- to 2-mm pieces and incubated with 0.5% polyvinylpyrrolidone in 0.5% acetic acid solution for 1 h at 37°C. The 3A4-containing membrane pieces were rinsed five times with water, and they were then digested by incubation with 30 mg of CNBr in 100 μl of 70% trifluoroacetic acid (TFA) at room temperature for 18 h. The CNBr and TFA were then removed by repeated washing with water and evaporation in a Savant SpeedVac (Thermo Fisher Scientific, Waltham, MA) (Roberts et al., 1993). The peptides generated by treatment with CNBr were eluted out of the membrane with sample buffer (0.5 M Tris-HCl, 1% SDS, 2% glycerol, 0.02% bromphenol blue, 5% β-mercaptoethanol, pH 6.8) and analyzed by Tricine-SDS-PAGE (Roberts et al., 1993).
Digestion of [14C]BG-Labeled Apo-3A4 by Lys C on NC Membranes.
The same procedure as described above was used for these studies, except that the proteins were transferred to a NC membrane after SDS-PAGE. The 3A4-containing samples were then digested with 2 μg of Lys C in 100 μl of 50 mM Tris buffer, pH 8.0, for 18 h at 37°C. The resulting peptides were eluted with sample buffer as described above for Tricine-SDS-PAGE analysis.
Tricine-SDS-PAGE Analysis of the Peptides from the Digests of the BG-Adducted Proteins.
All of the peptides that were eluted from the PVDF membrane and the NC membrane were subjected to Tricine-SDS-PAGE (Roberts et al., 1993). After electrophoresis, the peptides were electroblotted onto an NC membrane and exposed to a Kodak BioMax MR film (Eastman Kodak, Rochester, NY) at −80°C for 3 days before developing.
LC-MS/MS Analysis of GSH Conjugates.
The control (−NADPH) and BG-inactivation samples (+NADPH) containing CYP3A4 or its Q273V mutant were prepared as described above with 50 μM BG for 30 min. After the incubation, samples containing 1 nmol each of P450 were acidified with 60 μl of 10% TFA and applied to a 1-ml AccuBond ODS-C18 solid-phase extraction cartridge (Agilent Technologies). The cartridges were previously washed with 2 ml of methanol followed by 2 ml of water. After the samples were loaded, the cartridges were washed with 2 ml of water, and the GSH conjugates were then eluted with 2 ml of methanol followed by 0.3 ml of acetonitrile. The eluted samples were dried under N2 gas and resuspended in 80 μl of a 1:1 mixture of solvent A (0.1% acetic acid in H2O) and solvent B (0.1% acetic acid in acetonitrile). The samples were analyzed on a C18 reverse-phase column (Luna, 3 μm, 4.6 ′ 100 mm; Phenomenex, Torrance, CA) using a gradient of 20 to 30% B over 5 min, followed by a gradient to 40% B over 15 min, and then increasing linearly to 90% B over 15 min at a flow rate of 0.3 ml/min in the Agilent 1100 series HPLC system (Agilent Technologies). The column effluent was directed into the ESI source of a LCQ mass spectrometer (Thermo Fisher Scientific). The ESI conditions were as follows: sheath gas flow rate, 90 arbitrary units; auxiliary gas, 30 arbitrary units; spray voltage, 4.5 kV; capillary temperature, 170°C; capillary voltage, 30 V; tube lens offset, 25 V. Data were acquired in positive ion mode using Xcalibur software (Thermo Fisher Scientific) with one full scan followed by two data-dependent scans of the most intense and the second most intense ions.
ESI-LC-MS Analysis of the Apoprotein.
Control (−NADPH) and samples inactivated by incubation with 50 μM BG and NADPH for 10 min were prepared. Aliquots containing 50 pmol of P450 were analyzed on a C3 reverse-phase column (Zorbax 300SB-C3, 3.5 μm, 3.0 ′ 150 mm; Agilent Technologies) equilibrated with 30% acetonitrile/0.1% TFA at flow rate 0.2 ml/min in Agilent 1100 series HPLC system (Agilent Technologies). After 5 min, the column effluent was directed into the mass analyzer of a LCQ mass spectrometer (Thermo Fisher Scientific) as described previously (Lin et al., 2005). The acetonitrile concentration was increased linearly to 90% over the next 30 min to separate the components of the incubation mixture, and the mass spectra of the peaks were recorded. The m/z spectra corresponding to the protein envelopes for the CYP3A4 samples were deconvoluted to give the masses associated with each protein envelope using the Bioworks software package (Thermo Fisher Scientific). The ESI source conditions were as follows: sheath gas, 90 arbitrary units; auxiliary gas, 30 arbitrary units; spray voltage, 3.5 kV; capillary temperature, 200°C.
LC-MS/MS Analysis of the BG-Modified Residue.
