Abstract
Stress and psychiatric illness have been associated with a dysregulation of glutamatergic neurotransmission. Recently, positive allosteric modulators (PAMs) of the metabotropic glutamate 2 (mGlu2) receptor have been found to exert antidepressant-like activity in rats performing under a differential reinforcement of low rate (DRL) 72-s schedule. An autoreceptor role at glutamatergic synapses is the most salient physiological role played by the mGlu2 receptor. Adenosine A1 receptors play a heteroreceptor role at many of the same forebrain synapses where mGlu2 autoreceptors are found. Agonists and/or PAMs of mGlu2 receptors act similarly to adenosine A1 receptor agonists with respect to a wide range of electrophysiological, biochemical, and behavioral responses mediated by limbic circuitry thought to play a role in the pathophysiology of neuropsychiatric disease and to mediate therapeutic drug effects. Therefore, the role of adenosine A1 receptor activation on rat DRL 72-s behavior was explored to provide preclinical evidence consistent or inconsistent with potential antidepressant effects. The adenosine A1 receptor agonist N6-cyclohexyladenosine (CHA) increased the reinforcement rate, decreased the response rate, and induced a rightward shift in inter-response time distributions in a dose-dependent fashion similar to most known antidepressant drugs. The adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) blocked these antidepressant-like effects. These novel observations with CHA and DPCPX suggest that activation of adenosine A1 receptors could contribute to antidepressant effects, in addition to previous preclinical reports of anxiolytic and antipsychotic effects. By implication, targeting a dysregulated glutamatergic system may be an important principle in discovering novel antidepressant agents that may also possess anti-impulsive activity.
Introduction
Stress is known to play a major role in psychiatric illness. Major depression and bipolar depression are associated with increased or decreased blood flow in the medial orbital prefrontal cortex (PFC) and a number of other limbic-related brain regions, suggesting dysregulation of glutamatergic neurotransmission. Furthermore, antidepressant treatments normalize increased cortical blood flow (Price and Drevets, 2010). Metabotropic glutamate 2/3 (mGlu2/3) receptor agonism is an effective therapeutic approach for neuropsychiatric illness with divergent etiology/pathophysiology such as generalized anxiety disorder and schizophrenia (Patil et al., 2007; Dunayevich et al., 2008), disorders where stress and/or dysregulated glutamate release at key forebrain regions plays a key pathophysiological role. Activation of mGlu2 receptors plays a role in preclinical anxiety and psychosis “efficacy” models extrapolating from experiments with transgenic mice lacking mGlu2 and/or mGlu3 receptors or from comparisons of mGlu2 receptor positive allosteric modulators (PAMs) with orthosteric mGlu2/3 receptor agonists. An glutamatergic autoreceptor function is the most prominent physiological role played by mGlu2 receptors; mGlu2 receptor activation suppresses glutamate release at many synapses throughout the limbic forebrain (Schoepp, 2001). Preclinical studies with mGlu2 receptor PAMs have suggested that this novel class of drugs may possess antidepressant properties (Nikiforuk et al., 2010; Fell et al., 2011).
Although activation of mGlu2 receptors may play a therapeutic role in the treatment of schizophrenia and generalized anxiety disorder, preclinical evidence suggests that activation of mGlu2 and/or mGlu3 receptors could play a role in antinociceptive effects (including antimigraine effects), attenuation of opioid withdrawal effects, and general neuroprotective and antiepileptic effects. Preclinical evidence suggests that the activation of adenosine A1 receptors plays a similar role for this wide range of neuropsychiatric indications (Ribeiro et al., 2002; Kaster et al., 2004; Marek, 2009). The similar distribution of mGlu2 and adenosine A1 receptors throughout the limbic forebrain probably is an important underpinning to similar effects of activating these receptors across a wide range of neuropsychiatric preclinical models (Bauer et al., 2003; Richards et al., 2005). Hallucinogen-induced head shakes and hallucinogen/(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (MK-801)-induced immediate early gene expression are modulated by mGlu2 or adenosine A1 receptors in the PFC or on thalamocortical pathways (Scruggs et al., 2000; Gotoh et al., 2002; Pei et al., 2004; Benneyworth et al., 2007; Marek, 2009; Wischhof and Koch, 2012). Although adenosine A1 receptors are located at both postsynaptic and presynaptic sites, these receptors are known to play a prominent role as heteroreceptors, decreasing excitatory amino acid release, similar to the mGlu2 receptor role as an autoreceptor.
