Abstract
Prior studies in breast cancer cells have shown that lapatinib and obatoclax interact in a greater than additive fashion to cause cell death and do so through a toxic form of autophagy. The present studies sought to extend our analyses to the central nervous system (CNS) tumor cells and to further define mechanisms of drug action. Lapatinib and obatoclax killed multiple CNS tumor isolates. Cells lacking PTEN (phosphatase and tensin homolog on chromosome 10) function were relatively resistant to drug combination lethality; expression of PTEN in PTEN-null cells restored drug sensitivity, and knockdown of PTEN promoted drug resistance. On the basis of knockdown of ERBB1-4 (erythroblastic leukemia viral oncogene homolog 1–4), we discovered that the inhibition of ERBB1/3/4 receptors were most important for enhancing obatoclax lethality rather than ERBB2. In parallel, we noted in CNS tumor cells that knockdown of BCL-xL (B-cell lymphoma-extra large)and MCL-1 (myeloid cell leukemia-1) interacted in an additive fashion to facilitate lapatinib lethality. Pretreatment of tumor cells with obatoclax enhanced the lethality of lapatinib to a greater extent than concomitant treatment. Treatment of animals carrying orthotopic CNS tumor isolates with lapatinib- and obatoclax-prolonged survival. Altogether, our data show that lapatinib and obatoclax therapy could be of use in the treatment of tumors located in the CNS.
Introduction
Glioblastoma (GBM) has an abysmal 5-year survival rate and is an incurable malignancy. Even under optimal circumstances, in which essentially all of a glial tumor can be surgically removed and the patients are maximally treated with radiation and “state of the art” chemotherapeutic agents, such as temozolomide (Temodar) or carmustine (Gliadel wafers), the median survival of this disease is only extended from ∼3 months to 12 to 15 months (Parkin et al., 2005; Robins et al., 2007). Although in some reports, up to ∼80% of pediatric medulloblastoma patients can be “cured” based on a 5-year survival; linked to this is a very high degree of patient morbidity associated with the elevated levels of radio- and chemotherapy required to treat the disease, resulting in profound and sustained life-altering effects on the surviving/growing child (Onvani et al., 2010; Schmidt et al., 2010). It is obvious that better therapeutic approaches are required for both malignancies.
In general, the effect of a single target anticancer kinase inhibitor in vitro most often is to elicit a cytostatic rather than a cytotoxic effect (Martin et al., 2009; Mitchell et al., 2010; and references therein). To achieve greater effects on survival, inhibition of multiple growth factor receptors and intracellular pathways has to be targeted. It has been noted that tumors presenting with alterations in ERBB receptors are often more aggressive and frequently convey poorer clinical outcome (Bigner and Vogelstein, 1990; Hynes and Lane, 2005; Martin et al., 2008). In addition, it is known that medulloblastoma and malignant glioma often exhibit expression of constitutively activated or altered ERBB receptors; therefore, the use of small molecule kinase inhibitors, such as lapatinib and gefitinib, as anticancer therapeutics in CNS tumor types is logical (Miller, 2004; Hynes and Lane, 2005).
Lapatinib, a dual ERBB1/ERBB2 inhibitor, is currently clinically approved for use in ERBB2 overexpressing metastatic breast cancer combined with capecitabine (Geyer et al., 2006; Kong et al., 2008). Over time, resistance to ERBB inhibitor therapeutics develops through secondary mutations within ERBB receptors, through the initiation of alternative receptor tyrosine kinase signaling pathways, or through the up-regulation of prosurvival proteins in the BCL-2 family (Ware et al., 2010).
The BCL-2 family of proteins consists of protective BCL-2 family proteins (BCL-2, BCL-xL, MCL-1) and proapoptotic proteins in the family, such as BAX, BAK, PUMA, and NOXA, (Martin et al., 2009; van Delft and Huang, 2009; Mitchell et al., 2010; and references therein). Most frequently reported is that the release of BAK and BAX from protective BCL-2 proteins results in pore formation, mitochondrial stress with reactive oxygen species generation, leading to the release of cytochrome c, and the activation of apoptosis. This effect can also be induced by target-specific therapeutics such as obatoclax (GX15-070) that act by inhibiting the interaction between protective BCL-2 family members and toxic members of the family. In theory, this approach would also increase the toxicity of other therapies that act to promote mitochondrial dysfunction (Martin et al., 2009). However, in our previous studies combining lapatinib and obatoclax, we demonstrated that cell killing, despite activation of BAX and BAK, was the result of a toxic form of autophagy, and caspase inhibitors such as z-VAD had no effect on suppressing the cell-killing effect (Martin et al., 2009; Mitchell et al., 2010).
The interplay between apoptosis and autophagy is still not fully understood; however, many of the same pathways are implicated within both of these death processes. Autophagy is an evolutionarily conserved catabolic pathway that recycles or removes damaged or excess organelles as well as break down proteins into their amino acid constituents. Cancer cells often display reduced levels of autophagy, allowing continuing malignant progression and proliferation (Mathew et al., 2007) but also providing an anticancer role by limiting tumor size/growth (Hippert et al., 2006). This leaves the question whether autophagy in tumor cells is a cytoprotective or cytotoxic event. As noted above, we have found that the BCL-2 inhibitor obatoclax, either alone or in combination with the ERBB1/2/4 inhibitor lapatinib, kills through a toxic form of autophagy that correlated with the activation of toxic BH3 domain proteins, such as BAX and BAK (Martin et al., 2009; Mitchell et al., 2010).
The present study aimed to establish further mechanisms of action of lapatinib and obatoclax toxicity in CNS tumor cells, the importance of PTEN status in drug toxicity, and the role of autophagy in cancer cell survival and/or death. We have established that cancer cells lacking PTEN are inherently resistant to drug treatment. Our findings have also shown that in medulloblastoma and glioblastoma cells, down-regulation of ERBB1/3/4 by lapatinib and inhibition of MCL-1 by obatoclax are central to drug combination effects.
Materials and Methods
Materials.