The control and BG-inactivated CYP3A4 (500 pmol) were digested with 2 μg of trypsin in 100 μl of 50 mM ammonium bicarbonate buffer, pH 8.0, at 37°C for 18 h, as described previously (Lin et al., 2010). The digested peptides were centrifuged at 16,000g, and the clear supernatant was subjected to analysis using a C18 reversed-phase column (Jupiter, 5 μm, 2.0 ′ 100 mm; Phenomenex). The mobile phase consisted of solvent A (0.05% formic acid and 0.05% TFA in water) and solvent B (0.05% formic acid and 0.05% TFA in acetonitrile). The initial gradient was 10 to 25% B for 25 min followed by a linear gradient to 65% B over 35 min, and then it was increased linearly to 95% B over 10 min at a flow rate of 0.3 ml/min on an LC-10AD system (Shimadzu, Kyoto, Japan). The column effluent was directed into a LCQ Deca XP mass spectrometer (Thermo Fisher Scientific). The electrospray ionization conditions were as follows: sheath gas flow rate, 60 arbitrary units; auxiliary gas, 10 arbitrary units; spray voltage, 4.5 kV; capillary voltage, 30 V; capillary temperature, 220°C; tube lens offset, 60 V. Data were acquired in positive mode using Xcalibur software (Thermo Fisher Scientific) in a data-dependent experiment where MS/MS data were collected on the six most abundant ions in the survey scan.
Metabolism of BG.
WT 3A4 and the Q273V mutant were reconstituted as described above and incubated with 25 μM BG in the presence or absence of NADPH. After incubation for 30 min at 37°C, the reaction mixtures were extracted with 3 ml of ethyl acetate. The organic phase was dried under N2 and dissolved in 0.1% acetic acid/50% acetonitrile for HPLC analysis. The metabolites and BG were separated on a C8 column and monitored at 310 nm according to previous methods (Lin et al., 2005; Kent et al., 2006).
Results
Identification of [14C]BG-Labeled Peptides.
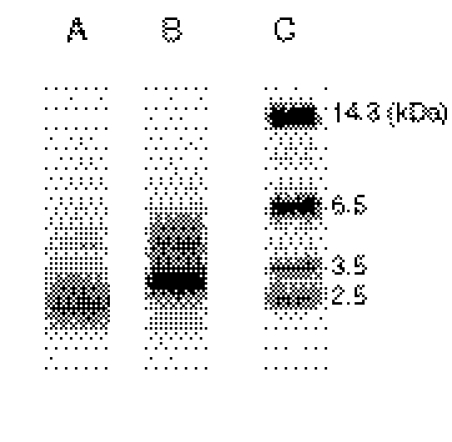
After inactivation by [14C]BG in the reconstituted system, the reaction mixtures were subjected to SDS-PAGE analysis and electroblotted onto a PVDF membrane or NC membrane to separate CYP3A4 from other components. The CYP3A4 was then recovered from the PVDF or NC membranes and cleaved by CNBr or Lys C, respectively. The resulting peptides from the digestions were analyzed by Tricine-SDS-PAGE. As shown in Fig. 1, a radiolabeled band was observed at approximately 2.0 to 3.0 kDa after CNBr cleavage, and a major band at approximately 2.5 to 3.5 kDa, with a minor band at ∼4.5 kDa, was observed after Lys C cleavage. The results suggested that BG-adducted peptides were relatively stable and able to be used for further studies.
Fig. 1.
Autoradiography after Tricine-SDS-PAGE separation of the peptides formed by digestion of [14C]BG-inactivated CYP3A4 with CNBr or Lys C. The SDS-PAGE of the reconstituted system, the digestion of CYP3A4, and Tricine-SDS-PAGE of the digested peptides were carried out as described under Materials and Methods. Lane A, separation of peptides from CNBr cleavage; lane B, separation of peptides from Lys C cleavage; lane C, molecular weight markers.
LC-MS/MS Analysis of the GSH Conjugates Formed during Metabolism of BG.
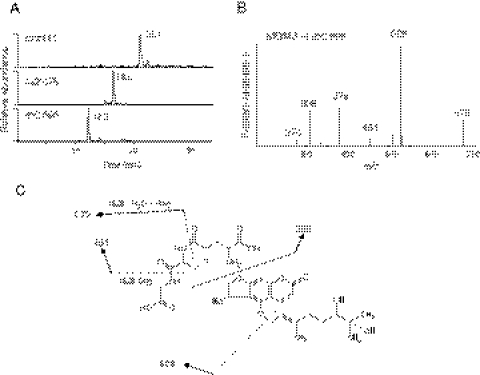
The extracted ion chromatograms for the three major GSH conjugates formed during the metabolism of BG by CYP3A4 are shown in Fig. 2A. The masses of BG, monohydroxy BG, and DHBG are 338, 354, and 372 Da, respectively. The molecular ions at m/z 662, 678, and 696 are from the reaction of GSH with the furanoepoxide of BG, furanoepoxide of monohydroxy BG, and furanoepoxide of DHBG, respectively. The two GSH conjugates with MH+ values at m/z 662 and 678 are similar to those previously reported for the GSH conjugates formed by CYP2B6 and 3A5 (Lin et al., 2005; Kent et al., 2006). The MS/MS spectrum of the GSH conjugate with MH+ at m/z 696 is shown in Fig. 2B. This GSH conjugate exhibited a predominant fragment ion of m/z 526, corresponding to GSH conjugated to the furanoepoxide of the 5-hydroxypsoralen moiety. The neutral losses of 129 and 75 Da correspond to the loss of the pyroglutamate and glycine moieties of GSH, respectively, and are characteristic features of GSH conjugates (Baillie and Davis, 1993). The fragment ion at m/z 526 underwent a loss of Gly (75 Da) to form an ion at m/z 451 and underwent a loss of Glu (129 Da) and a water (18 Da) to form an ion at m/z 379. The fragment ion at m/z 276 is formed from the further loss of Gly (75 Da) and CO (28 Da) from the ion at m/z 379. The combination loss of 129 and 75 Da from the fragment ion at m/z 526 indicates that the GSH is bound to the furanocoumarin core structure. The fragment ion at m/z 308 is due to protonated GSH. Together, these data are consistent with the structures shown in Fig. 2C for the GSH conjugate with MH+ at m/z 696.