Although mGlu2/3 receptor agonist prodrugs have been demonstrated in double-blind, placebo-controlled trials to exert antipsychotic or anxiolytic action, adenosine A1 receptor agonists act similarly to many of the mGlu2/3 receptor agonists in a wide variety of the same preclinical screens detecting antipsychotic (reviewed by Marek, 2009) or anxiolytic drugs. Adenosine A1 receptor agonists possess antidepressant-like tests in mice in the forced swim test (FST) and the tail suspension test (Kaster et al., 2004). An additional reason for testing potential antidepressant activity of adenosine A1 receptor agonists is that these compounds presynaptically suppress glutamate release induced by 5-HT2A receptor activation in the PFC, similarly to activation of mGlu2 autoreceptors (Marek et al., 2000; Stutzmann et al., 2001). The DRL 72-s schedule is an antidepressant screen where 5-HT2A receptor antagonists exert frank antidepressant effects and also enhance the antidepressant-like effects of tricyclic antidepressants, selective serotonin reuptake inhibitors, and monoamine oxidase inhibitors (Marek et al., 2005; Ardayfio et al., 2008). From a clinical standpoint, blockade of 5-HT2A receptors may contribute to the antidepressant effects of tricyclic antidepressants, heterocyclic antidepressants (e.g., mianserin, mirtazapine, trazodone, and nefazodone), and atypical antipsychotics (e.g., quetiapine, risperidone, olanzapine, and aripiprazole).
Because the activation of mGlu2 receptors (Nikiforuk et al., 2010; Fell et al., 2011) and the blockade of 5-HT2A receptors results in antidepressant activity in rats performing under a DRL 72-s schedule potentially by attenuating motoric impulsivity, the effects of a selective adenosine A1 receptor agonist, N6-cyclohexyladenosine (CHA), both alone and with a selective adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX or PD116,948), were tested in rats trained to perform on a DRL 72-s schedule. The results with CHA are consistent with previous literature suggesting antidepressant-like activity with the activation of adenosine A1 receptors (Kaster et al., 2004). The present results on DRL 72-s behavior also are consistent with the working hypothesis that the mechanism underlying these antidepressant-like effects involves an attenuation of motoric impulsivity. These results add to a growing literature suggesting that modulation of glutamatergic activity may lead to the development of truly novel antidepressant and/or mood-stabilizing drugs.
Materials and Methods
Animals.
For the DRL experiments, 21 male Sprague-Dawley rats weighing between 300 and 350 g at the beginning of the behavioral experiments (Holtzman, Madison, WI) were housed in suspended stainless-steel wire cages (18 × 36 × 20 cm) with two rats occupying each cage. The colony room was maintained at 20°C and relative humidity (60%). The room was illuminated 12 h/day (7:00 AM–7:00 PM). All rats had free access to laboratory chow (Teklad 4% rat diet; Harlan Laboratories, Madison, WI) except during experimental sessions. Water was available for only a 20-min period after the daily behavioral session except for Friday after the DRL session when water was available ad libitum until approximately noon Sunday. All animals were treated in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996), and all protocols were approved by the Yale University Animal Care and Use Committee.
Apparatus.