Lapatinib was provided by GlaxoSmithKline (King of Prussia, PA). Obatoclax was provided by Cephalon (Frazer, PA). Other drugs were purchased from Selleck Chemicals LLC (Houston, TX). Trypsin-EDTA, Dulbecco's modified Eagle's medium, minimal essential medium, RPMI 1640 medium, and penicillin-streptomycin were purchased from Invitrogen (Carlsbad, CA). All established tumor cells were purchased from the American Type Culture Collection (Manassas, VA). Primary human GBM and medulloblastoma cells were from Virginia Commonwealth University and Mayo Clinic (Hamed et al., 2010). Short hairpin PTEN, plasmid expressing green fluorescent protein (GFP)-tagged PTEN, B-Raf, MEK, p70, mTOR, and plasmid-expressing luciferase were purchased from Addgene (Cambridge, MA). Dominant-negative MEK1 was purchased from Thermo Fisher Scientific (Waltham, MA), and the plasmid expressing GFP-tagged human LC3 was kindly provided by Dr. S Spiegel, Virginia Commonwealth University. Commercially available validated siRNA to ErbB1, ErbB2, mTOR, Mcl-1, Bcl-xL, Beclin1, ATG5, NOXA, PUMA, apoptosis-inducing factor, and BAK were purchased from QIAGEN (Valencia, CA) whereas si-smart pools targeting ErbB3 and ErbB4 were purchased from Dharmacon (Lafayette, CO). Mcl-1, Bcl-xL, ErbB2, ErbB3, ErbB4, p-ErbB2, p-mTOR, and ATG5 antibodies were purchased from Cell Signaling Technology (Danvers, MA), and glyceraldehyde-3-phosphate dehydrogenase, p-AKT, Beclin1, p-ERK1/2, NOXA, and PUMA antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). ErbB1 and p-p70 (Thr421) antibodies were purchased from Invitrogen, whereas p62, p-p70 (Thr389), and p-ErbB1 were purchased from R&D Systems (Minneapolis, MN) and LC3 antibody was purchased from Novus Biologicals, Inc. (Littleton, CO). All secondary antibodies were purchased from Santa Cruz Biotechnology. Reagents and performance of experimental procedures were described in Park et al., 2008, 2009, 2010; Zhang et al., 2008; Martin et al., 2009; and Eulitt et al., 2011.
Culture and In Vitro Exposure of Cells to Drugs.
All established cell lines were cultured at 37°C [5% (v/v) CO2] in vitro using RPMI 1640 medium (BT474/BT549), Dulbecco's modified Eagle's medium (GBMs), or minimal essential medium (DAOY and D283) supplemented with 5% (v/v) fetal calf serum and 10% (v/v) nonessential amino acids. For short-term cell killing assays and immunoblotting studies, cells were plated at ∼2 × 105 cells/well of a 12-well plate and 48 h after plating were treated with various drugs, as indicated. In vitro drug treatments were from 100 mM stock solutions of each drug, and the maximal concentration of vehicle (DMSO) in medium was 0.02% (v/v). Cells were not cultured in reduced serum medium during any study described in this article.
In Vitro Cell Treatments, Microscopy, SDS-Polyacrylamide Gel Electrophoresis, and Western Blot Analysis.
For in vitro analyses of short-term cell death effects, cells were treated with vehicle or lapatinib/obatoclax or the combination of lapatinib/obatoclax with the addition of either rapamycin or BEZ-235 for the indicated times in the figure legends. For apoptosis assays where indicated, cells were isolated at the indicated times and subjected to trypan blue cell viability assay by counting in a light microscope. For SDS-PAGE and immunoblotting, cells were plated at 5 × 105 cells/cm2 and treated with drugs at the indicated concentrations and after the indicated time of treatment and lysed in whole-cell lysis buffer (0.5 M Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 0.02% bromphenol blue), and the samples were boiled for 30 min. The boiled samples were loaded onto 8 to 14% SDS-PAGE, and electrophoresis was run for approximately 1.5 h. Proteins were electrophoretically transferred onto 0.22-μm nitrocellulose and immunoblotted with various primary antibodies against different proteins. Blots were visualized using an Odyssey infrared imaging system.
Transfection of Cells with siRNA or with Plasmids.
Cells were plated as described above and transfected 24 h after plating. For all cell types (0.5 μg), plasmids expressing a specific mRNA (or siRNA) or appropriate vector control plasmid DNA was diluted in 50 μl of serum-free and antibiotic-free medium (1 portion for each sample). At the same time, 2 μl of Lipofectamine 2000 (Invitrogen) was diluted into 50 μl of serum-free and antibiotic-free medium (1 portion for each sample). Diluted DNA was added to the diluted Lipofectamine 2000 for each sample and incubated at room temperature for 30 min. This mixture was added to each well per dish of cells containing 200 μl of serum-free and antibiotic-free medium for a total volume of 300 μl, and the cells were incubated for 4 h at 37°C. An equal volume of medium was then added to each well. Cells were incubated for 48 h and then treated with drugs.
Transfection with siRNA.
Cells were plated in 60-mm dishes from a fresh culture growing in log phase as described above and transfected 24 h after plating. Before transfection, the medium was aspirated, and 1 ml of serum-free medium was added to each plate. For transfection, a 10 nM concentration of the annealed siRNA, the positive sense control doubled-stranded siRNA targeting glyceraldehyde-3-phosphate dehydrogenase or the negative control (a “scrambled” sequence with no significant homology to any known gene sequences from mouse, rat, or human cell lines) was used. Scrambled or experimental siRNA (10 nM) was diluted in serum-free medium. Lipofectamine (4 μl; QIAGEN) was added to this mixture, and the solution was mixed by pipetting up and down several times. This solution was incubated at room temperature for 10 min and then added dropwise to each dish. The medium in each dish was swirled gently to mix and then incubated at 37°C for 2 h. One milliliter of 10% (v/v) serum-containing medium was added to each plate, and cells were incubated at 37°C for 48 h before replating (50 × 103 cells each) onto 12-well plates. Cells were allowed to attach overnight and then treated with drugs (0–48 h). Trypan blue exclusion/ terminal deoxynucleotidyl transferase dUTP nick-end labeling and SDS-PAGE/immunoblotting analyses were performed at the indicated time points.
Microscopy for LC3-GFP Expression.
Cells were transfected with a plasmid to express an LC3-GFP fusion protein and were then cultured for 24 h. Cells were then treated with drugs, as indicated. LC3-GFP transfected cells were visualized at the indicated time points on the Zeiss Axiovert 200 microscope using the fluorescein isothiocyanate filter.
Intracerebral Inoculation of GBM6 and BT474 Cells.
Athymic female NCr-nu/nu mice (National Cancer Institute, Frederick, MD) weighing ∼20 g were used for this study. Mice were maintained under pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the U.S. Department of Agriculture (Washington, DC), the U.S. Department of Health and Human Services (Washington, DC), and the National Institutes of Health (Bethesda, MD). The GBM6-luc and BT474 cell lines were used for these studies. Mice were anesthetized via intraperitoneal administration of ketamine (40 mg/kg) and xylazine (3 mg/kg) and immobilized in a stereotactic frame (David Kopf Instruments, Tujunga, CA). A 24-gauge needle attached to a Hamilton syringe was inserted into the right basal ganglia to a depth of 3.5-mm and then withdrawn 0.5-mm to make space for tumor cell accumulation. The entry point at the skull was 2-mm lateral and 1-mm dorsal to the bregma. Intracerebral injection of BT474, DAOY, and GBM6-luc cells in 2 μl of PBS was performed over 10 min. The skull opening was enclosed with sterile bone wax, and the skin incision was closed using sterile surgical staples. Two to four weeks after tumor cell implantation, animals were segregated into treatment groups. For animal administration, lapatinib and obatoclax were first dissolved in DMSO, and an equal volume of 50:50 Cremophor EL/ethanol (Sigma-Aldrich, St. Louis) was added. After mixing, a 1:10 dilution was made with sterile PBS. Animals were treated with vehicle (PBS/Cremophor EL/ethanol/DMSO), lapatinib, obatoclax, or a combination of lapatinib and obatoclax using oral gavage to a final concentration of 5 mg/kg q.i.d. body mass for obatoclax and 100 mg/kg b.i.d. for lapatinib. Animals were imaged as indicated. Mice were injected intraperitoneally with luciferin at 175 mg/kg in a volume of 100 μl and were anesthetized with isoflurane before and during imaging. Animals were imaged at a peak time of 15 min after luciferin injection via a Xenogen IVIS instrument (Caliper Life Sciences, Alameda, CA).