Fig. 2.
LC-MS/MS analysis of the GSH conjugates formed during the metabolism of BG. A reaction mixture containing CYP3A4 was incubated with BG in the presence of NADPH, the reactive intermediates were trapped by reaction with GSH, and the conjugates were analyzed as described under Materials and Methods. A, extracted ion chromatograms of the GSH conjugates with MH+ ions at m/z 662, m/z 678, and m/z 696, which elute at 21.1, 16.6, and 12.2 min, respectively. GSH eluted at 5 min under these conditions and exhibits a MH+ ion at m/z 308 (data not shown). B, MS/MS spectrum of the GSH conjugate with the MH+ ion at m/z 696. C, the proposed structure of the GSH conjugate with the MH+ ion at m/z 696. The MS/MS spectrum was obtained in the positive mode and analyzed using the Xcalibur software package. The sites of fragmentation are indicated by dashed lines and are explained in the text.
Characterization of the Apoprotein Adduct of BG with 3A4 by ESI-LC-MS Analysis.
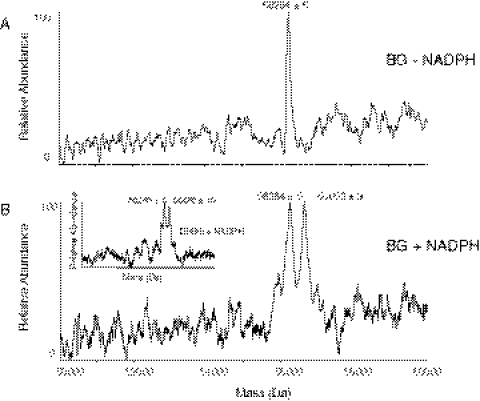
The P450 reaction mixtures were incubated with BG in the absence or presence of NADPH and analyzed by ESI-LC-MS, and the m/z spectra were deconvoluted. Figure 3A shows the control CYP3A4 apoprotein with a mass of 56,264 ± 5 Da, and Fig. 3B shows the BG-inactivated CYP3A4 apoprotein with the mass of 56,652 ± 9 Da. The difference in mass of 388 ± 2 Da is consistent with the addition of the mass of one DHBG molecule plus one oxygen atom. BG is the parent compound of DHBG. If the reactive intermediate were formed from the furanoepoxide of the parent compound BG, the mass increase would be 354 Da. Therefore, it seems that the reactive intermediate with a mass of 388 Da is formed from the furanoepoxide of DHBG. To test our hypothesis, CYP3A4 was inactivated by incubation with DHBG, and the mass increase of the apoprotein was 386 ± 3 Da, as shown in the inset of Fig. 3B, confirming our hypothesis that the inactivation is due to modification of 3A4 by the furanoepoxide of DHBG.
Fig. 3.
Detection of the BG-adducted CYP3A4 apoprotein by ESI-LC-MS analysis. The incubation conditions, HPLC, and MS analysis conditions were as described under Materials and Methods. A, representative deconvoluted mass spectrum of CYP3A4 incubated with BG in the absence of NADPH (control). B, representative deconvoluted mass spectrum of CYP3A4 incubated with BG in the presence of NADPH (inactivated). The inset shows a representative deconvoluted mass spectrum of CYP3A4 incubated with DHBG in the presence of NADPH.
MS/MS Analysis of the BG-Modified Peptide.
The BG-inactivated CYP3A4 was digested with trypsin, and the resulting peptides were analyzed as described under Materials and Methods. The identification of a BG-GSH conjugate with an m/z 696 and the BG-apoprotein adduct have indicated that the mass of adduct is 388 Da. Therefore, the modification of a nucleophilic residue in the target peptide by the addition of a reactive metabolite of BG would be expected to result in a mass increase for the modified peptide of 388 Da. Using this mass increase, a SEQUEST search (Bioworks; Thermo Fisher Scientific) of the data suggested the presence of one modified peptide showing this mass increase in the BG-modified sample. None was identified in the control sample. The MS/MS spectrum of the modified peptide was further analyzed using Xcalibur software as described under Materials and Methods.
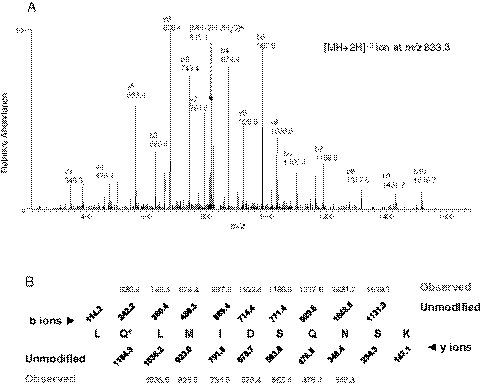
The results of the SEQUEST search suggested that the covalent modification of the apoprotein by the reactive intermediate of BG occurred at residue Gln273 in the peptide sequence 272LQLMIDSQNSK282, which has a MH+ ion at m/z 1665.5. The MS/MS spectrum is shown in Fig. 4A. Using ProteinProspector software, the b and the y ion series for the unmodified peptide with a MH+ ion at m/z 1277.5 were predicted and displayed in Fig. 4B. Shown in red are the observed b and y ions for the modified peptide. The identification of the modified peptide and the amino acid residue covalently modified by BG were confirmed by MS/MS fragmentation of the doubly charged precursor ion at m/z 833.3. Almost all the singly charged fragment ions and a doubly charged ion for [MH + 2H − 2H2O]+2 for this modified peptide can be identified in the MS/MS spectrum. The mass increase of 388 Da observed in the b2 to b10 ions, whereas the lack of any mass increase in the y3 through y9 ions observed in the modified peptide, indicates that Gln273 is the amino acid residue modified by a reactive intermediate of DHBG, which is a metabolite of BG (Goosen et al., 2004; Kent et al., 2006).