Eight operant-conditioning chambers (30.5 × 24.1 × 29.2 cm; MED Associates, St. Albans, VT) were used for the DRL experiments. The levers in these chambers were mounted on one wall with the water access next to the levers in the middle of the wall. A reinforced response caused the dipper (0.02-cc cup) to be lifted from a water trough to an opening in the floor of the access port for 4 s. The house light (which was mounted on the opposite wall) was turned on when the session began, remained on throughout the entire session, and was turned off at the end of the session. Each experimental chamber was enclosed in a melamine sound-attenuating cubicle and equipped with a white-noise generator to provide masking noise.
Operant Training.
Rats were water-deprived for ∼22.5 h before each session. Each rat was initially trained under an alternating fixed-ratio 1, fixed-time 1-min schedule for water reinforcement. Thus, each response was reinforced, and water was also provided every minute if a response did not occur. The few rats that did not acquire lever-pressing behavior after three daily 1-h sessions under this schedule were trained by the experimenter using the method of successive approximation. After the rats had acquired lever-pressing behavior they were trained during daily DRL 18-s sessions for ∼2 weeks before moving directly to DRL 72-s sessions. The responding on these sessions became stable after ∼8 weeks. Experimental sessions lasted for 1 h and were conducted 5 days/week during light hours.
Schedule Control and Statistical Analyses.
The experimental chambers were connected to a computer via a MED-SYS-8 interface and software package (Med Associates). All behavioral data are expressed as the mean ± S.E.M., in some cases normalized to the vehicle or vehicle–vehicle condition. The DRL data analysis was performed on nontransformed data. The effects of CHA (15.6–125 μg/kg) and DPCPX (0.313 mg/kg) alone were analyzed with a one-factor repeated-measures analysis of variance. The effects of the combined treatment of CHA and DPCPX were analyzed by using a two-factor repeated-measures analysis of variance with CHA and DPCPX as within-subject factors. Significant main effects or interactions were analyzed by the Newman-Keuls or Dunnett test where appropriate. The level of significance was set for p < 0.05 for all analyses. The cumulative inter-response time (IRT) distribution for the entire group of rats treated with a within-subject design were analyzed with the nonparametric Kolmogorov-Smirnov (K-S) test to make comparisons between different treatment conditions. The level of significance was set for p < 0.05.
Drugs.
Doses were calculated on the basis of the salt forms. The drugs were dissolved in saline, neutralized to a pH ∼7.4, and injected by using a volume of 1 ml/kg body weight. The adenosine A1 receptor agonist CHA and the adenosine A1 receptor antagonist DPCPX were purchased from Sigma-Aldrich (St. Louis, MO) and Tocris Bioscience (Ellisville, MO), respectively. Drugs were administered to the animals only once weekly by an intraperitoneal route of administration 1 h before the behavioral session (Thursday). All drug treatments were carried out only once weekly to minimize possible carryover effects.
Results
The behavior of each rat seemed to return to baseline the day after drug administration.
Antidepressant-Like Actions of CHA on DRL 72-s Behavior.
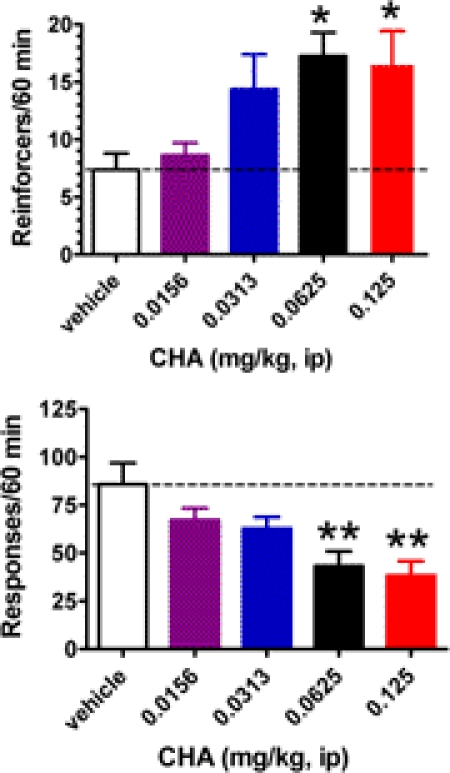
The adenosine A1 receptor agonist CHA (0.0156–0.125 mg/kg i.p.) increased the number of reinforcers earned for rats performing under a DRL 72-s operant schedule in a dose-dependent manner (F4,28 = 3.91; p < 0.05; Fig. 1). Post hoc testing revealed significant increases at the 0.0625 and 0.125 mg/kg dose conditions (Dunnett t test; p < 0.05) compared with the vehicle condition. CHA also decreased the total lever press responses in a dose-dependent manner (F4,28 = 8.27; p < 0.001) with significant decreases at the 0.0625 and 0.125 mg/kg dose conditions (Dunnett t test; p < 0.01).