Data Analysis.
Comparison of the effects of various treatments was performed using analysis of variance and the Student's t test. Differences with a P value of <0.05 were considered statistically significant. Experiments shown are the means of multiple individual points (± S.E.M.).
Results
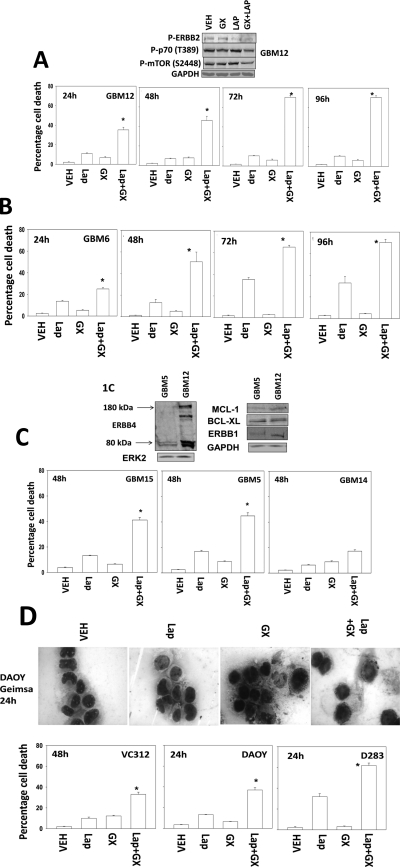
Treatment of GBM cells (GBM6, GBM12) with lapatinib increased the toxicity of obatoclax in a time-dependent fashion (Fig. 1,A and B). Similar data were observed in GBM5 and GBM15 cells (Fig. 1C). In contrast to GBM5/6/12/15 cells, GBM14 cells, which lack PTEN function, were relatively resistant to drug combination toxicity (Fig. 1C). Similar findings to those in short-term viability measurements were made in colony formation assays (Supplemental Fig. 1). Findings comparable with those found in GBM cells were noted in pediatric medulloblastoma cell lines (Fig. 1D). Of interest, even in cell lines such as DAOY that were exhibiting ∼40% killing by the drug combination via trypan blue staining, no evidence of “classic” morphological signs of apoptosis were evident. This is in agreement with our previous findings wherein the pan-caspase inhibitor z-VAD did not protect cells from this drug combination and no cleavage of procaspase 3 or poly(ADP-ribose) polymerase was evident (Martin et al., 2009; Mitchell et al., 2010).
Fig. 1.
Lapatinib and obatoclax interact to kill multiple CNS tumor cells but not those cells lacking PTEN function/expression. A, GBM12 cells were treated with vehicle (VEH, DMSO), lapatinib (lap, 1 μM), and/or obatoclax (GX, 50 nM) as indicated. Cells were isolated 24 to 96 h after exposure, and viability was determined by trypan blue exclusion (n = 3, ±S.E.M.).*, P < 0.05 greater than vehicle control. Top blot, drug combination causes inactivation of ERBB2, p70 S6K, and mTOR. B, GBM6 cells were treated with vehicle (DMSO), lapatinib (lap, 1 μM), and/or obatoclax (GX, 50 nM) as indicated. Cells were isolated 24 to 96 h after exposure, and viability was determined by trypan blue exclusion (n = 3, ±S.E.M.).*, P < 0.05 greater than vehicle control. C, GBM5, GBM14, and GBM15 cells were treated with vehicle (DMSO), lapatinib (1 μM), and/or obatoclax (GX, 50 nM) as indicated. Cells were isolated 48 h after exposure, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.).*, P < 0.05 greater than vehicle control. Top blot, the expression of ERBB1, ERBB4, MCL-1, and BCL-xL in GBM5 and GBM12 cells. D, VC312, DAOY, and D283 pediatric CNS tumor cells were treated with vehicle (DMSO), lapatinib (1 μM), and/or obatoclax (GX, 50 nM) as indicated. Cells were isolated 24 to 48 h after exposure, and viability was determined by trypan blue exclusion (n = 3, ±S.E.M.).*, P < 0.05 greater than vehicle control. Top, Giemsa staining of DAOY cells 24 h after drug treatment.
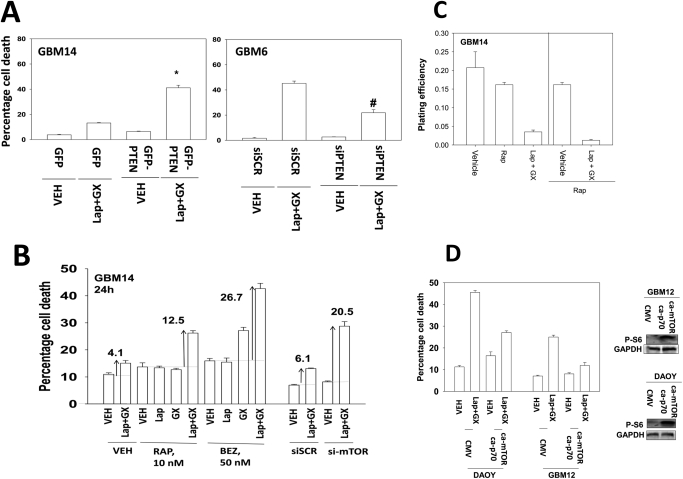
Loss of PTEN function has often been observed in several tumor cell types, including brain, breast, and prostate cancers (Depowski et al., 2001; Höland et al., 2011). Re-expression of PTEN in PTEN-null GBM14 cells facilitated lapatinib and obatoclax lethality (Fig. 2A; Supplemental Fig. 2) (Leslie and Foti, 2011). In contrast, knockdown of PTEN in GBM6 cells inhibited drug combination lethality. Identical data were obtained in BT474 (PTEN wild type) and BT549 (PTEN-null) breast cancer cell lines (data not shown). As loss of PTEN was causing resistance to the drug combination, we hypothesized that inhibition of a drugable downstream effector of PTEN, mTOR, could restore the lethal effects of lapatinib and obatoclax treatment. In agreement with our hypothesis, treatment of GBM14 cells with either rapamycin or BEZ-235 caused significant enhancements in lapatinib and obatoclax lethality compared with vehicle-treated cells (Fig. 2B). Knockdown of mTOR expression caused a similar effect to the use of either rapamycin or BEZ-235. In colony-formation assays, rapamycin also enhanced lapatinib and obatoclax toxicity (Fig. 2C). On the basis of our findings examining PTEN and mTOR, we next tested whether molecular activation of signaling pathways protected against the lapatinib and obatoclax drug combination. Expression of activated forms of p70 S6K and mTOR protected tumor cells from drug lethality (Fig. 2D). B-RAF V600E is a known oncogene upstream of MEK1 and can also promote activation of PI3K-dependent pathways through paracrine signaling (Matallanas et al., 2011). Expression of B-RAF V600E modestly suppressed drug lethality, suggesting that the ERK1/2 pathway was not a key mediator of drug resistance (Supplemental Fig. 3). Expression of dominant-negative MEK1 partially abrogated the modest protective effects of B-RAF V600E expression.