Fig. 4.
LC-MS/MS analysis of the peptide 272LQLMIDSQNSK282 isolated from BG-inactivated CYP3A4 with the reactive intermediate of BG covalently bound to the Gln273. A, the observed fragment ions in the MS/MS spectrum are from the doubly charged precursor ion at m/z 833.3 obtained in the positive mode using the Xcalibur software, as discussed under Materials and Methods. B, predicted fragment ion series (b and y ions) for the unmodified peptide 272–282. The b and y ions observed for the modified peptide are indicated in red. The residue modified is indicated by the asterisk.
Mutagenesis Studies of Gln273 to Val in CYP3A4.
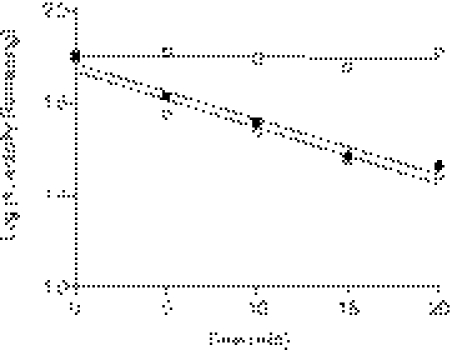
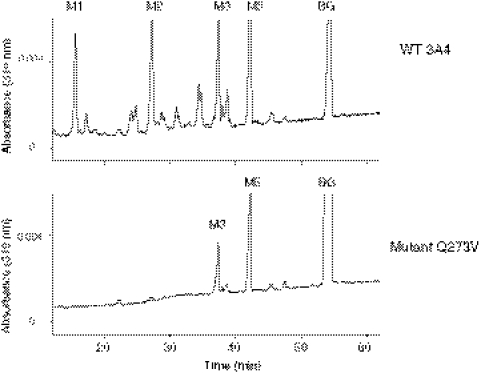
The Q273V mutant was constructed, expressed in E. coli, and purified to investigate whether covalent modification of the Gln273 residue was responsible the mechanism-based inactivation of CYP3A4 by BG. The testosterone 6β-hydroxylation activity remaining after incubation of the WT and Q273V mutant of 3A4 with 5 to 50 μM BG for 5 to 20 min was determined. As calculated from the data shown in Fig. 5, the rate constant for the inactivation is 0.055 min−1 for the WT enzyme incubated with either 5 or 10 μM BG, whereas the rate constant for the Q273V mutant when it is incubated with 50 μM BG is 0.002 min−1, suggesting that the inactivation of the mutant protein by BG was essentially eliminated by this mutation. The metabolism of BG by the WT and Q273V mutant was also determined by HPLC analysis (Fig. 6). As reported previously (Kent et al., 2006), the WT 3A4 generated four major metabolites (M1, M2, M3, and M5), but the Q273V mutant only generated two major metabolites (M3 and M5). On the basis of our previous reports on the metabolism of BG by CYP3A5 and CYP2B6, M1 is bergaptol, M2 is DHBG, M3 is 5′-OH-BG, and M5 is 2′-OH-BG (Kent et al., 2006). From the mass increase due to covalent modification of the apoprotein and the mass of the BG-GSH conjugate as described previously, the reactive intermediate for BG inactivation seems to be derived from oxygenation of DHBG. Therefore, the generation of metabolite M2 from BG is a critical step for the inactivation by BG. Because the formation of M2 by the Q273V mutant is almost undetectable, the lack of inactivation after the inactivation of Gln273 to Val conversion is not unexpected. Therefore, we investigated the ability of DHBG to cause mechanism-based inactivation of WT 3A4 and the Q273V mutant and to form the GSH adduct of the reactive intermediate. Although both proteins formed the GSH conjugate having MH+ at m/z 696 and DHBG caused mechanism-based inactivation of WT 3A4, no inactivation of the Q273Vmutant was observed (data not shown). Together, these results suggested that Gln273 is adducted by the reactive intermediate of DHBG, which may be involved in the mechanism-based inactivation.
Fig. 5.
Loss of testosterone 6β-hydroxylation activity of the WT 3A4 and Q273V mutant P450 after the reconstituted reaction mixtures were inactivated by incubation with 5 μM BG (●) or 10 μM BG (○) for the WT and 50 μM BG (□) for the Q273V mutant for the times indicated. The testosterone 6β-hydroxylation activity at time 0 for each condition was used as 100% control. The results presented here were from two experiments done with duplicates. The specific activity of WT 3A4 and Q273V mutant in the reconstituted system was 14 and 3 nmol of 6β-hydroxytestosterone per minute per nanomole of P450, respectively.
Fig. 6.
