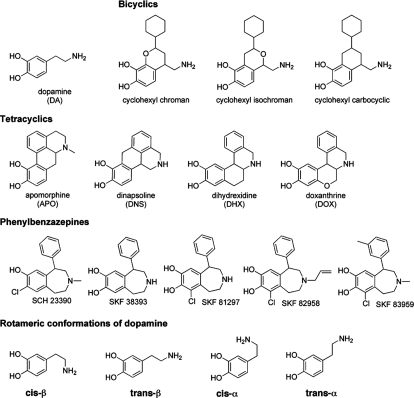
Abstract
To refine further the structure-activity relationships of D1 dopamine receptor agonists, we investigated the roles of three conserved serine residues [Ser198(5.42), Ser199(5.43), and Ser202(5.46)] in agonist binding and receptor activation. These transmembrane domain 5 (TM5) residues are believed to engage catechol ligands through polar interactions. We stably expressed wild-type or mutant (S198A, S199A, and S202A) D1 receptors in human embryonic kidney cells. These receptors were expressed at similar levels (approximately 2000 fmol/mg) and bound the radioligand [3H]R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SCH 23390), although S198A and S199A displayed significant losses of affinity compared with that for wild-type receptors. The endogenous agonist, dopamine, had losses of potency at each of the mutant receptors. We tested cyclohexyl-substituted isochroman, carbocyclic, and chroman bicyclic dopamine analogs and found that the mutations affected the chroman to a lesser extent than the other compounds. These results support our hypothesis that the decreased D1 activity of chroman analogs results from a ligand intramolecular hydrogen bond that impairs the ability of the catechol to engage the receptor. Sensitivities of these rigid catechol agonists to the effects of the serine mutations were dependent on ligand geometry, particularly with respect to the rotameric conformation of the ethylamine side chain and the distance between the amino group and each catechol hydroxyl. Functional experiments in striatal tissue suggest that the ability to engage TM5 serines is largely correlated with agonist efficacy for cAMP stimulation. These results provide a new understanding of the complexities of D1 ligand recognition and agonist activation and have implications for the design of rigid catechol ligands.
Introduction
Dopamine (DA) is an important neurotransmitter that plays numerous roles in the central and peripheral nervous systems (Missale et al., 1998). The receptors for dopamine are members of the class A (rhodopsin-like) group of seven-transmembrane domain G protein-coupled receptors. To date, five distinct subtypes of dopamine receptors have been identified (Civelli et al., 1993). The D1-like subclass of DA receptors includes D1 and D5, which couple to Gαs and stimulate the production of cAMP through the activation of adenylyl cyclases (Clark and White, 1987). The D2-like receptors, D2, D3, and D4, couple to Gαi, thereby inhibiting the production of cAMP (Neve et al., 2004). Of the five receptor subtypes, D1 and D2 have arguably received the most scientific attention.
Dopamine has been implicated in a number of neuropsychiatric conditions including addiction, schizophrenia, Parkinson's disease, and attention-deficit hyperactivity disorder (Kienast and Heinz, 2006). Deficient D1 receptor expression or signaling is thought to be an important component of the pathology of cognitive deficits and motor dysfunctions associated with aging, Huntington's disease, Alzheimer's disease, and Parkinson's disease. Thus, understanding the molecular requirements of D1 receptor binding and activation may aid in the development of novel therapeutics for these disorders.
Early attempts to define the topography of monoamine neurotransmitter binding pockets used site-directed mutagenesis to probe adrenergic receptors (Strader et al., 1989; Wang et al., 1991; Liapakis et al., 2000). Those studies demonstrated that the primary ligand contact sites are in the third and fifth transmembrane domains (TM3 and TM5). In particular, Asp3.32 in TM3 is important for coordinating the amino functionality, and serine residues in TM5 interact with the catechol moiety. Strader et al. (1989) demonstrated that Ser5.43 and Ser5.46 of the β2-adrenergic receptor interact with the meta- and para-hydroxyl groups of catecholamine ligands, respectively. It was later demonstrated by Liapakis et al. (2000) that the m-OH also interacts with Ser5.42, possibly in a bifurcated fashion.
Previous mutagenesis studies exploring the TM5 serines in D1 receptors have been somewhat limited in their choice of ligands (Pollock et al., 1992; Tomic et al., 1993; O'Dowd et al., 2005). Pollock et al. (1992) individually mutated Ser198(5.42), Ser199(5.43), and Ser202(5.46) to alanine and examined the effects on ligand binding and potency. That study, however, used relatively few test ligands and found no detectable radioligand binding with S198A. They concluded that S202A has profound effects on the affinity and potency of dopamine and little to no effect on the phenylbenzazepines [R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SCH 23390), (±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol (SKF 38393), and (±)-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SKF 82958)]. In contrast, S199A adversely affected the affinity and potency of all test compounds. Despite the lack of demonstrable radioligand binding by the S198A mutant, Northern blot analysis revealed that it was expressed at levels similar to those of the other mutant receptors. Furthermore, functional assays (cAMP accumulation) demonstrated that S198A was functional but had profoundly disrupted ligand potency. Tomic et al. (1993) created the S199V/S202A double mutant, which drastically decreased the affinity of dopamine and, to a lesser extent, of SCH 23390. Finally, O'Dowd et al. (2005) used the S198A/S199A double mutant, which bound (+)-butaclamol, but not SCH 23390 or dopamine, to study dopamine receptor oligomerization.
The aim of the present study was to broaden these earlier studies by using many structurally diverse agonist ligands to provide a greater understanding of the molecular interactions of the TM5 serines of D1 receptors. The ligands used for this study are illustrated in Fig. 1. We stably expressed wild-type and mutant D1 dopamine receptors in human embryonic kidney (HEK) cells and used competitive binding and cAMP accumulation assays to evaluate the effects of the S198A, S199A, and S202A mutations on agonist affinity and potency. We found that the effects of these mutations were structure-specific, suggesting that the engagement of these residues in the wild-type receptor is determined by ligand structure. Furthermore, measurements of agonist efficacy for striatal D1-like receptors suggest that the trans-β conformation of the ethylamine side chain is optimal for full efficacy and that inability of the catechol moiety to engage one or more TM5 serine residues may result in partial agonism. The results of this study demonstrate that these TM5 serine residues of the D1 dopamine receptor play critical, ligand-specific roles in agonist binding and receptor activation.
Fig. 1.
Structures of D1 dopamine receptor ligands used in this study.
Materials and Methods
Materials.
[3H]SCH 23390 was purchased from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK) and PerkinElmer Life and Analytical Sciences (Waltham, MA). [3H]cAMP, [3H]methylspiperone, and MicroScint-O were purchased from PerkinElmer Life and Analytical Science. (±)-SKF 38393 HCl, (±)-SKF 82958 HBr, R-(+)-SCH 23390 HCl, (+)-butaclamol HCl, R-(−)-apomorphine, ketanserin tartrate, and dopamine HCl were purchased from Sigma-Aldrich (St. Louis, MO). (±)-6-Chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine) [(±)-SKF 81297 HBr] and 6-chloro-7,8-dihydroxy-3-methyl-1-(3-methylphenyl)-2,3,4,5-tetrahydro-1H-3-benzazepine [(±)-SKF 83959 HBr] were purchased from Tocris Bioscience (Ellisville, MO). All isochroman compounds (as racemic HCl salts) were kindly provided by Abbott Laboratories (Abbott Park, IL). All other test compounds (as racemates) were synthesized in our own laboratory and verified for identity and purity by thin-layer chromatography, melting point, NMR, mass spectrometry, and elemental analyses. All compounds synthesized by our laboratory were prepared as racemic HCl salts. Bovine calf serum and fetal clone 1 serum were obtained from VWR (West Chester, PA). Unless otherwise noted, cell culture reagents, including media and antibiotics, were purchased from Invitrogen (Carlsbad, CA). All restriction and polymerase enzymes were obtained from New England Biolabs (Ipswich, MA). BCA Protein Assay kits were purchased from Thermo Fisher Scientific (Waltham, MA), and 96-well, glass fiber MultiScreen Harvest APFB plates were obtained from Millipore Corporation (Billerica, MA).