Fig. 2.
Loss of PTEN function renders tumor cells resistant to lapatinib and obatoclax treatment. A, GBM14 cells were transfected with either control (GFP) or with a plasmid to express PTEN (GFP-PTEN). GBM6 cells were transfected with either a control plasmid short hairpin RNA or a plasmid to express a short hairpin RNA to knock down PTEN. Twenty-four hours after transfection, cells were treated with vehicle (VEH, DMSO) or with lapatinib (lap, 1 μM) and obatoclax (GX, 50 nM). Cells were isolated 24 h after exposure, and viability was determined by trypan blue exclusion (n = 3, ±S.E.M.). B, GBM14 cells were treated with vehicle (DMSO) or with lapatinib (1 μM) and obatoclax (GX, 50 nM) in the presence or absence of rapamycin (Rap, 10 nM) or BEZ-235 (BEZ, 50 nM). Parallel sets of cells were transfected to knock down mTOR. Cells were isolated 24 after exposure, and viability was determined by trypan blue exclusion (n = 3, ±S.E.M.). C, GBM14 cells were plated as single cells in sextuplicate and were treated for 48 h with vehicle, lapatinib (1 μM) + obatoclax (GX, 50 nM), rapamycin (10 nM), or the drug combination as indicated. Colony formation 14 days later was determined (n = 3, ±S.E.M.). D, DAOY and GBM12 cells were transfected with empty vector [cytomegalovirus (CMV)] or with plasmids to express activated p70 S6K and activated mTOR. Cells were treated with vehicle (DMSO) or with lapatinib (1 μM) and obatoclax (GX, 50 nM). Cells were isolated 24 h after exposure, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.).
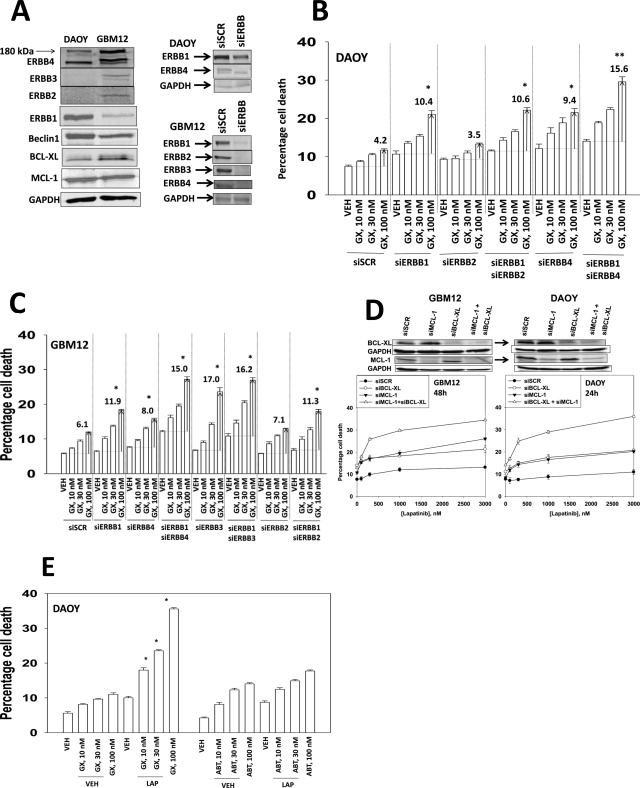
Lapatinib can inhibit ERBB1, ERBB2, and ERBB4 kinase activities (Berezowska and Schlegel, 2011 and references therein). Tao and Maruyama (2008) next defined in several cell lines the relative contribution of ERBB1-4 in the biological actions of lapatinib. DAOY cells expressed ERBB1 and ERBB4; GBM12 cells expressed ERBB1, ERBB2, ERBB3, and ERBB4 (Fig. 3A). In DAOY cells, knockdown of ERBB1 or ERBB4 and to a greater extent both ERBB1 and ERBB4 enhanced obatoclax lethality in a dose-dependent fashion (Fig. 3B). In GBM12 cells, it was apparent that a similar ERBB1/ERBB4-protective signaling axis was present, as had been observed in DAOY cells, although knockdown of ERBB3 alone in GBM12 cells permitted a strong toxic interaction with obatoclax (Fig. 3C). After examining the relative roles of ERBB receptors, we then examined which protective BCL-2 family proteins most influenced lapatinib sensitivity. Knockdown of BCL-xL or MCL-1 increased lapatinib toxicity in DAOY and GBM12 cells (Fig. 3D). Knockdown of both BCL-xL and MCL-1 caused an additive increase in killing compared with knockdown of the individual proteins. Finally, we compared and contrasted the abilities of various small molecule BCL-2 inhibitors to promote lapatinib toxicity. In a dose-dependent manner, the BCL-2/BCL-xL/MCL-1 inhibitor obatoclax enhanced lapatinib lethality in DAOY and GBM12 cells (Fig. 3E, data not shown). However, at similar mole-for-mole drug concentrations, the BCL-2/BCL-xL inhibitor ABT-263 did not alter lapatinib lethality (Fig. 3E, data not shown).
Fig. 3.
Dissecting the roles of ERBB receptors and protective BCL-2 family members in the toxic interaction between lapatinib and obatoclax. A, expression of ERBB1-4, BCL-xL, and MCL-1 in CNS tumor cells and the knock down of these proteins by siRNA. DAOY (B) and GBM12 (C) cells had expression of the indicated receptors knocked down. Cells were treated with vehicle (DMSO) or with obatoclax (GX, 10–100 nM). Cells were isolated 24 h after exposure, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.); the values shown indicate the true percentage of cell death above its matched vehicle control:*, P < 0.05 greater than vehicle control; **, P < 0.05 greater than the same value under parallel other conditions. D, DAOY and GBM12 cells had expression of the indicated protective BCL-2 family proteins (MCL-1, BCL-xL) knocked down. Cells were treated with vehicle (DMSO) or with lapatinib (100–3000 nM). Cells were isolated 24 h after exposure, and viability was determined by trypan blue exclusion (n = 3, ±S.E.M.); the values shown indicate the true percentage of cell death above its matched vehicle control. E, DAOY cells were treated with 1 μM lapatinib in the presence of obatoclax (GX, 10–100 nM) and ABT-263 (ABT, 10–100 nM). Cells were isolated 24 h after exposure, and viability was determined by trypan blue exclusion (n = 3, ±S.E.M.).*, P < 0.05 greater than vehicle control.