Metabolism of BG by the WT and Q273V mutant analyzed by HPLC. WT 3A4 (top) and the Q273V mutant (bottom) were incubated with BG and NADPH in the reconstituted system at 37°C for 30 min, and the reaction mixtures were extracted with ethyl acetate and analyzed by HPLC as described under Materials and Methods.
Discussion
Initial studies on the inactivation of CYPCYP2B1, CYP2B4, CYP2B6, CYP3A4, and CYP3A5 using [14C]BG, followed by SDS-PAGE separation of the proteins and autoradiography, demonstrated that modification of the apoproteins of these P450s contributed to the mechanism-based inactivation (Lin et al., 2005). Mass spectral analysis of BG-inactivated CYP2B1, CYP2B4, CYP2B6, and CYP3A5 showed that the mass increases due to the formation of a protein adduct were 380 to 390 Da, but attempts to elucidate the mass of the BG-modified 3A4 apoprotein were not successful. In the present study, we modified the conditions for the inactivation reaction and the ESI operating parameters and used a different LC column with a different gradient and were able to observe the 3A4 apoprotein with a covalent adduct attached. The mass increase observed of 388 ± 2 Da for the adduct of BG with CYP3A4 is in good agreement with that characterized for CYP2B1, CYP2B4, CYP2B6, and CYP3A5. Studies on the masses and structures of the reactive intermediates trapped as GSH conjugates provided valuable information on the structure of the protein adduct and the identity of the reactive intermediate. After incubation of BG with CYP3A4 and GSH, a GSH conjugate with an MH+ ion at m/z 696, corresponding to a mass increase of 388 Da, was identified by LC-MS/MS analysis. This mass increase indicates that the reactive intermediate is a furanoepoxide of DHBG. Because DHBG is also a mechanism-based inactivator of CYP3A4 (Schmiedlin-Ren et al., 1997; Paine et al., 2004), LC-MS analysis of the protein adduct formed with DHBG was evaluated for comparison, and a mass increase of 386 ± 3 Da was obtained (Fig. 3B, inset). In another study, the mass increase for the DHBG-inactivated CYP3A4 was reported to be ∼387 Da (Bateman et al., 2004). The mass increase for the DHBG-inactivated CYP3A5 was determined to be ∼392 (Lin et al., 2005). Together, these data suggest that BG (mass, 338 Da) is initially metabolized to give DHBG (mass, 372 Da) before the generation of a furanoepoxide reactive intermediate (mass, 388 Da) that then reacts with the CYP3A4 apoprotein leading to inactivation. These findings lead us to conclude that with both compounds, the reactive intermediate is the epoxygenated DHBG, having a mass of 388 Da, which is responsible for the mechanism-based inactivation of CYP3A4.
Because radiolabeled BG was readily available, we used it initially to detect the presence of the labeled peptides by Tricine-SDS-PAGE analysis. CNBr cleavage at the methionine residues of 3A4 resulted in five peptides in the mass range of 2.0 to 3.0 kDa, corresponding to the peptides spanning amino acid residues: 1) 63 to 89; 2) 90 to 114; 3) 257 to 275; 4) 354 to 371; and 5) 372 to 395. Lys C cleavage of 3A4 resulted in more than 10 peptides in the mass range of 2.5 to 3.5 kDa, but only 5 peptides spanning those residues (36–66, 68–91, 92–115, 258–282, and 379–406) that correspond to the residues in the CNBr digest. These methods clearly demonstrate that the BG-modified peptides are stable to enzymatic digestion and amenable to subsequent analysis. However, some BG-adducted peptides may not be stable under our experimental conditions and may elude detection during LC-MS/MS analysis.
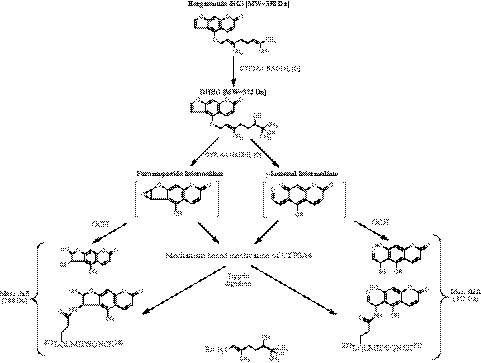
After digestion of the BG-inactivated CYP3A4 with trypsin, LC-MS/MS analysis followed by a SEQUEST database search revealed that the peptide 272LQLMIDSQNSK282 exhibited the expected mass shift of 388 Da due to the formation of an adduct with the epoxy-DHBG, and MS/MS analysis of this adducted peptide indicated that the modified residue is Gln273. Under the same conditions, the covalent modification of Gln273 was also determined by LC-MS/MS analysis of CYP3A5 to be the site modified during the inactivation by BG (data not shown). The BG-modified Gln273 detected in the peptide isolated after trypsin digest is also found in the peptides identified by Tricine-SDS-PAGE analysis after CNBr or Lys C cleavage, as shown in Fig. 1. Thus, on the basis of these studies, we hypothesized that 1) BG is hydroxylated to give DHBG before the formation of the reactive intermediate and 2) the site of bioactivation to form the reactive intermediate is the double bond of the intact furan ring. A scheme showing possible pathways for the inactivation and the formation of the GSH conjugates is shown in Fig. 7. DHBG, a hydroxylated product of BG, is more hydrophilic than BG (Paine et al., 2004); therefore, the furanoepoxide intermediate or r-ketoenal intermediate may diffuse out of the active site to react with GSH to form GSH conjugates in addition to binding to the 3A4 apoprotein.