Creation of D1 Mutants.
Wild-type human D1 cDNA in the pcDNA3.1/V5-His TOPO vector (Invitrogen) was obtained from Dr. Bryan Roth (University of North Carolina, Chapel Hill, NC). XL1-Blue competent cells (Stratagene, La Jolla, CA) and the QIAprep Spin Miniprep and Midiprep Kits (QIAGEN, Valencia, CA) were used to transform, amplify, and isolate DNA. Mutagenesis was planned using vector NTI 9 (Invitrogen) and performed using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). The following primers (and corresponding antisense primers) were used according to the QuikChange protocol to generate the mutants (Integrated DNA Technologies, Inc., Coralville, IA): S198(5.42)A, CCTCAGCAGGACCTATGCCATCTCAGCCTCTGTAATAAGC; S199(5.43)A, CCTCAGCAGGACCTATGCCATCTCATCCGCTGTAATAAGC; and S202(5.46)A, CCATCTCATCCTCTGTAATAGCCTTTTACATCCCTGTGGC.
The accuracy of mutant cDNA was validated by sequencing by the Purdue University DNA Sequencing Low Throughput Laboratory (West Lafayette, IN) using the T7 and BGH reverse primers.
Cell Culture and Creation of Pooled Cell Lines.
HEK293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 5% fetal clone serum, 5% bovine calf serum, 0.05 μg/ml penicillin, 50 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. Cells were grown in a humidified incubator with 5% CO2. HEK cells were stably transfected by combining 3 μg of pcDNA3.1/V5-His TOPO hD1 (WT or mutant) with 15 μl of Lipofectamine 2000 (Invitrogen) in OptiMEM I medium according to the manufacturer's protocol. This mixture was added dropwise to 10-cm2 tissue culture plates containing ∼70% confluent HEK cells. Twenty-four hours later, these cells were split into new 10-cm2 plates at various seeding densities. On the following day and every 3 days thereafter, the medium was replaced with fresh selection medium containing 600 μg/ml G418. After approximately 4 weeks, when colonies were visible to the naked eye, the entire plate was resuspended and transferred to a new 10-cm2 plate. These plates were grown to 90% confluence in maintenance medium containing 300 μg/ml G418 and further split into additional plates to enable receptor evaluation.
Membrane Preparation.
HEK cells expressing either WT or mutant D1 receptors were grown to confluence in 15-cm2 plates. Membrane pellets for radioligand binding assays were prepared as described previously (Chemel et al., 2006). In brief, medium was decanted, and 10 ml of ice-cold lysis buffer (1 mM HEPES, pH 7.4, and 2 mM EDTA) was added. After 10 min, cells were scraped and centrifuged at 30,000g and 4°C for 20 min. The supernatant was discarded, and the pellet was resuspended by mechanical homogenization in 4 ml/15-cm2 plate receptor binding buffer (50 mM Tris-HCl, pH 7.4, and 4 mM MgCl2). Then 1-ml aliquots were transferred to prechilled microcentrifuge tubes and centrifuged at 13,000g for 10 min, followed by aspiration of the supernatant. These pellets were frozen at −80°C until use.
Radioligand Saturation Binding.
Membrane preparation pellets were resuspended by trituration and mechanical homogenization in receptor binding buffer (approximately 50 μg of protein/100 μl) and added in duplicate to assay tubes containing 0.2 to 5.0 nM [3H]SCH 23390 and either buffer (total binding) or 5 μM (+)-butaclamol (nonspecific binding) in a total volume of 500 μl. Assay tubes were incubated at 37°C for 30 min before termination by harvesting by filtration (MultiScreen Harvest APFB plates) using a 96-well Packard FilterMate cell harvester (PerkinElmer Life and Analytical Sciences). After addition of 10 μl of each radioligand concentration in duplicate to empty wells to determine accurately the total radioligand added, filter plates were dried overnight. After addition of 30 μl of Packard MicroScint-O scintillation fluid to each well, a Packard TopCount scintillation counter (PerkinElmer Life and Analytical Sciences) was used to determine counts per minute per well. The actual protein concentration for resuspended membranes was calculated using the BCA Protein Assay kit (Thermo Fisher Scientific). These values were used to calculate and plot specific binding (femtomoles per milligram) versus free radioligand concentration.
Homologous Competition (Cold Saturation) Binding.
Traditional radioligand saturation binding experiments could not be used to generate affinity (Kd) and expression levels (Bmax) for the S198A and S199A cell lines because of to the dramatic loss of radioligand affinity at these mutant receptors. Therefore, we used homologous competition (cold saturation) binding assays that use only one concentration of radioligand and enable the practical determination of Kd and Bmax when the radioligand is expensive, is in short supply, or lacks high affinity. Cells were grown and membranes were prepared as described for radioligand saturation binding. [3H]SCH 23390 at 2 to 3 nM was added to each well, and nine concentrations of nonradioactive SCH 23390 (10 pM–10 μM) were added in duplicate to a total volume of 250 μl. Total binding was defined in the absence of competing ligand, and nonspecific binding was defined by the addition of 5 μM (+)-butaclamol. Assays were incubated at 37°C for 30 min before harvesting and scintillation counting as described above for radioligand saturation assays.
Heterologous Competition Binding.
Heterologous competition binding assays were performed to estimate the binding affinity (Ki) values of test compounds in essentially the same manner as that described for homologous binding. Nine concentrations of test compounds, ranging from 1 pM to 100 μM, were added in duplicate to wells containing approximately 1 to 3 nM [3H]SCH 23390. Drugs were evaluated at mutant and wild-type receptors in parallel to facilitate normalization.
HEK cAMP Stimulation Assays.
When cells reached 100% confluence in 48-well plates, growth medium was decanted, and plates were placed on ice. Ten concentrations of test compounds were made in Earle's balanced salt solution buffer (Earle's balanced salt solution with 2% bovine calf serum, 0.025% ascorbic acid, and 15 mM HEPES, pH 7.4) and added in duplicate to a total volume of 200 μl in the presence of 500 μM 3-isobutyl-1-methylxanthine. To facilitate normalization, mutant receptors were assayed in parallel with wild-type, and wells containing vehicle (basal) and 100 μM dopamine were included alongside each test drug as controls. Assays were incubated for 15 min at 37°C in a water bath and were terminated by decanting followed by the addition of 100 μl of ice-cold 3% trichloroacetic acid on ice. Plates were stored at 4°C for at least 1 h before quantification of cAMP.
Striatal cAMP Stimulation Assays.
The striatal adenylate cyclase assay was performed as described previously (Przybyla et al., 2009). Assays were performed in 96-well assay tubes containing (final concentration) reaction buffer (5 mM MgCl2, 2 mM EDTA, 1 mM 3-isobutyl-1-methylxanthine, 0.01% ascorbic acid, 10 μM pargyline, and 15 mM HEPES, pH 7.4), reaction mix [1.25 mM ATP, 21.5 mM N-[imino(phosphonoamino)methyl]-N-methylglycine disodium salt (phosphocreatine), and 3 U of creatine phosphokinase], 1 μM guanosine 5′-(β,γ-imido)triphosphate, 30 μg of striatal protein, and the indicated drugs (10 μM) in a total volume of 100 μl. Propranolol and prazosin (1 μM each) were included to block adrenergic receptors. Triplicate samples for each treatment were incubated in a 30°C water bath for 15 min. Adenylate cyclase activity was terminated by the addition of 200 μl of 3% trichloroacetic acid. The reaction tubes were covered with Parafilm and stored at 4°C for at least 1 h before the concentration of cAMP was quantified.
cAMP Quantification.
A previously described protocol was followed to quantify levels of cAMP production in each well (Watts and Neve, 1996). In brief, 10 to 15 μl of lysate was added in duplicate to assay tubes with cAMP binding buffer (100 mM Tris-HCl, pH 7.4, 100 mM NaCl, and 5 mM EDTA) containing 1 nM [3H]cAMP and 100 μg of bovine cAMP binding protein in a total volume of 500 μl. Assays were incubated at 4°C for 2 to 3 h and were harvested and counted by scintillation as described above. The concentration of cAMP in each sample was estimated from a standard curve ranging from 0.01 to 300 pmol of cAMP.