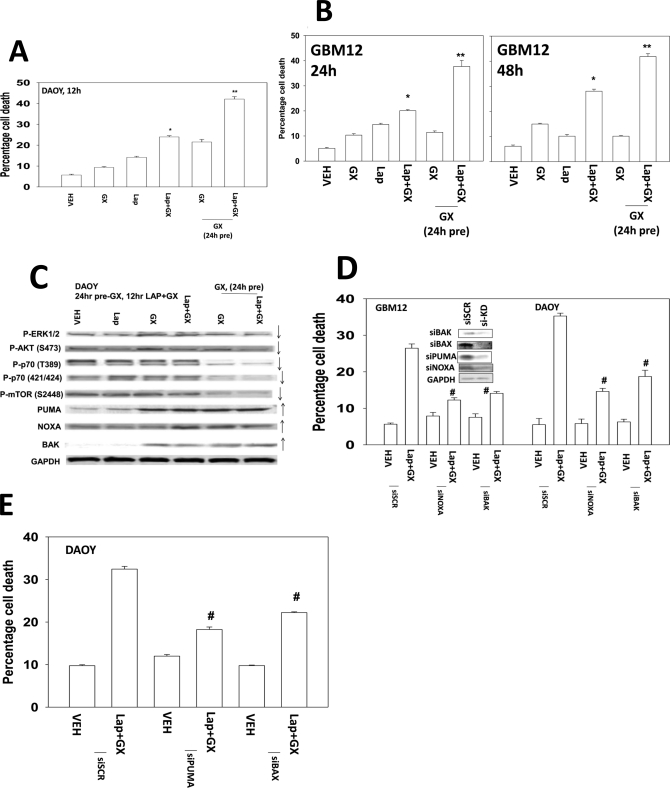
Many therapeutic drugs are administered in a sequential fashion to achieve their greatest antitumor effect in vitro and in vivo. We next tested whether pretreatment of cells with obatoclax could enhance the overall lethal response to a subsequent lapatinib treatment. A 24-h pretreatment of DAOY cells with obatoclax caused a significant rise in the percentage of cells killed by a later exposure to lapatinib (Fig. 4A). Similar data were also observed in GBM12 cells (Fig. 4B). We then performed immunoblotting to define alterations in protein expression and protein phosphorylation. Concomitant treatment of cells with lapatinib and obatoclax after 12 h reduced mTOR, p70 S6K, and AKT activity and increased the levels of PUMA and NOXA (Fig. 4C). In cells pretreated with obatoclax for 24 h, the levels of mTOR, p70 S6K, AKT, and ERK1/2 activity were further reduced; the levels of NOXA and PUMA remained elevated. We next determined whether NOXA, PUMA, and other survival regulatory factors altered viability after drug exposure. Knockdown of NOXA, PUMA, BAK, or BAX protected cells from the drug combination (Fig. 4, D and E).
Fig. 4.
Pretreatment of cells with obatoclax promotes greater drug combination toxicity than concomitant drug treatment. A, DAOY cells were pretreated with either vehicle (VEH, DMSO) or with obatoclax (GX, 50 nM). After 24 h, as indicated, cells were treated with lapatinib (1 μM) and/or obatoclax (GX, 50 nM). Cells were isolated after 12 h, and viability was determined by trypan blue exclusion (n = 3, ±S.E.M.)*, P < 0.05 greater than vehicle control; **, P < 0.05 greater than parallel value in nonpretreated cells. B, GBM12 cells were pretreated with either vehicle (DMSO) or with obatoclax (50 nM). After 24 h, as indicated, cells were treated with lapatinib (1 μM) and/or obatoclax (GX, 50 nM). Cells were isolated after 24 to 48 h, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.).*, P < 0.05 greater than vehicle control; **, P < 0.05 greater than parallel value in nonpretreated cells. C, DAOY cells (see A) were isolated, and immunoblotting performed on cell lysates. D and E, DAOY and GBM12 cells were transfected to knock down PUMA and/or NOXA expression or to knock down BAK or BAX expression. Twenty-four hours later, cells were treated with vehicle (DMSO) or with lapatinib (1 μM) and obatoclax (GX, 50 nM). Cells were isolated 24 h later, and viability was determined by trypan blue exclusion (n = 3, ±S.E.M.). #, P < 0.05 less than vehicle control value.
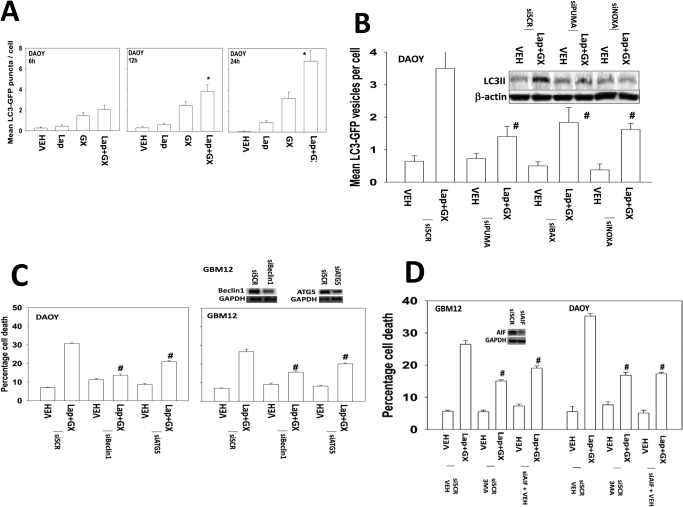
Previous studies in breast cancer cells have shown that the induction of autophagy plays an important role in lapatinib and obatoclax lethality (Martin et al., 2009; Mitchell et al., 2010). In DAOY cells that are particularly sensitive to the lapatinib/obatoclax drug combination, we noted a rapid induction of autophagy as judged by the formation of LC3-GFP punctae (Fig. 5A). Loss of PUMA, NOXA, or BAX prevented the drug-induced induction of LC3-GFP punctae (Fig. 5B). This also correlated with a reduction in LC3-II formation by blotting cell lysates. Incubation with 3-methyl-adenine and knockdown of ATG5, Beclin1, or apoptosis-inducing factor all protected cells from lapatinib and obatoclax toxicity (Figs. 5, C and D).
Fig. 5.
Lapatinib and obatoclax interact to kill CNS tumor cells through a toxic form of autophagy. A, DAOY cells were transfected with a plasmid to express LC3-GFP. Cells were treated with lapatinib (1 μM) and/or obatoclax (GX, 50 nM), and the number of LC3-GFP punctae was determined over 6 to 24 h (n = 3, ±S.E.M.)*, P < 0.05 greater than vehicle control. B, DAOY cells were transfected to knock down PUMA and/or NOXA expression or to knock down BAX expression in parallel with a plasmid to express LC3-GFP. Cells were treated with lapatinib (1 μM) and/or obatoclax (GX, 50 nM), and the number of LC3-GFP punctae was determined over 24 h (n = 3, ±S.E.M.). #, P < 0.05 less than vehicle control. C, DAOY and GBM12 cells were transfected with a scrambled siRNA (siSCR) or siRNA molecules to knock down Beclin-1 or ATG5. Twenty-four hours after transfection, cells were treated with vehicle (DMSO) or with lapatinib (1 μM) and obatoclax (GX, 50 nM). Cells were isolated 24 h later, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). #, P < 0.05 less than vehicle control. D, DAOY and GBM12 cells were transfected with a scrambled siRNA (siSCR) or a siRNA molecule to knock down apoptosis-inducing factor (AIF). Twenty-four hours after transfection, cells were treated vehicle (PBS) followed as indicated by 3-methyl adenine (3MA, 5 mM) followed by with vehicle (DMSO) or with lapatinib (1 μM) and obatoclax (GX, 50 nM). Cells were isolated 24 h later, and viability was determined by trypan blue exclusion (n = 3, ± S.E.M.). #, P < 0.05 less than vehicle control.