Fig. 7.
Pathways proposed for the formation of the reactive intermediate responsible for covalently modifying the protein, leading to inactivation during the metabolism of BG by CYP3A4 (Mays et al., 1989; Koenigs and Trager 1998a,b).
Mutagenesis studies showed that mutation of Gln273 to Val in 3A4 altered the profiles of the products formed during the metabolism of BG with four metabolites formed by the WT 3A4, whereas only two were formed by the Q273V mutant. The formation of bergaptol (M1) is undetectable with the Q273V mutant, suggesting that the modification of the Gln273 may alter the protein conformation such that BG is no longer bound in an orientation that favors cleavage of the geranyl group. DHBG (M2) is formed at extremely low levels by the Q273V mutant, suggesting that not enough of the reactive intermediate is formed under the incubation conditions we used to cause the inactivation. To test whether the lack of inactivation of Q273V mutant was due primarily to the very slow formation of DHBG or due to the removal of Gln273 preventing formation of the protein adduct, the following experiments were performed with the Q273V mutant in the reconstituted system: 1) when the mutant was incubated with BG, GSH, and NADPH, the only GSH conjugate detected, was one having MH+ at m/z 662, and none were observed with MH+ at m/z 678 or 696 (data not shown); and 2) when the mutant was incubated with DHBG, GSH, and NADPH, a GSH conjugate with MH+ at m/z 696 was observed even though no inactivation of the Q273V mutant by DHBG was observed (data not shown). These results suggest that the Q273V mutant is capable of generating reactive intermediates from BG and DHBG. Therefore, the reason that inactivation is not observed with the Q273V mutant is due not only to the low level of DHBG produced from BG but also to the absence of the Gln273 target. For DHBG, the lack of inactivation is due to the absence of the Gln273 target. The modification of the Gln273 by the relatively bulky reactive intermediate of DHBG most likely affects substrate binding and subsequent catalysis. Thus, our results suggest that the Gln273 residue is required for the generation of the dihydroxylated product, which is then converted to the reactive intermediate responsible for the inactivation of CYP3A4 by BG.
To better understand the structural basis for the effect of Gln273 modification by the reactive intermediate of DHBG on the catalytic activity, we used the known crystal structure of CYP3A4 (Protein Data Bank code 1TQN). As shown in Fig. 8A, Gln273 is far away from the heme iron (∼20 Å) and not in the active site. However, the hydrogen bonding distance between the amine group of Gln273 and the carboxylate side chain of Asp277 is 2.4 Å (Fig. 8B). It is reasonable to propose that covalent formation of an amide bond between the NH2 group of Gln273 and the furanoepoxide of DHBG, as shown in Fig. 7, would disrupt this hydrogen bond interaction and compromise the formation of the preferred secondary and tertiary structures of CYP3A4, thereby leading to impaired catalysis. It is also interesting to note from this structure that the van der Waals radii of the carbonyl oxygen of Asp277 and the imidazole ring of His267 partially shield Gln273 from complete exposure to the solvent (Fig. 8C). Therefore, it is possible that the reactive DHBG intermediate may react with the glutamine during its egress from the active site. In addition, the reactive intermediate may be prevented from coming into contact with GSH in the solvent during its egress from the active site because of shielding by His267 and Asp277.
Fig. 8.
The location of Gln273 in the CYP3A4 crystal structure. A, ribbon diagram showing the spatial relationships between Gln273 and the C-, E-, F-, G-, H-, and I-helices. B, Gln273 and Asp277 are within hydrogen bonding distance from each other (2.4 Å). This interaction may be disrupted by covalent adduction of Gln273 by the reactive intermediate (epoxide of DHBG). C, Gln273 is partially shielded from the solvent by His267 and Asp277.
It is also of interest to note the differences in the positions of adduct formation between the P450 families. For example, the Thr302 residue modified in CYP2B1, CYP2B4, and CYP2B6 by tert-butylphenylacetylene is located in the I-helix and is only ∼6 Å away from the heme iron (Zhang et al., 2009, 2011; Lin et al., 2010, 2011), whereas the residues of 3A4 modified by BG and raloxifene are all relatively far away from the heme iron. For mechanism-based inactivation of CYP3A4 by raloxifene, Tyr75 (∼25 Å away from heme iron) and Cys239 (∼21 Å away from heme iron) were identified as the two sites that are covalently modified by the diquinone methide intermediate of raloxifene (Pearson et al., 2007; Yukinaga et al., 2007). Here, Gln273 is ∼20 Å away from heme iron. Perhaps, because the CYP3A4 active site is relatively large and malleable, not only can it accommodate a wide range of structurally diverse substrates, but it may also be possible for the reactive intermediate to “tumble” after its formation and as it exits from the protein active site. Thus, it may not be restrained in the vicinity of the highly conserved Thr in the I-helix where it would be more likely to react because of its proximity.
In conclusion, we have identified the reactive intermediate of BG and the CYP3A4 residue covalently modified by BG. BG must first be hydroxylated to DHBG and then epoxygenated to form the furanoepoxide intermediate responsible for the mechanism-based inactivation of CYP3A4 to occur. Thus, the two major furanocoumarins, BG and DHBG, both contribute to the grapefruit juice effect through the formation of the same ultimate reactive intermediate species.