Molecular Modeling.
Molecules were built and minimized using the software package Spartan '06 (Wavefunction, Inc., Irvine, CA). All molecules were minimized as their protonated forms in a vacuum, using AM1 semiempirical potential functions. If two ring conformations were possible, those were built manually and minimized, and the lowest energy final conformation was used. Minimized structures were overlaid, manually aligned, and measured using MacPyMol (DeLano Scientific, San Carlos, CA).
Data Analysis.
GraphPad Prism 4.0 was used to generate curves for saturation, competition, and cAMP experiments. Data from cAMP accumulation assays were normalized to percent maximum dopamine stimulation (100 μM) at each receptor and graphed using sigmoidal dose-response curves with a Hill slope fixed to unity to generate EC50 and intrinsic activity (percentage of maximum DA stimulation) values. Emax and basal values of cAMP accumulation were generated from the tops and bottoms, respectively, of fixed Hill slope sigmoidal dose-response curves of raw dopamine-stimulated cAMP values as defined by Prism. Within each striatal cyclase assay, cAMP levels produced in response to each drug (10 μM) were normalized to percentage of stimulation by 10 μM dopamine over vehicle levels (1 μM guanosine 5′-(β,γ-imido)triphosphate alone).
Saturation binding experiments were analyzed using a one-site binding (hyperbola) model to generate values for Kd and Bmax. For homologous competition binding assays, IC50 values, as well as top and bottom values, were determined from one-site, variable slope sigmoidal dose-response curves. Kd values were calculated as follows: Kd = IC50 − [radioligand]. Bmax values were determined as follows: Bmax = (top − bottom)/([radioligand]/(Kd − [radioligand])). Bmax values were then converted from counts per minute to picomoles per milligram. Competition binding experiments were analyzed using variable slope, one-site sigmoidal curves to calculate Ki values from IC50 values using the Cheng-Prusoff equation (Cheng and Prusoff, 1973). The Hill slopes (not reported) for the agonists evaluated were mostly <1 at each of the receptors, but these data were not reliably resolved by a two-site model. Therefore, the Ki values reported herein reflect contributions of high- and low-affinity states. When necessary (for low-affinity compounds), the bottom limits of curves were constrained to average nonspecific binding values.
Prism also was used to perform one-way ANOVA calculations with Dunnett's post-tests comparing mutant with wild-type values. The significance threshold was p < 0.05. Within individual competitive binding and cAMP accumulation experiments, changes in affinity and potency values were calculated for each mutant relative to that for the wild type. To aid visualization, mutation-induced changes in binding affinities (Ki) were expressed as changes in the standard Gibbs free energy (ΔΔGo), calculated from Ki values as follows: ΔΔGo = ΔGomutant − ΔGoWT = −RT ln(Ki-mutant/Ki-WT), where R is the gas constant and T is absolute temperature. To enable statistical analysis, changes in −log Ki were calculated for each mutant relative to that for the wild type for independent experiments performed in parallel as follows: ΔpKi = pKi-mutant − pKi-WT = −log Ki-mutant − (−log Ki-WT). Changes in potency were transformed by calculating the differences of the log EC50 values for independent experiments performed in parallel as follows: ΔpEC50 = pEC50-mutant − pEC50-WT = −log EC50-mutant − (−log EC50-WT). ΔΔGo, ΔpKi, and ΔpEC50 values, calculated from the corresponding affinity or potency values of each replicate experiment, were used to generate the mean and S.E.M. values displayed in the figures.
Results
Characterization of Cell Lines.
D1 WT, S198A, S199A, and S202A stable cell lines were constructed as described under Materials and Methods section. Radioligand saturation assays were performed on these cell lines to evaluate their receptor expression (Bmax) levels and their affinities for [3H]SCH 23390 (Table 1). The wild-type D1 cell line displayed saturable radioligand binding with mean values of 1.2 nM and 1840 fmol/mg for Kd and Bmax, respectively. The S202A mutant exhibited values for radioligand affinity and expression that were very similar to those for the wild type (1.1 nM and 1890 fmol/mg, respectively). In contrast, despite the substantial specific binding displayed by the S198A and S199A mutants, we were unable to generate affinity and expression data using radioligand saturation analysis because the specific binding was not saturable. Thus, we used homologous competitive binding (cold saturation binding) to measure these values. Table 1 demonstrates that SCH 23390 possessed significantly lower affinity for S198A and S199A (52 and 28 nM, respectively). The use of this approach was supported by the observations that the Kd/Ki values generated by homologous competition experiments for wild-type and S202A were identical to those generated through radioligand saturation binding (data not shown). These experiments confirmed that all four cell lines express similar receptor levels (1600–2000 fmol/mg).
TABLE 1.
Characterization of radioligand affinity and expression for human D1 WT, S198A, S199A, and S202A cell lines
Experiments were performed with [3H]SCH 23390 at D1 receptors stably expressed in HEK cells. Values for Kd and Bmax are expressed as means ± S.E.M. as calculated from at least seven independent experiments.
| Cell Line | Kd | Bmax |
|---|---|---|
| nM | fmol/mg | |
| HEK hD1 WTa | 1.2 ± 0.2 | 1840 ± 120 |
| HEK hD1 S198Ab | 51.6 ± 7.8** | 1610 ± 350 |
| HEK hD1 S199Ab | 27.5 ± 4.1** | 1990 ± 160 |
| HEK hD1 S202Aa | 1.1 ± 0.1 | 1890 ± 150 |
Significantly different from wild-type (p < 0.01, one-way ANOVA with Dunnett's post-test).
Generated by radioligand saturation binding.
Generated by homologous competition binding.
The functional properties of the D1 receptors were evaluated using the endogenous agonist, dopamine, by measuring cAMP accumulation in response to D1-stimulated Gαs activation of adenylyl cyclase (Table 2). Dopamine dose dependently increased cAMP accumulation in each cell line but not in mock-transfected cells (data not shown). The EC50 value for dopamine at the wild-type D1 receptor was 22 nM. In contrast, dopamine was dramatically less potent at all three mutant receptors. The S199A mutation resulted in the smallest loss of potency (∼100-fold). S198A and S202A led to greater than 300- and 500-fold losses in potency, respectively. Consistent with previous reports (Tiberi and Caron, 1994), the wild-type D1 receptor did not display appreciable levels of basal activity. Mean basal levels of cAMP for the mutant cell lines also were less than 5 pmol/well, indicating that these mutations did not lead to increased constitutive activity. In addition, the inverse agonists (+)-butaclamol, chlorpromazine, and haloperidol (Kozell et al., 1994; Cai et al., 1999) had no effect on basal levels of WT D1 receptor activity (data not shown). Dopamine receptor stimulation resulted in similar maximum levels of cAMP in the wild-type, S198A, and S199A cell lines (134, 117, and 111 pmol/well, respectively). In addition to yielding the greatest loss of potency for dopamine, S202A displayed significantly reduced levels of maximum dopamine-stimulated cAMP (55 pmol/well).
TABLE 2.
Effects of human D1 receptor TM5 serine mutations on DA-stimulated cAMP production
Dopamine dose-response curves were obtained in the presence of 500 μM IBMX. Experiments were performed in 48-well plates and cAMP levels were calculated for each well (total volume of 100 μl). Data represent means ± S.E.M. as calculated from at least six independent experiments.
| Cell Line | Basal cAMP | Dopamine |
|
|---|---|---|---|
| EC50 | Emax | ||
| pmol/well | nM | pmol/well | |
| HEK hD1 WT | 3.7 ± 0.7 | 22 ± 3.4 | 134 ± 10 |
| HEK hD1 S198A | 1.8 ± 0.6 | 7800 ± 570** | 117 ± 10 |
| HEK hD1 S199A | 1.3 ± 0.3* | 2800 ± 400** | 111 ± 5.9 |
| HEK hD1 S202A | 1.4 ± 0.4 | 12000 ± 370** | 55 ± 3.1** |
Significantly different from wild-type (p < 0.01, one-way ANOVA with Dunnett's post-test).