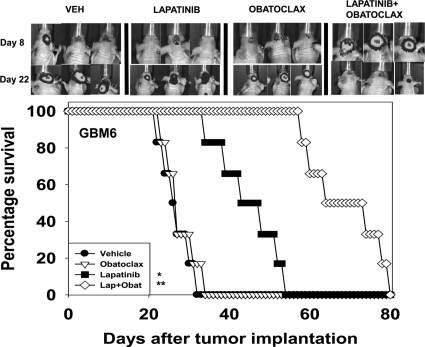
We next determined whether the lapatinib and obatoclax drug combination could prolong animal survival, with animals carrying intracranial tumors (Awada et al., 2011; Taskar et al., 2012). In GBM6 and DAOY tumors, lapatinib as a single agent enhanced animal survival (Fig. 6; Supplemental Fig. 4). Lapatinib combined with obatoclax extended survival further than lapatinib alone. Drug combination treatment did not alter mouse body mass (Supplemental Figure S5). In multiple normal tissues known to be targets for chemotherapy toxicity, no obvious toxic effects were observed (Supplemental Figs. 6–11).
Fig. 6.
Lapatinib and obatoclax prolong survival of animals with intracranial tumors. GBM6-luc cells (106) were implanted into the brains of mice. Eight days after implantation animals were treated with vehicle (VEH, Cremophor EL), lapatinib (100 mg/kg b.i.d.), obatoclax (5 mg/kg q.i.d.), or the drug combination for 5 days. Top, animals carrying tumors show regression 14 days after initiation of drug combination exposure as judged by bioluminescent imaging. Bottom, animals exposed to lapatinib show elevated survival compared with vehicle control (two studies, n = 6, ±S.E.M.).*, P < 0.05 greater than vehicle treatment; **, P < 0.05 exposure to lapatinib and obatoclax causes greater survival than lapatinib alone.
Discussion
In the present article, we aimed to define the importance of each ERBB protein to the lapatinib and obatoclax drug combination response, the PTEN status as an outcome for the response to drug combination treatment, to define whether MCL-1 and/or BCL-xL expression affects the response to drug combination treatment of GBM and medulloblastoma cell lines and to establish the involvement of autophagy in response to this drug combination in CNS tumor cells.
Lapatinib inhibits ERBB1/ERBB2, the expression of which is frequently elevated in CNS tumors; however, CNS and other tumor types often demonstrate limited toxicity to lapatinib as a single agent through de novo mechanisms and the acquirement of drug resistance (Duhem-Tonnelle et al., 2010). Lapatinib resistance can be attributed to further mutations, both point and splicing, within ERBB receptors, the initiation of alternative receptor tyrosine kinase signaling pathways, or the up-regulation of prosurvival proteins (BCL-2 family) (Martin et al., 2008; Ware et al., 2010). These multifactorial resistance mechanisms demonstrate the importance of simultaneously targeting multiple proteins vital in cell survival signaling pathways to enhance tumor cell killing. Our previous research using a variety of drug combinations has emphasized the success of targeting multiple prosurvival signaling pathways through combinational treatment of CNS and other tumor types both in vivo and in vitro.
The ERBB1-4 receptors can homo- or heterodimerize with each other. Lapatinib, a known ErbB1/2/4 receptor inhibitor, can thus affect multiple ERBB receptor combinations dependent on cell type, and by implication the downstream pathways controlled by a combination of ERBB1/2 and ERBB3/4 could also be regulated by lapatinib treatment. Our findings showed that knockdown of ERBB1/4 in DAOY and ERBB1/3/4 in GBM12 cells was responsible for cell killing in combination with obatoclax. Accompanying this, knockdown of MCL-1 and subsequent treatment with lapatinib greatly enhanced or restored lapatinib toxicity in CNS tumor cells, suggesting MCL-1 as a key target of obatoclax. Obatoclax acts to inhibit protective BCL-2 family proteins, MCL-1, BCL-2, and BCL-xL, thereby releasing proapoptotic proteins, such as NOXA, BAX, and BAK. Inhibition or down-regulation of MCL-1 results in the release of these toxic BH3 domain proteins, which form pores, increasing mitochondrial permeability (Supplemental Fig. 12).
Obatoclax has recently completed its phase 2 trial evaluation with some clinical responses evident but has yet to be tested for antitumor effects in CNS tumor types. Combinational treatment of lapatinib with another BCL-2 family inhibitor, ABT-263 (inhibitor of BCL-2 and BCL-xL), did not demonstrate the same enhanced level of killing as observed with obatoclax. This emphasizes the importance of subverting the protective effect of MCL-1, the main target of obatoclax, to induce cell death. This was confirmed using molecular approaches. Pretreatment of CNS tumor cells with obatoclax before treatment with lapatinib and obatoclax further enhanced cell killing. In vitro, by use of molecular tools, we attributed the sequence-dependent effect due to lower activity levels of p70 S6K and mTOR and enhanced levels of the toxic BH3 domain proteins BAK, PUMA, and NOXA (Mitchell et al., 2010) (Supplemental Fig. 12).
Although combination treatment with lapatinib and obatoclax shows promise, we noted that certain cell lines, e.g., GBM14 and BT549, remained relatively resistant to the drugs. PTEN, a tumor suppressor that negatively regulates the PI3K/AKT pathway, is often lost or mutated in cancer brain and breast cancer cells, resulting in the constitutive activation of the PI3K pathway and its downstream cell survival effectors (Seminario et al., 2003). Restoration of PTEN in PTEN-null cancer cell lines restored drug combination sensitivity, and in parallel, silencing of PTEN in lapatinib-sensitive cells decreased drug lethality, demonstrating the importance of PTEN in the therapeutic response. The involvement of this signaling pathway was further confirmed by targeting downstream effectors such as mTOR and p70 S6K (see below).