Acknowledgments
We thank Drs. Yoshihiro Morishima and Jaime D'Agostino (Department of Pharmacology, University of Michigan, Ann Arbor, MI) for advice during the preparation of this manuscript.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant CA16954] (to P.F.H.).
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
- BG
- bergamottin
- DHBG
- 6′,7′-dihydroxybergamottin
- WT
- wild type
- Q273V
- Gln273 to Val mutation of 3A4
- TFA
- trifluoroacetic acid
- Lys C
- lysyl endopeptidase
- CNBr
- cyanogen bromide
- PVDF
- polyvinylidene difluoride
- P450
- cytochrome P450
- NC
- nitrocellulose
- HPLC
- high-pressure liquid chromatography
- PAGE
- polyacrylamide gel electrophoresis
- ESI
- electrospray ionization
- LC-MS
- liquid chromatography-mass spectrometry
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- apo
- apoprotein.
Authorship Contributions
Participated in research design: Lin, Kenaan, and Hollenberg.
Conducted experiments: Lin and Kenaan.
Contributed new reagents or analytic tools: Lin and Kenaan.
Performed data analysis: Lin and Kenaan.
Wrote or contributed to the writing of the manuscript: Lin, Kenaan, and Hollenberg.
References
- Baer BR, Wienkers LC, Rock DA. (2007) Time-dependent inactivation of P450 3A4 by raloxifene: identification of Cys239 as the site of apoprotein alkylation. Chem Res Toxicol 20:954–964 [DOI] [PubMed] [Google Scholar]
- Bailey DG, Malcolm J, Arnold O, Spence JD. (1998) Grapefruit juice-drug interactions. Br J Clin Pharmacol 46:101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DG, Dresser GK, Kreeft JH, Munoz C, Freeman DJ, Bend JR. (2000) Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther 68:468–477 [DOI] [PubMed] [Google Scholar]
- Baillie TA, Davis MR. (1993) Mass spectrometry in the analysis of glutathione conjugates. Biol Mass Spectrom 22:319–325 [DOI] [PubMed] [Google Scholar]
- Bateman KP, Baker J, Wilke M, Lee J, LeRiche T, Seto C, Day S, Chauret N, Ouellet M, Nicoll-Griffith DA. (2004) Detection of covalent adducts to cytochrome P450 3A4 using liquid chromatography mass spectrometry. Chem Res Toxicol 10:1356–1361 [DOI] [PubMed] [Google Scholar]
- Benton RE, Honig PK, Zamani K, Cantilena LR, Woosley RL. (1996) Grapefruit juice alters terfenadine pharmacokinetics, resulting in prolongation of repolarization on the electrocardiogram. Clin Pharmacol Ther 59:383–388 [DOI] [PubMed] [Google Scholar]
- Ducharme MP, Warbasse LH, Edwards DJ. (1995) Disposition of intravenous and oral cyclosporine after administration with grapefruit juice. Clin Pharmacol Ther 57:485–491 [DOI] [PubMed] [Google Scholar]
- Goosen TC, Cillié D, Bailey DG, Yu C, He K, Hollenberg PF, Woster PM, Cohen L, Williams JA, Rheeders M, et al. (2004) Bergamottin contribution to the grapefruit juice-felodipine interaction and disposition in human. Clin Pharmacol Ther 76:607–617 [DOI] [PubMed] [Google Scholar]
- Guo LQ, Fukuda K, Ohta T, Yamazoe Y. (2000) Role of furanocoumarin derivatives on grapefruit juice-mediated inhibition of human CYP3A activity. Drug Metab Dispos 28:766–771 [PubMed] [Google Scholar]
- He K, Iyer KR, Hayes RN, Sinz MW, Woolf TF, Hollenberg PF. (1998) Inactivation of cytochrome P450 3A4 by bergamottin, a component of grapefruit juice. Chem Res Toxicol 11:252–259 [DOI] [PubMed] [Google Scholar]
- Kakar SM, Paine MF, Stewart PW, Watkins PB. (2004) 6′7′-Dihydroxybergamottin contributes to the grapefruit juice effect. Clin Pharmacol Ther 75:569–579 [DOI] [PubMed] [Google Scholar]
- Kent UM, Lin HL, Noon KR, Harris DL, Hollenberg PF. (2006) Metabolism of bergamottin by cytochromes P450 2B6 and 3A5. J Pharmacol Exp Ther 318:992–1005 [DOI] [PubMed] [Google Scholar]
- Koenigs LL, Trager WF. (1998a) Mechanism-based inactivation of P450 2A6 by furanocoumarins. Biochemistry 37:10047–10061 [DOI] [PubMed] [Google Scholar]
- Koenigs LL, Trager WF. (1998b) Mechanism-based inactivation of cytochrome P450 2B1 by 8-methoxypsoralen and several other furanocoumarins. Biochemistry 37:13184–13193 [DOI] [PubMed] [Google Scholar]
- Kupferschmidt HH, Ha HR, Ziegler WH, Meier PJ, Krähenbühl S. (1995) Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther 58:20–28 [DOI] [PubMed] [Google Scholar]
- Lightning LK, Jones JP, Friedberg T, Pritchard MP, Shou M, Rushmore TH, Trager WF. (2000) Mechanism-based inactivation of cytochrome P450 3A4 by L-754,394. Biochemistry 39:4276–4287 [DOI] [PubMed] [Google Scholar]