TM5 Serine to Alanine Mutations Differentially Disrupt the Binding of Catechol Agonists.
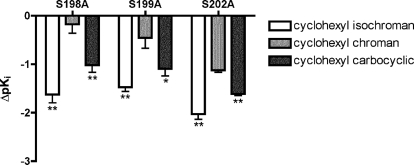
Competitive binding experiments with [3H]SCH 23390 were used to evaluate the binding affinities (Ki) of structurally diverse catechol agonists for wild-type and mutant D1 receptors (Fig. 1; Table 3). To compare the extent of affinity loss caused by each mutation on the cyclohexyl-substituted bicyclic compounds, we calculated changes in pKi values relative to that for the wild type from matched experiments (Fig. 2). These data demonstrate that each of the mutations affected the cyclohexyl-substituted isochroman and carbocyclic compounds to significantly greater extents than the cyclohexyl chroman (p < 0.05, one-way ANOVA with Dunnett's post-test).
TABLE 3.
Binding affinities of catechol agonists for wild-type human D1 and TM5 serine mutant receptors
Data represent means ± S.E.M. from at least four independent competitive binding experiments performed with [3H]SCH 23390. Ki values were calculated from IC50 values of one-site sigmoidal curves (with Hill slopes <1) as described under Materials and Methods. Statistical significance was determined from pKi values.
| Ligand |
Ki |
|||
|---|---|---|---|---|
| hD1 WT | S198A | S199A | S202A | |
| nM | ||||
| DA | 1010 ± 230 | 4700 ± 1200** | 13,000 ± 3700** | 54,400 ± 1500** |
| Cyclohexyl isochroman | 13.1 ± 2.4 | 464 ± 140** | 402 ± 70** | 1200 ± 91** |
| Cyclohexyl carbocylic | 183 ± 13 | 2800 ± 690** | 3240 ± 690** | 7610 ± 620** |
| Cyclohexyl chroman | 2110 ± 140 | 5210 ± 1400* | 10,500 ± 2400** | 27,900 ± 2900** |
| Apomorphine | 274 ± 52 | 700 ± 150* | 669 ± 99* | 4780 ± 360** |
| Dinapsoline | 110 ± 16 | 689 ± 190** | 885 ± 180** | 3950 ± 420** |
| Dihydrexidine | 114 ± 11 | 1370 ± 170** | 2680 ± 470** | 3430 ± 260** |
| Doxanthrine | 238 ± 69 | 1150 ± 320* | 2660 ± 660** | 6160 ± 400** |
| SKF 38393 | 290 ± 28 | 2140 ± 600** | 1440 ± 310** | 860 ± 110* |
| SKF 81297 | 18.8 ± 3.6 | 213 ± 50** | 365 ± 29** | 68.5 ± 12** |
| SKF 82958 | 9.16 ± 2.7 | 390 ± 190** | 173 ± 17** | 42.2 ± 9.7* |
| SKF 83959 | 1.19 ± 0.3 | 66.6 ± 15** | 29.5 ± 11** | 5.23 ± 1.7* |
Significantly different from wild-type (p < 0.05).
Significantly different from wild-type (p < 0.01).
Fig. 2.
Relative effects of TM5 serine to alanine mutations on binding affinity of cyclohexyl-substituted bicyclic compounds. Data represent ΔpKi values (mean and S.E.M.) for each mutant relative to that for the wild type of the cyclohexyl-substituted isochroman (□), carbocyclic (▩), and chroman (■) (n = 4 matched experiments). *, p < 0.05; **, p < 0.01, significantly different from cyclohexyl chroman (one-way ANOVA with Dunnett's post-test).
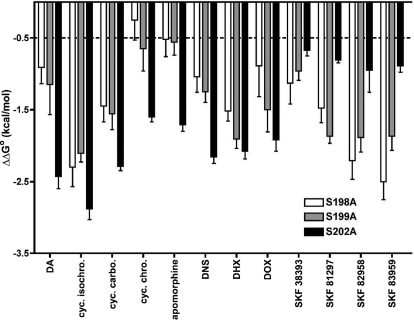
Ki values from independent experiments were converted to changes (from the wild type) in standard Gibbs free energy (ΔΔGo) of binding to illustrate graphically the effects of these mutations (Fig. 3), for which the energetic threshold for the disruption of a hydrogen bond is equivalent to ∼0.5 kcal/mol (Fersht, 1988). S202A produced the largest loss of affinity for dopamine, the cyclohexyl-substituted bicyclic (isochroman, chroman, and carbocyclic), and the tetracyclic [apomorphine, dinapsoline, dihydrexidine (DHX), and doxanthrine] compounds (magnitude of affinity loss: S198A ≤ S199A < S202A). The cyclohexyl chroman and apomorphine were somewhat unique in that they were relatively weakly affected by S198A and S199A. Furthermore, the change in affinity of the cyclohexyl chroman caused by S198A did not exceed the energetic threshold of a disrupted hydrogen bond. Although S202A caused the greatest relative losses of affinity for the δ-cyclohexyl and tetracyclic compounds, this mutation caused comparatively minor reductions in affinities for the phenylbenzazepine agonists SKF 38393, SKF 81297, SKF 82958, and SKF 83959. The effects of S202A on these compounds were substantially less than those caused by S198A and S199A (S202A < S199A ≈ S198A), but all were above the threshold for the loss of a hydrogen bond.
Fig. 3.
Effects of D1 receptor TM5 serine mutations on catechol agonist binding affinity. ΔΔGo values for the S198A (□), S199A (▩), and S202A (■) D1 receptor mutants relative to that for wild-type receptors were calculated from mutant and wild-type Ki values generated in parallel (see Materials and Methods). Negative values indicate detrimental effects on affinity. The dashed line illustrates the lower energetic limit corresponding to the loss of a hydrogen bond (Fersht, 1988). Data represent the mean and S.E.M for at least three matched experiments. The corresponding pKi values were all significantly different from wild-type values (p < 0.05; one-way ANOVA with Dunnett's post-test) (Table 4). cyc. isochrom, cyclohexyl isochroman; cyc. carbo., cyclohexyl chroman; cyc. chro., cyclohexyl chroman; DNS, dinapsoline; DHX, dihydrexidine; DOX, doxanthrine.
TM5 Serine to Alanine Mutations Differentially Disrupt the Functional Properties of Catechol Agonists.
Table 4 lists the results of cAMP functional assays performed on wild-type and mutant cell lines with structurally diverse catechol D1 agonists. SKF 38393 and SKF 83959 displayed partial agonism at wild-type D1 receptors. The relatively high intrinsic activity of SKF 38393, which is a well known partial agonist at D1 receptors (Andersen and Jansen, 1990), probably reflects receptor reserve as a result of high levels of receptor expression (Watts et al., 1995). Of interest, each serine mutation reduced the intrinsic activity of this partial agonist. The intrinsic activities of the other test ligands were not drastically altered by S199A, and many were modestly enhanced by S198A. S202A, however, produced ligand-dependent effects on intrinsic activity. Although S202A significantly reduced the maximum levels of cAMP produced by dopamine (Table 2), a number of compounds exhibited very high levels of intrinsic activity (>200%). This result probably reflects the reduced efficacy of dopamine but highlights the fact that the cyclohexyl isochroman, SKF 81297, and SKF 82958 were resistant to the negative impact of this mutant on efficacy.
TABLE 4.