The kinase mTOR acts to balance nutrient availability and cell growth through phosphorylation of p70 S6K and autophagy regulatory proteins, ensuring regulation of protein synthesis, degradation, and survival. Two mTOR-specific inhibitors, rapamycin and BEZ-235, can block the PI3K pathway downstream of PTEN, causing growth arrest and, in some cells, cell death (Alva et al., 2004). Both mTOR inhibitors enhanced cell killing by lapatinib and obatoclax in PTEN-null cells. Knocking down the expression of mTOR in PTEN-null cells also increased cell death, replicating results seen with rapamycin and BEZ-235. Furthermore, lapatinib and obatoclax-mediated cell death was reduced by expression of activated forms of mTOR or p70 S6K, validating that inactivation of PI3K pathway is essential in lapatinib and obatoclax lethality. However, expression of mutated active B-RAF (V600E), which caused further activation of the MEK/ERK1/2 pathway, only slightly decreased sensitivity to lapatinib and obatoclax, suggesting that signaling by ERK1/2 was a peripheral pathway to the drug combination effects.
Autophagy occurs at a basal rate in all cells and involves the sequestration of cytoplasmic constituents such as damaged or excess organelles that are then removed, degraded, and recycled to supply the cell with nutrients; this enables cancer cells to survive under extreme stress (Hippert et al., 2006). Autophagy involves the initiation, nucleation, cycling, and expansion of an autophagosome (Wang and Klionsky, 2003; Pattingre et al., 2008; Yoshimori and Noda, 2008; Jia et al., 2010; Mehrpour et al., 2010). The autophagosome fuses with a lysosome, forming an autolysosome, which acts to degrade and recycle the vesicle contents (Graf et al., 2009). Giemsa staining of lapatinib- and obatoclax-treated CNS tumor cells revealed a relative lack of morphological changes, e.g., profound nuclei fragmentation normally associated with apoptosis (Alva et al., 2004).
Increased levels of BCL-2 family members in lapatinib resistant cells can prevent autophagy through the sequestration and inhibition of Beclin-1 (Hippert et al., 2006; Martin et al., 2009). Pretreatment of CNS tumor cells with 3-methyladenine, which inhibits the class III PI3K Vps34, resulted in reduced sensitivity of tumor cells to lapatinib and obatoclax treatment. Furthermore, knockdown of Beclin-1 and ATG5 using siRNA abrogated lapatinib- and obatoclax-induced cell death. These findings collectively suggest that autophagic flux is significant in lapatinib- and obatoclax-induced cell death.
Medulloblastoma and glioblastoma are tumor types that use high doses of chemotherapy and radiotherapy to achieve a therapeutic response. Approaches to using less toxic drug and radiation regimens would be beneficial to patients. In orthotopic glioblastoma tumors, lapatinib and obatoclax treatment prolonged animal survival to a greater extent than either drug individually. Altogether these data argue that the lapatinib and obatoclax drug combination has efficacy in vivo using multiple tumor models.
In conclusion, the drug combination of lapatinib and obatoclax kills multiple CNS and mammary tumor types. The death-promoting effects of lapatinib in this combination, in two of the cell lines tested, were dependent on inhibition of ERBB1/3/4 and not on ERBB2. On the basis of molecular as well as drug interactions, MCL-1 was shown to be the key protective BCL-2 family protein that was inhibited by obatoclax. Downstream of the receptors, loss of PTEN function reduced drug combination toxicity. Downstream of PTEN, both p70 S6K and mTOR were shown to play an essential regulatory role in the actions of the drug combination. In addition, in agreement with reduced mTOR activity, the drug combination killed tumor cells through a form of toxic autophagy and that was also dependent on the actions of NOXA, PUMA, and BAK. Given that both lapatinib and obatoclax are clinically relevant agents, translation into the clinic of this drug combination is warranted.
Supplementary Material
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Cancer Institute [Grants R01-CA141704, R01-CA150214]; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK52825]; and the Department of Defense [Grant DAMD17-03-1-0262].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- GBM
- glioblastoma
- ERBB1-4
- erythroblastic leukemia viral oncogene homolog 1–4
- BCL
- B-cell lymphoma
- BCL-xL
- B-cell lymphoma-extra large
- MCL
- MCL-1, myeloid cell leukemia-1
- GX15-070
- obatoclax
- ERK
- extracellular-regulated kinase
- MEK
- mitogen-activated extracellular regulated kinase
- PI3K
- phosphatidylinositol 3-kinase
- z-VAD
- N-benzyloxycarbonyl-Val-Arg-Asp
- siRNA
- small interfering RNA
- PTEN
- phosphatase and tensin homolog on chromosome 10
- PAGE
- polyacrylamide gel electrophoresis
- DMSO
- dimethyl sulfoxide
- GFP
- green fluorescent protein
- BEZ-235
- 2-methyl-2-{4-[3-methyl-2-oxo-8-(quinolin-3-yl)-2,3-dihydro-1H-imidazo[4,5-c]quinolin-1-yl]phenyl}propanenitrile
- PBS
- phosphate-buffered saline
- ABT-263
- navitoclax
- CNS
- central nervous system.
Authorship Contributions
Participated in research design: Poklepovic, Fisher, Grant, and Dent.
Conducted experiments: Cruickshanks, Hamed, and Bareford.
Performed data analysis: Cruickshanks and Dent.
Wrote or contributed to the writing of the manuscript: Cruickshanks and Dent.
References
- Alva AS, Gultekin SH, Baehrecke EH. (2004) Autophagy in human tumors: cell survival or death? Cell Death Differ 11:1046–1048 [DOI] [PubMed] [Google Scholar]
- Awada A, Saliba W, Bozovic-Spasojevic I. (2011) Lapatinib ditosylate: expanding therapeutic options for receptor tyrosine-protein kinase erbB-2-positive breast cancer. Drugs Today (Barc) 47:335–345 [DOI] [PubMed] [Google Scholar]
- Berezowska S, Schlegel J. (2011) Targeting ErbB receptors in high-grade glioma. Curr Pharm Des 17:2468–2487 [DOI] [PubMed] [Google Scholar]
- Bigner SH, Vogelstein B. (1990) Cytogenetics and molecular genetics of malignant gliomas and medulloblastoma. Brain Pathol 1:12–18 [DOI] [PubMed] [Google Scholar]
- Depowski PL, Rosenthal SI, Ross JS. (2001) Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol 14:672–676 [DOI] [PubMed] [Google Scholar]
- Duhem-Tonnelle V, Bièche I, Vacher S, Loyens A, Maurage CA, Collier F, Baroncini M, Blond S, Prevot V, Sharif A. (2010) Differential distribution of erbB receptors in human glioblastoma multiforme: expression of erbB3 in CD133-positive putative cancer stem cells. J Neuropathol Exp Neurol 69:606–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulitt PJ, Park MA, Hossein H, Cruikshanks N, Yang C, Dmitriev IP, Yacoub A, Curiel DT, Fisher PB, Dent P. (2011) Enhancing mda-7/IL-24 therapy in renal carcinoma cells by inhibiting multiple protective signaling pathways using sorafenib and by Ad.5/3 gene delivery. Cancer Biol Ther 10:1290–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, et al. (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743 [DOI] [PubMed] [Google Scholar]