- Lin HL, Kent UM, Hollenberg PF. (2005) The grapefruit juice effect is not limited to cytochrome P450 (P450) 3A4: evidence for bergamottin-dependent inactivation, heme destruction, and covalent binding to protein in P450s 2B6 and 3A5. J Pharmacol Exp Ther 313:154–164 [DOI] [PubMed] [Google Scholar]
- Lin HL, Zhang H, Hollenberg PF. (2009) Metabolic activation of mifepristone [RU486; 17β-hydroxy-11β-(4-dimethylaminophenyl)-17α-(1-propynyl)-estra-4,9-dien-3-one] by mammalian cytochromes P450 and the mechanism-based inactivation of human CYP2B6. J Pharmacol Exp Ther 329:26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HL, Zhang H, Jushchyshyn M, Hollenberg PF. (2010) Covalent modification of Thr302 in cytochrome P450 2B1 by the mechanism-based inactivator 4-tert-butylphenylacetylene. J Pharmacol Exp Ther 333:663–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HL, Zhang H, Pratt-Hyatt MJ, Hollenberg PF. (2011) Thr302 is the site for the covalent modification of human cytochrome P450 2B6 leading to mechanism-based inactivation by tert-butylphenylacetylene. Drug Metab Dispos 39:2431–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays DC, Hilliard JB, Wong DD, Gerber N. (1989) Activation of 8-methoxypsoralen by cytochrome P-450. Enzyme kinetics of covalent binding and influence of inhibitors and inducers of drug metabolism. Biochem Pharmacol 38:1647–1655 [DOI] [PubMed] [Google Scholar]
- Ohnishi A, Matsuo H, Yamada S, Takanaga H, Morimoto S, Shoyama Y, Ohtani H, Sawada Y. (2000) Effect of furanocoumarin derivatives in grapefruit juice on the uptake of vinblastine by Caco-2 cells and on the activity of cytochrome P450 3A4. Br J Pharmacol 130:1369–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine MF, Criss AB, Watkins PB. (2004) Two major grapefruit juice components differ in intestinal CYP3A4 inhibition kinetic and binding properties. Drug Metab Dispos 32:1146–1153 [DOI] [PubMed] [Google Scholar]
- Paine MF, Widmer WW, Hart HL, Pusek SN, Beavers KL, Criss AB, Brown SS, Thomas BF, Watkins PB. (2006) A furanocoumarin-free grapefruit juice establishes furanocoumarins as the mediators of grapefruit juice-felodipine interaction. Am J Clin Nutr 83:1097–1105 [DOI] [PubMed] [Google Scholar]
- Pearson JT, Wahlstrom JL, Dickmann LJ, Kumar S, Halpert JR, Wienkers LC, Foti RS, Rock DA. (2007) Differential time-dependent inactivation of P450 3A4 and P450 3A5 by raloxifene: a key role for C239 in quenching reactive intermediates. Chem Res Toxicol 20:1778–1786 [DOI] [PubMed] [Google Scholar]
- Roberts ES, Hopkins NE, Alworth WL, Hollenberg PF. (1993) Mechanism-based inactivation of cytochrome P450 2B1 by 2-ethnylnaphthalene: identification of an active-site peptide. Chem Res Toxicol 6:470–479 [DOI] [PubMed] [Google Scholar]
- Sahali-Sahly Y, Balani SK, Lin JH, Baillie TA. (1996) In vitro studies on the metabolic activation of the furanopyridine L-754,394, a highly potent and selective mechanism-based inhibitor of cytochrome P450 3A4. Chem Res Toxicol 9:1007–1012 [DOI] [PubMed] [Google Scholar]
- Schmiedlin-Ren P, Edwards DJ, Fitzsimmons ME, He K, Lown KS, Woster PM, Rahman A, Thummel KE, Fisher JM, Hollenberg PF, et al. (1997) Mechanisms of enhanced oral availability of CYP3A4 substrates by grapefruit constituents. Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins. Drug Metab Dispos 25:1228–1233 [PubMed] [Google Scholar]
- Tassaneeyakul W, Guo LQ, Fukuda K, Ohta T, Yamazoe Y. (2000) Inhibition selectivity of grapefruit juice components on human cytochromes P450. Arch Biochem Biophys 378:356–363 [DOI] [PubMed] [Google Scholar]
- Wen YH, Sahi J, Urda E, Kulkarni S, Rose K, Zheng X, Sinclair JF, Cai H, Strom SC, Kostrubsky VE. (2002) Effects of bergamottin on human and monkey drug-metabolizing enzymes in primary cultured hepatocytes. Drug Metab Dispos 30:977–984 [DOI] [PubMed] [Google Scholar]
- Yukinaga H, Takami T, Shioyama SH, Tozuka Z, Masumoto H, Okazaki O, Sudo K. (2007) Identification of cytochrome P450 3A4 modification site with reactive metabolite using linear ion trap-Fourier transform mass spectrometry. Chem Res Toxicol 20:1373–1378 [DOI] [PubMed] [Google Scholar]
- Zhang H, Lin HL, Walker VJ, Hamdane D, Hollenberg PF. (2009) tert-Butylphenylacetylene is a potent mechanism-based inactivator of cytochrome P450 2B4: inhibition of cytochrome P450 catalysis by steric hindrance. Mol Pharmacol 76:1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lin HL, Kenaan C, Hollenberg PF. (2011) Targeting of the highly conserved threonine 302 residue of cytochromes P450 2B family during mechanism-based inactivation by aryl acetylenes. Arch Biochem Biophys 507:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]