Functional properties of catechol agonists for wild-type human D1 and TM5 serine mutant receptors
Data represent means ± S.E.M. of potency (EC50) and intrinsic activity (normalized to percentage of 100 μM dopamine) in response to D1 receptor-stimulated cAMP accumulation (n ≥ 3). Statistical significance was determined from pEC50 values. The dopamine EC50 values are taken from Table 2.
| Ligand | hD1 WT |
S198A |
S199A |
S202A |
||||
|---|---|---|---|---|---|---|---|---|
| EC50 | IA | EC50 | IA | EC50 | IA | EC50 | IA | |
| nM | % DA | nM | % DA | nM | % DA | nM | % DA | |
| DAa | 22 ± 3.4 | 100 | 7800 ± 570** | 100 | 2800 ± 400** | 100 | 12,000 ± 370** | 100 |
| Cyclohexyl isochroman | 1.6 ± 0.1 | 114 ± 4.2 | 430 ± 88** | 157 ± 5.7 | 75 ± 12** | 119 ± 6.9 | 570 ± 74** | 231 ± 14 |
| Cyclohexyl carbocyclic | 120 ± 7.4 | 108 ± 5.2 | 14,000 ± 1500** | 149 ± 15 | 7900 ± 730** | 132 ± 6.7 | 17,000 ± 1700** | 175 ± 9.8 |
| Cyclohexyl chroman | 820 ± 110 | 125 ± 2.9 | 49,000 ± 7500** | 76 ± 5.5 | 12,000 ± 1900** | 124 ± 5.3 | 34,000 ± 3900** | 48 ± 1.1 |
| Apomorphine | 70 ± 9.4 | 110 ± 6.1 | 14,000 ± 810** | 78 ± 4.2 | 1200 ± 310** | 124 ± 6.4 | 14,000 ± 2200** | 108 ± 8.1 |
| Dinapsoline | 6.3 ± 1.0 | 113 ± 3.6 | 1600 ± 130** | 132 ± 9.7 | 190 ± 34** | 132 ± 10 | 3000 ± 400** | 145 ± 5.9 |
| Dihydrexidine | 5.2 ± 0.8 | 104 ± 2.9 | 940 ± 106** | 132 ± 10 | 240 ± 18** | 109 ± 12 | 750 ± 140** | 101 ± 3.3 |
| Doxanthrine | 6.4 ± 1.2 | 101 ± 3.4 | 470 ± 80** | 125 ± 16 | 240 ± 49** | 101 ± 4.2 | 240 ± 33** | 95 ± 4.5 |
| SKF 38393 | 38 ± 3.1 | 92 ± 3.5 | 2600 ± 340** | 58 ± 3.3 | 1000 ± 190** | 51 ± 1.7 | 180 ± 6.7** | 62 ± 3.5 |
| SKF 81297 | 2.1 ± 0.4 | 107 ± 5.9 | 480 ± 29** | 139 ± 5.4 | 64 ± 9.2** | 122 ± 1.9 | 7.6 ± 1.2** | 223 ± 16 |
| SKF 82958 | 2.6 ± 0.9 | 115 ± 1.3 | 200 ± 35** | 137 ± 2.8 | 63 ± 3.4** | 123 ± 7.4 | 11 ± 2.9** | 211 ± 8.8 |
| SKF 83959 | 1.8 ± 0.2 | 82 ± 3.5 | 130 ± 4.3** | 90 ± 4.1 | 25 ± 0.5** | 74 ± 6.6 | 6.5 ± 2.4** | 103 ± 4.9 |
Significantly different from wild-type (p < 0.05).
Significantly different from wild-type (p < 0.01).
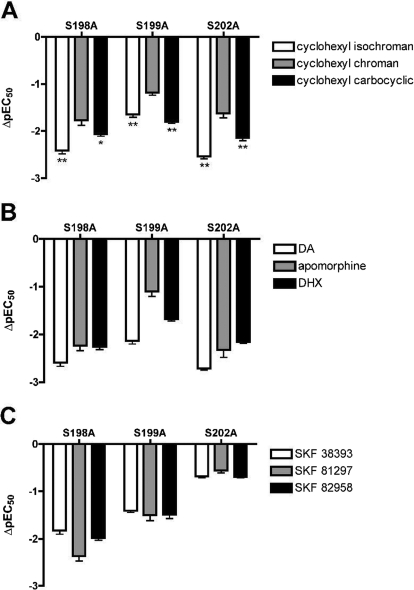
The EC50 values reported in Table 4 for each compound at the mutant receptors were significantly different from wild-type receptors (p < 0.05, one-way ANOVA with Dunnett's post-test). Changes in pEC50 values, relative to those for the wild type, were calculated from independent matched experiments to illustrate the effects of each mutant (Fig. 4). For most compounds, the disruption of potency caused by each serine mutation was similar to the effect on binding affinity, with a few noted exceptions. Of interest, S198A caused a relatively greater disruption of the potencies for these compounds than it did their affinities. The cyclohexyl-substituted bicyclics and the tetracyclic compounds displayed similar trends in ΔpEC50 values (Fig. 4, A and B). Like dopamine, they were less affected by S199A than by S198A and S202A (magnitude of potency loss: S199A < S198A ≈ S202A). Similar to the results obtained from binding assays, we demonstrated that each mutant disrupted the potency of the cyclohexyl chroman to a significantly lesser extent than the isochroman or carbocyclic compounds (Fig. 4A). The phenylbenzazepine agonists exhibited only minor potency losses in the S202A cell line (Fig. 4C). In contrast to the binding results, S198A caused a greater loss of potency for these compounds than S199A (S202A < S199A < S198A).
Fig. 4.
Effects of TM5 serine mutations on potencies of catechol agonists. ΔpEC50 values of cAMP accumulation for the S198A, S199A, and S202A D1 receptor mutants relative to that for the wild-type receptor, were calculated from independent experiments performed in parallel. Data represent means and S.E.M for at least three matched experiments. The corresponding pEC50 values were all significantly different from the wild-type value (p < 0.05; one-way ANOVA with Dunnett's post-test). A, ΔpEC50 values at each mutant relative to the wild-type value, for the cyclohexyl-substituted isochroman (□), chroman (▩), and carbocyclic (■). *, p < 0.05; **, p < 0.01 significantly different from cyclohexyl chroman (one-way ANOVA with Dunnett's post-test). B, ΔpEC50 values at each mutant relative to the wild-type value for dopamine, apomorphine, and DHX. C, ΔpEC50 values at each mutant relative to the wild-type value for three phenylbenzazepine ligands.
Intrinsic Activities of Agonists at Striatal D1 Dopamine Receptors.
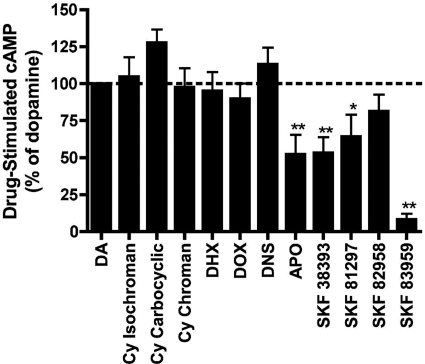
Partial agonists often behave as full agonists in recombinant cell lines with high levels of receptor expression that are due to receptor reserve (Watts et al., 1995). To provide a better understanding of the efficacies of test ligands at wild-type D1 dopamine receptors, we evaluated cAMP production in porcine striatal homogenates (Fig. 5). Saturating concentrations (10 μM) of all agonists were used, and data were normalized to percentage dopamine. These studies revealed that the bicyclic and the trans-β tetracyclic ligands were full agonists with efficacies that were statistically indistinguishable from that of dopamine. Apomorphine and the phenylbenzazepines behaved as partial agonists with varying degrees of efficacy.
Fig. 5.
D1 dopamine receptor agonist efficacy for cAMP production in porcine striatal homogenates. Data represent means and S.E. for cAMP levels produced in response to 10 μM concentrations of each test compound, normalized to dopamine (n = 6). *, p < 0.05; **, p < 0.01, significantly different from dopamine (100%) (one-way ANOVA with Dunnett's post-test). Cy, cyclohexyl; DHX, dihydrexidine; DOX, doxanthrine; DNS, dinapsoline; APO, apomorphine.