- Graf MR, Jia W, Johnson RS, Dent P, Mitchell C, Loria RM. (2009) Autophagy and the functional roles of Atg5 and beclin-1 in the anti-tumor effects of 3β androstene 17α diol neuro-steriod on malignant glioma cells. J Steroid Biochem Mol Biol 115:137–145 [DOI] [PubMed] [Google Scholar]
- Hamed HA, Yacoub A, Park MA, Eulitt P, Sarkar D, Dimitrie IP, Chen CS, Grant S, Curiel DT, Fisher PB, Dent P. (2010) OSU-03012 enhances Ad.7-induced GBM cell killing via ER stress and autophagy and by decreasing expression of mitochondrial protective proteins. Cancer Biol Ther 9:526–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippert MM, O'Toole PS, Thorburn A. (2006) Autophagy in cancer: good, bad, or both? Cancer Res 66:9349–9351 [DOI] [PubMed] [Google Scholar]
- Höland K, Salm F, Arcaro A. (2011) The phosphoinositide 3-kinase signaling pathway as a therapeutic target in grade IV brain tumors. Curr Cancer Drug Targets 11:894–918 [DOI] [PubMed] [Google Scholar]
- Hynes NE, Lane HA. (2005) ERBB receptors and cancer: the complexity of targeted inhibitors [published erratum appears in Nat Rev Cancer 5:580, 2005]. Nat Rev Cancer 5:341–354 [DOI] [PubMed] [Google Scholar]
- Jia W, Loria RM, Park MA, Yacoub A, Dent P, Graf MR. (2010) The neuro-steroid, 5-androstene 3â,17á diol; induces endoplasmic reticulum stress and autophagy through PERK/eIF2á signaling in malignant glioma cells and transformed fibroblasts. Int J Biochem Cell Biol 42:2019–2029 [DOI] [PubMed] [Google Scholar]
- Kong A, Calleja V, Leboucher P, Harris A, Parker PJ, Larijani B. (2008) HER2 oncogenic function escapes EGFR tyrosine kinase inhibitors via activation of alternative HER receptors in breast cancer cells. PloS ONE 3:e2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie NR, Foti M. (2011) Non-genomic loss of PTEN function in cancer: not in my genes. Trends Pharmacol Sci 32:131–140 [DOI] [PubMed] [Google Scholar]
- Martin AP, Miller A, Emad L, Rahmani M, Walker T, Mitchell C, Hagan MP, Park MA, Yacoub A, Fisher PB, et al. (2008) Lapatinib resistance in HCT116 cells is mediated by elevated MCL-1 expression and decreased BAK activation and not by ERBB receptor kinase mutation. Mol Pharmacol 74:807–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AP, Mitchell C, Rahmani M, Nephew KP, Grant S, Dent P. (2009) Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol Ther 8:2084–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Karantza-Wadsworth V, White E. (2007) Role of autophagy in cancer. Nat Rev Cancer 7:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matallanas D, Birtwistle M, Romano D, Zebisch A, Rauch J, von Kriegsheim A, Kolch W. (2011) Raf family kinases: old dogs have learned new tricks. Genes Cancer 2:232–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrpour M, Esclatine A, Beau I, Codogno P. (2010) Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. Am J Physiol Cell Physiol 298:C776–C785 [DOI] [PubMed] [Google Scholar]
- Miller KD. (2004) The role of ErbB inhibitors in trastuzumab resistance. The Oncologist 9:16–19 [DOI] [PubMed] [Google Scholar]
- Mitchell C, Yacoub A, Hossein H, Martin AP, Bareford MD, Eulitt P, Yang C, Nephew KP, Dent P. (2010) Inhibition of MCL-1 in breast cancer cells promotes cell death in vitro and in vivo. Cancer Biol Ther 10:903–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onvani S, Etame AB, Smith CA, Rutka JT. (2010) Genetics of medulloblastoma: clues for novel therapies. Expert Rev Neurother 10:811–823 [DOI] [PubMed] [Google Scholar]
- Park MA, Reinehr R, Häussinger D, Voelkel-Johnson C, Ogretmen B, Yacoub A, Grant S, Dent P. (2010) Sorafenib activates CD95 and promotes autophagy and cell death via Src family kinases in gastrointestinal tumor cells. Mol Cancer Ther 9:2220–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Walker T, Martin AP, Allegood J, Vozhilla N, Emdad L, Sarkar D, Rahmani M, Graf M, Yacoub A, et al. (2009) MDA-7/IL-24-induced cell killing in malignant renal carcinoma cells occurs by a ceramide/CD95/PERK-dependent mechanism. Mol Cancer Ther 8:1280–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, Graf M, Rahmani M, Ryan K, Liu X, et al. (2008) Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther 7:1648–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108 [DOI] [PubMed] [Google Scholar]
- Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. (2008) Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie 90:313–323 [DOI] [PubMed] [Google Scholar]
- Robins HI, Chang S, Butowski N, Mehta M. (2007) Therapeutic advances for glioblastoma multiforme: current status and future prospects. Curr Oncol Rep 9:66–70 [DOI] [PubMed] [Google Scholar]
- Schmidt AL, Brunetto AL, Schwartsmann G, Roesler R, Abujamra AL. (2010) Recent therapeutic advances for treating medulloblastoma: focus on new molecular targets. CNS Neurol Disord Drug Targets 9:335–348 [DOI] [PubMed] [Google Scholar]
- Seminario MC, Precht P, Wersto RP, Gorospe M, Wange RL. (2003) PTEN expression in PTEN-null leukaemic T cell lines leads to reduced proliferation via slowed cell cycle progression. Oncogene 22:8195–8204 [DOI] [PubMed] [Google Scholar]
- Tao RH, Maruyama IN. (2008) All EGF(ErbB) receptors have preformed homo- and heterodimeric structures in living cells. J Cell Sci 121:3207–3217 [DOI] [PubMed] [Google Scholar]
- Taskar KS, Rudraraju V, Mittapalli RK, Samala R, Thorsheim HR, Lockman J, Gril B, Hua E, Palmieri D, Polli JW, Castellino S, Rubin SD, Lockman PR, Steeg PS, Smith QR. (2012) Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res 29:770–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft MF, Huang DC. (2009) How the BCL-2 family of proteins interact to regulate apoptosis. Cell Res 16:203–213 [DOI] [PubMed] [Google Scholar]
- Wang CW, Klionsky DJ. (2003) The molecular mechanism of autophagy. Mol Med 9:65–76 [PMC free article] [PubMed] [Google Scholar]
- Ware KE, Marshall ME, Heasley LR, Marek L, Hinz TK, Hercule P, Helfrich BA, Doebele RC, Heasley LE. (2010) Rapidly acquired resistance to EGFR tyrosine kinase inhibitors in NSCLC cell lines through de-repression of FGFR2 and FGFR3 expression. PLoS ONE 5:e14117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T, Noda T. (2008) Toward unraveling membrane biogenesis in mammalian autophagy. Curr Opin Cell Biol 20:401–407 [DOI] [PubMed] [Google Scholar]
- Zhang G, Park MA, Mitchell C, Hamed H, Rahmani M, Martin AP, Curiel DT, Yacoub A, Graf M, Lee R, et al. (2008) Vorinostat and sorafenib synergistically kill tumor cells via FLIP suppression and CD95 activation. Clin Cancer Res 14:5385–5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.