Discussion
Few studies have been performed to investigate the interactions between structurally diverse catechol agonists and serine residues S198(5.42), S199(5.43), and S202(5.46) in TM5 of D1 receptors (Pollock et al., 1992; Tomic et al., 1993; O'Dowd et al., 2005). These residues are largely conserved in catecholamine-binding G protein-coupled receptors. Early studies with adrenergic receptors suggested that these residues (S5.42, S5.43, and S5.46) are involved in important hydrogen bond interactions with the hydroxyls of catecholamine ligands (Strader et al., 1989; Liapakis et al., 2000). Because D1 receptors remain attractive, but elusive, therapeutic targets (Lewis et al., 2006; Przybyla et al., 2009; Zhang et al., 2009), exploring these molecular interactions may aid in the development of novel, subtype-selective, and bioavailable compounds.
The amino acid substitution of alanine for serine was chosen under the assumption that it ablates the potential for specific polar ligand-receptor interactions without disrupting the global protein structure (Fersht et al., 1987). Our findings gave results in agreement with the findings of Pollock et al. (1992), who reported that S199A and S198A, but not S202A, severely disrupted the affinity of [3H]SCH 23390. The S202A and wild-type cell lines displayed similar Ki values for [3H]SCH 23390 (∼1 nM), consistent with previous reports (Manik et al., 1988; Ryman-Rasmussen et al., 2007). Homologous competition binding was used to estimate the Ki and Bmax for S198A and S199A (Table 1), which produced 50- and 25-fold losses of affinity, respectively. These results strongly suggest that in the wild-type D1 receptor, the phenolic OH of SCH 23390 interacts with both Ser198 and Ser199, but, unlike catechol agonists, SCH 23390 does not engage Ser202.
These experiments were initially designed to explore the unexpected pharmacological profiles exhibited by structurally similar isochroman, chroman, and tetralin dopamine analogs (Bonner et al., 2011). Abbott Laboratories had developed bicyclic isochroman ligands with high-affinity and selectivity for D1-like receptors (DeNinno et al., 1991). A variety of hydrophobic substituents at the C3 position of the isochromans increase D1-like selectivity, presumably by interacting with the same accessory binding region that is exploited by the β-phenyl moiety that is common to many D1 receptor-selective agonists (Nichols, 2010). The active enantiomer of the adamantyl isochroman [(1R,3S)-3-(1′-adamantyl)-1-aminomethyl-3,4-dihydro-5,6-dihydroxy-1H-2-benzopyran (A-77636)] is effective in rodent and primate models of Parkinson's disease (Kebabian et al., 1992). It was later shown, however, that A-77636 rapidly induces tolerance and loses effectiveness in these models (Asin and Wirtshafter, 1993; Blanchet et al., 1996).
Encouraged by the creation of dinoxyline (Grubbs et al., 2004), an oxygen bioisostere of dinapsoline (Ghosh et al., 1996), we created the chroman analogs of the isochromans by repositioning the heterocyclic ring oxygen adjacent to the m-OH (Bonner et al., 2011). Surprisingly, this modification severely disrupted D1 receptor affinity and selectivity of the chroman compounds. To explore this effect further, we synthesized carbocyclic analogs, which demonstrated that removal of the heterocyclic oxygen largely rescued D1 affinity and selectivity. These data suggest that the poor D1 binding of the chromans is due, at least in part, to a detrimental effect of the heterocyclic oxygen atom when it is adjacent to the catechol ring. As proposed by Bonner et al. (2011), this effect is probably due to an intramolecular hydrogen bond between the chroman oxygen and the m-OH, which may limit the ability of the catechol to interact productively with the TM5 serines.
To test this hypothesis, we evaluated the potencies and affinities of the cyclohexyl-substituted compounds from each of the three bicyclic series at the TM5 serine to alanine mutant D1 receptors, assuming that negative effects reflect the loss of favorable interactions between the ligand and receptor. The EC50 and Ki values of these compounds in the WT and mutant receptor cell lines paralleled their D1-like binding affinities in native tissues (isochroman < carbocyclic < chroman). Comparing the magnitudes of mutation-induced changes in binding affinity (ΔpKi) and potency (ΔpEC50) for each compound revealed that the chroman was the ligand least affected at each mutant (Figs. 2 and 4A, respectively).
The small changes in Gibbs standard free energy of binding (≤0.5 kcal/mol) for the chroman at S198A and S199A are consistent with weak or nonexistent hydrogen bond interactions between its m-OH and these serines in the native receptor (Fersht, 1988). The effect of S202A on the cyclohexyl chroman was substantially greater but was significantly less than the changes observed for the isochroman and carbocyclic compounds at this same mutant. Although we cannot completely rule out the potential contribution of solvation effects, these data support the hypothesis that the chroman oxygen disrupts the catechol interactions with critical TM5 serine residues by altering the orientation of the m-OH through an intramolecular hydrogen bond.
This finding and the loss of D1-like receptor selectivity for the chroman among the unsubstituted bicyclic compounds (Bonner et al., 2011) underscore important differences between the structural requirements of D1-like and D2-like receptors. Unlike D1 receptor ligands, many of the prototypical D2-like receptor full agonists are noncatechol (e.g., quinpirole), indicating that the catechol hydrogen bond requirements of D2 receptors are less demanding. Furthermore, the loss of D2 affinity and reciprocal increase of D1 affinity upon hydrophobic substitution of the bicyclic ligands highlights the absence of an accessory binding region in the D2 binding site.
We evaluated a number of ligands in which the ethylamine side chain is constrained into different orientations (Fig. 1). The trans-β (e.g., dihydrexidine) tetracyclic and bicyclic (e.g., isochoman) ligands behaved like dopamine at the mutant receptors (Figs. 3 and 4), which suggests that their catechol hydroxyls interact with these residues in a similar fashion and that they adopt similar orientations in the ligand binding pocket. The greater detrimental effect on affinity of S202A suggests that when S198A or S199A is individually mutated, the adjacent residue can interact with the m-OH in a compensatory fashion. In addition, S198A produced greater relative disruption of potency than affinity, suggesting that Ser198 plays a unique role in the activation of D1 receptors by these compounds. Consistent with their apparent similar modes of interaction within the D1 binding site, these ligands all behaved as full agonists in native tissues (Fig. 5).
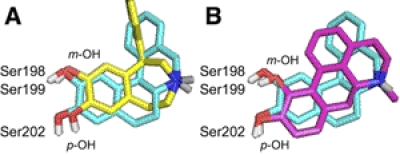
All the phenylbenzazepine agonists were similarly affected by the D1 receptor mutations (Figs. 3 and 4), but as a whole were affected differently than the bicyclic or tetracyclic ligands. Of interest, S202A produced the smallest changes in binding and potency for these ligands compared with the relatively large effects of this mutation on the nonbenzazepine agonists. This finding suggests that benzazepine ligands adopt unique orientations in the D1 receptor binding pocket, perhaps because of the constraint of their ethylamine side chain into a “cis-β-like” orientation. Analyzing the energetically preferred conformations of the benzazepine agonists revealed that, although the azepine ring is somewhat flexible, it constrains the amino group above the plane of the catechol ring. Comparison with DHX illustrates that the cis-β-like orientation of the azepine ring places the nitrogen substantially closer to the catechol ring than the trans orientation shared by most other nonbenzazepine D1 agonists (Fig. 6A). The distances between the amino group and the m- or p-O in SKF 38393 are 0.3 and 0.8 Å shorter, respectively, than in DHX. This geometry may limit the ability of these compounds to engage Asp103 and the TM5 serines simultaneously.
Fig. 6.
Comparison of the low energy conformations of catechol agonists. DHX (blue) and (A) SKF 38393 (yellow) or (B) apomorphine (pink) were manually aligned with priority for the amino proton that interacts with Asp103. Nonpolar hydrogens have been omitted for clarity. TM5 serines are included to illustrate the proposed interacting partners of the meta-OH and para-OH of the catechol moieties.
The basic dopamine pharmacophore has four important interaction points within the dopamine receptor binding site corresponding to 1) the protonated amine with Asp103, 2) the m-OH with Ser198 and Ser199, 3) the p-OH with Ser202, and 4) the catechol ring with TM6 aromatic residues. The most important interaction for an amine ligand is the salt bridge with Asp103 (Strader et al., 1988), and the relative rigidity of TM3 suggests that the protonated amine of different ligands will occupy approximately the same space when bound. Ser198/Ser199 seems to be the more important interaction for the catechol moiety because it offers the potential for more hydrogen bond interactions than Ser202 alone. This proposal is supported by a study that examined the binding affinities of monohydroxy DHX analogs (Jassen et al., 2000). The removal of the p-OH of DHX resulted in approximately 20-fold lower affinity, whereas removal of the m-OH reduced affinity by more than 200-fold.
As a result of their constrained geometry, the benzazepine ligands are unable to engage both of their catechol hydroxyl groups with TM5 serine residues and appear preferentially to engage Ser198 and Ser199 with the m-OH over Ser202 with the p-OH. When the amino groups of DHX and SKF 38393 are aligned (Fig. 6A), it is apparent that the p-OH of SKF 38393 will be substantially further away from Ser202, perhaps explaining the relatively modest detrimental effects of S202A on the phenylbenzazepine agonists.
To provide additional support for the hypothesis that ligand geometry determines the extent of engagement of TM5 serine residues by the catechol moiety, we aligned the minimized structures of apomorphine and DHX (Fig. 6B). Apomorphine was unique among the tetracyclic agonists in that S198A and S199A had only modest effects on ligand binding, which barely exceeded the threshold for hydrogen bonding (Fig. 3). The minimized structure of apomorphine demonstrates that its trans-α orientation reduces the distance between its protonated amine and m-OH. Thus, in contrast to the benzazepines, the m-OH, not the p-OH, of apomorphine is, in essence, pulled away from TM5. This increased distance could be expected to reduce the strength of the interaction of apomorphine's m-OH with S198A or S199A but not with S202A.
It has been proposed that activation of catecholamine receptors occurs sequentially and that the engagement of TM3 and TM5 by the amine and catechol moieties, respectively, of catechol agonists stabilizes the ligand-receptor complex (Swaminath et al., 2004). Once these primary contacts are established, the top of TM6 is pulled toward the ligand by interactions between aromatic residues and the catechol ring. The conformational constraints imposed by the ring systems used to rigidify the ethylamine side chains of apomorphine and the benzazepine compounds probably reduce the ability of these ligands to engage Asp103(3.32) and all three TM5 serine residues simultaneously. By reducing the stability of the ligand-receptor complex and impairing the engagement of the catechol ring with TM6 aromatic residues, these limitations may decrease the ability of these ligands to induce an active receptor conformation and may explain the partial agonism displayed by most phenylbenzazepine ligands and apomorphine (Andersen and Jansen, 1990; Watts et al., 1995). This reasoning suggests that the simultaneous engagement of Ser198/Ser199 and Ser202 is required for full D1 receptor activation. This conclusion is further supported by the fact that the efficacy of many full agonists (including dopamine and DHX) was decreased in S202A (Table 4). The partial agonist properties of the antagonist SCH 23390 (Tiberi and Caron, 1994) may be due to a relatively weak interaction of Ser202 with its p-Cl group. In addition, the apparent ability of the m-Cl to enhance the efficacy of some benzazepines (e.g., SKF 81297) may result from the ability of the chlorine atom to interact with Phe203(5.47), thereby compensating for the decreased ability of the p-OH to engage Ser202.
In addition to elucidating the molecular determinants of D1 receptor agonist activity and providing valuable empirical evidence to help refine future homology models, these studies may have direct therapeutic implications. The S199A mutation in D1 receptors has recently been identified as a naturally occurring single nucleotide polymorphism in the human population (Al-Fulaij et al., 2008). The ability of this mutation to reduce ligand binding and potency suggests that traditional D1 receptor drugs will be less effective for people with this polymorphism. The insights gained by these studies can guide the design of drugs that retain their activity at this mutant receptor, and can be used to screen novel ligands.
Acknowledgments
We thank Abbott Laboratories for the generous donation of the isochroman compounds, Dr. Uros Laban for the synthesis of the chroman compounds, and Drs. Mike Braden and Jason Parrish for valuable counsel. Dr. Bruce Craig and Jeff Li at the Purdue University Statistical Counseling Service provided helpful statistical guidance.
This work was supported by the National Institutes of Health National Institute of Mental Health [Grants MH42705, MH060397] (to D.E.N. and V.J.W., respectively) and the Purdue Research Foundation and the Department of Medicinal Chemistry and Molecular Pharmacology.
This work is part of the doctoral dissertation of B.C.: Chemel B (2010) Exploring the Molecular Determinants of Binding Selectivity and Efficacy for D1 Dopamine Receptor Agonists, Ph.D. thesis, Purdue University, West Lafayette, IN.
Parts of this work were previously presented at the following conferences: Chemel BR, Bonner LA, Watts VJ, and Nichols DE (2007) D1 versus D2 dopamine receptor selectivity is determined by intramolecular hydrogen bonding patterns in catechol-containing novel dopamine analogs (Poster 351.1). Neuroscience 2007; 2007 Nov 3–7; San Diego, CA. Society for Neuroscience, Washington, DC; Chemel BR, Bonner LA, Watts VJ, and Nichols DE (2008) D1 versus D2 dopamine receptor selectivity is determined by intramolecular hydrogen bonding patterns in catechol-containing novel dopamine analogs (Poster 1125.1). Experimental Biology 2008; 2008 Apr 5–9; San Diego, CA. Federation of the American Societies for Experimental Biology, Bethesda, MD; Chemel BR, Bonner LA, Watts VJ, and Nichols DE (2010) Ligand-specific roses for transmembrane 5 serine residues in D1 dopamine receptor binding and activation (Poster 584.2). Experimental Biology 2010, 2010 Apr 24–28; Anaheim, CA. Federation of the American Societies for Experimental Biology, Bethesda, MD.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- DA
- dopamine
- TM
- transmembrane domain
- SCH 23390
- R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- SKF 38393
- (±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol)
- SKF 82958
- (±)-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- HEK
- human embryonic kidney
- SKF 81297
- (±)-6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine)
- SKF 83959
- 6-chloro-7,8-dihydroxy-3-methyl-1-(3-methylphenyl)-2,3,4,5-tetrahydro-1H-3-benzazepine
- WT
- wild-type
- ANOVA
- analysis of variance
- DHX
- dihydrexidine
- A-77636
- (1R,3S)-3-(1′-adamantyl)-1-aminomethyl-3,4-dihydro-5,6-dihydroxy-1H-2-benzopyran.
Authorship Contributions
Participated in research design: Chemel, Watts, and Nichols.
Conducted experiments: Chemel.
Contributed new reagents or analytic tools: Bonner.
Performed data analysis: Chemel.
Wrote or contributed to the writing of the manuscript: Chemel, Bonner, Watts, and Nichols.
References
- Al-Fulaij MA, Ren Y, Beinborn M, Kopin AS. (2008) Pharmacological analysis of human D1 and D2 dopamine receptor missense variants. J Mol Neurosci 34:211–223 [DOI] [PubMed] [Google Scholar]
- Andersen PH, Jansen JA. (1990) Dopamine receptor agonists: selectivity and dopamine D1 receptor efficacy. Eur J Pharmacol 188:335–347 [DOI] [PubMed] [Google Scholar]
- Asin KE, Wirtshafter D. (1993) Effects of repeated dopamine D1 receptor stimulation on rotation and c-fos expression. Eur J Pharmacol 235:167–168 [DOI] [PubMed] [Google Scholar]
- Blanchet PJ, Grondin R, Bédard PJ, Shiosaki K, Britton DR. (1996) Dopamine D1 receptor desensitization profile in MPTP-lesioned primates. Eur J Pharmacol 309:13–20 [DOI] [PubMed] [Google Scholar]
- Bonner LA, Laban U, Chemel BR, Juncosa JI, Lill MA, Watts VJ, Nichols DE. (2011) Mapping the catechol binding site in dopamine D1 receptors: synthesis and evaluation of two parallel series of bicyclic dopamine analogues. ChemMedChem 6:1024–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Gurdal H, Smith C, Wang HY, Friedman E. (1999) Inverse agonist properties of dopaminergic antagonists at the D1A dopamine receptor: uncoupling of the D1A dopamine receptor from Gs protein. Mol Pharmacol 56:989–996 [DOI] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. (2006) WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology (Berl) 188:244–251 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Civelli O, Bunzow JR, Grandy DK. (1993) Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol 33:281–307 [DOI] [PubMed] [Google Scholar]
- Clark D, White FJ. (1987) D1 dopamine receptor—the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse 1:347–388 [DOI] [PubMed] [Google Scholar]
- DeNinno MP, Schoenleber R, Perner RJ, Lijewski L, Asin KE, Britton DR, MacKenzie R, Kebabian JW. (1991) Synthesis and dopaminergic activity of 3-substituted 1-(aminomethyl)-3,4-dihydro-5,6-dihydroxy-1H-2-benzopyrans: characterization of an auxiliary binding region in the D1 receptor. J Med Chem 34:2561–2569 [DOI] [PubMed] [Google Scholar]
- Fersht AR. (1988) Relationships between apparent binding energies measured in site-directed mutagenesis experiments and energetics of binding and catalysis. Biochemistry 27:1577–1580 [DOI] [PubMed] [Google Scholar]
- Fersht AR, Leatherbarrow RJ, Wells TN. (1987) Structure-activity relationships in engineered proteins: analysis of use of binding energy by linear free energy relationships. Biochemistry 26:6030–6038 [DOI] [PubMed] [Google Scholar]
- Ghosh D, Snyder SE, Watts VJ, Mailman RB, Nichols DE. (1996) 9-Dihydroxy-2,3,7,11b-tetrahydro-1H-naph[1,2,3-de]isoquinoline: a potent full dopamine D1 agonist containing a rigid-β-phenyldopamine pharmacophore. J Med Chem 39:549–555 [DOI] [PubMed] [Google Scholar]
- Grubbs RA, Lewis MM, Owens-Vance C, Gay EA, Jassen AK, Mailman RB, Nichols DE. (2004) 8,9-Dihydroxy-1,2,3,11b-tetrahydrochromeno[4,3,2,-de]isoquinoline (dinoxyline), a high affinity and potent agonist at all dopamine receptor isoforms. Bioorg Med Chem 12:1403–1412 [DOI] [PubMed] [Google Scholar]
- Jassen AK, Lewis MM, Miller DW, Leonard SK, Nicholas RA, Nichols DE, Tropsha A, Suzuki K, Mailman R. (2000) Molecular and pharmacological characterization of D1-like dopamine receptors reveals structural requirements for critical amino acid residues (Abstract). Soc Neurosci Abstr 27:532.9 [Google Scholar]
- Kebabian JW, Britton DR, DeNinno MP, Perner R, Smith L, Jenner P, Schoenleber R, Williams M. (1992) A-77636: a potent and selective dopamine D1 receptor agonist with antiparkinsonian activity in marmosets. Eur J Pharmacol 229:203–209 [DOI] [PubMed] [Google Scholar]
- Kienast T, Heinz A. (2006) Dopamine and the diseased brain. CNS Neurol Disord Drug Targets 5:109–131 [DOI] [PubMed] [Google Scholar]
- Kozell LB, Machida CA, Neve RL, Neve KA. (1994) Chimeric D1/D2 dopamine receptors. Distinct determinants of selective efficacy, potency, and signal transduction. J Biol Chem 269:30299–30306 [PubMed] [Google Scholar]
- Lewis MM, Huang X, Nichols DE, Mailman RB. (2006) D1 and functionally selective dopamine agonists as neuroprotective agents in Parkinson's disease. CNS Neurol Disord Drug Targets 5:345–353 [DOI] [PubMed] [Google Scholar]
- Liapakis G, Ballesteros JA, Papachristou S, Chan WC, Chen X, Javitch JA. (2000) The forgotten serine. A critical role for Ser-2035.42 in ligand binding to and activation of the β2-adrenergic receptor. J Biol Chem 275:37779–37788 [DOI] [PubMed] [Google Scholar]
- Manik CP, Molinoff PB, McGonigle P. (1988) Comparison of 125I-SCH 23982 and [3H]SCH 23390 as ligands for the D-1 dopamine receptor. J Neurochem 51:391–397 [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. (1998) Dopamine receptors: from structure to function. Physiol Rev 78:189–225 [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. (2004) Dopamine receptor signaling. J Recept Signal Transduct Res 24:165–205 [DOI] [PubMed] [Google Scholar]
- Nichols DE. (2010) Dopamine receptor subtype-selective drugs: D1-like receptors, in The Dopamine Receptors, 2nd ed (Neve KA. ed) Humana Press, New York [Google Scholar]
- O'Dowd BF, Ji X, Alijaniaram M, Rajaram RD, Kong MM, Rashid A, Nguyen T, George SR. (2005) Dopamine receptor oligomerization visualized in living cells. J Biol Chem 280:37225–37235 [DOI] [PubMed] [Google Scholar]
- Pollock NJ, Manelli AM, Hutchins CW, Steffey ME, MacKenzie RG, Frail DE. (1992) Serine mutations in transmembrane V of the dopamine D1 receptor affect ligand interactions and receptor activation. J Biol Chem 267:17780–17786 [PubMed] [Google Scholar]
- Przybyla JA, Cueva JP, Chemel BR, Hsu KJ, Riese DJ, 2nd, McCorvy JD, Chester JA, Nichols DE, Watts VJ. (2009) Comparison of the enantiomers of (+/−)-doxanthrine, a high efficacy full dopamine D1 receptor agonist, and a reversal of enantioselectivity at D1 versus α2C adrenergic receptors. Eur Neuropsychopharmacol 19:138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Griffith A, Oloff S, Vaidehi N, Brown JT, Goddard WA, 3rd, Mailman RB. (2007) Functional selectivity of dopamine D1 receptor agonists in regulating the fate of internalized receptors. Neuropharmacology 52:562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader CD, Candelore MR, Hill WS, Sigal IS, Dixon RA. (1989) Identification of two serine residues involved in agonist activation of the beta-adrenergic receptor. J Biol Chem 264:13572–13578 [PubMed] [Google Scholar]
- Strader CD, Sigal IS, Candelore MR, Rands E, Hill WS, Dixon RA. (1988) Conserved aspartic acid residues 79 and 113 of the β-adrenergic receptor have different roles in receptor function. J Biol Chem 263:10267–10271 [PubMed] [Google Scholar]
- Swaminath G, Xiang Y, Lee TW, Steenhuis J, Parnot C, Kobilka BK. (2004) Sequential binding of agonists to the β2 adrenoceptor. Kinetic evidence for intermediate conformational states. J Biol Chem 279:686–691 [DOI] [PubMed] [Google Scholar]
- Tiberi M, Caron MG. (1994) High agonist-independent activity is a distinguishing feature of the dopamine D1B receptor subtype. J Biol Chem 269:27925–27931 [PubMed] [Google Scholar]
- Tomic M, Seeman P, George SR, O'Dowd BF. (1993) Dopamine D1 receptor mutagenesis: role of amino acids in agonist and antagonist binding. Biochem Biophys Res Commun 191:1020–1027 [DOI] [PubMed] [Google Scholar]
- Wang CD, Buck MA, Fraser CM. (1991) Site-directed mutagenesis of α2A-adrenergic receptors: identification of amino acids involved in ligand binding and receptor activation by agonists. Mol Pharmacol 40:168–179 [PubMed] [Google Scholar]
- Watts VJ, Lawler CP, Gonzales AJ, Zhou QY, Civelli O, Nichols DE, Mailman RB. (1995) Spare receptors and intrinsic activity: studies with D1 dopamine receptor agonists. Synapse 21:177–187 [DOI] [PubMed] [Google Scholar]
- Watts VJ, Neve KA. (1996) Sensitization of endogenous and recombinant adenylate cyclase by activation of D2 dopamine receptors. Mol Pharmacol 50:966–976 [PubMed] [Google Scholar]
- Zhang J, Xiong B, Zhen X, Zhang A. (2009) Dopamine D1 receptor ligands: where are we now and where are we going. Med Res Rev 29:272–294 [DOI] [PubMed] [Google Scholar]