Fig. 1.
Dose-dependent changes in the frequency of reinforcers and responses induced by the adenosine A1 receptor agonist CHA (0.0156–0.125 mg/kg i.p.) for rats performing under a DRL 72-s schedule (n = 8). For the vehicle condition, the mean (± S.E.M.) number of reinforcers obtained was 7.9 (± 1.49) while the mean number of total responses was 86.8 (± 11.20). Rats were injected 1 h before a 1-h behavioral session. Significant changes from the vehicle control are indicated: *, p < 0.05, and **, p < 0.01, using the Dunnett test. Dotted lines indicate graphical continuation of the vehicle control value.
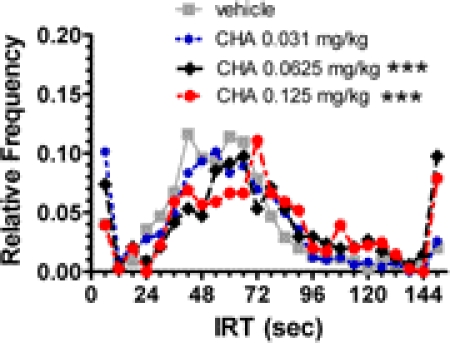
An apparent dose-dependent rightward shift in the IRT distributions was also observed after CHA administration (Fig. 2). This change was suggested by a significantly different IRT distribution for the 0.0625 and 0.125 mg/kg CHA doses compared with the vehicle condition when examining the cumulative IRT distributions for the entire group of eight rats (p < 0.001; K-S test). The changes in the temporal pattern of responding observed for the cumulative IRT distributions of the entire group were also seen when examining IRT distributions for the individual rats. Significantly different IRT distributions from the vehicle condition were observed for zero, four, and six of eight rats at the 0.0156, 0.0313, and 0.0625 mg/kg CHA doses, respectively (p < 0.001; K-S test). One rat at the 0.125 mg/kg CHA dose did not respond; five of the remaining seven rats had IRT distributions significantly different from the vehicle condition (p < 0.001). A numerical trend for a rightward shift in the mean IRT value approaching the 72-s schedule time requirement occurred after administration of the adenosine A1 receptor agonist CHA [mean IRTs (S.D.): vehicle, 57.5 (42.6) s; 0.0313 mg/kg, 56.0 (40.2) s; 0.0625 mg/kg, 81.9 (93.8) s; 0.125 mg/kg, 77.6 (62.8) s].
Fig. 2.
Effects of the adenosine A1 receptor agonist CHA (0.0313–0.125 mg/kg) on inter-response time (IRT) distributions for rats performing on a DRL 72-s schedule. Cumulative IRT for this group of rats obtaining the relative frequency of all responses for each rat separately for each dose condition. The cumulative IRT distribution for the entire group for each CHA dose was compared with the group cumulative IRT distribution for the vehicle condition by using the nonparametric Kolmogorov-Smirnov test. ***, p < 0.001.
Effects of the Adenosine A1 Receptor Antagonist DPCPX on DRL 72-s Behavior.
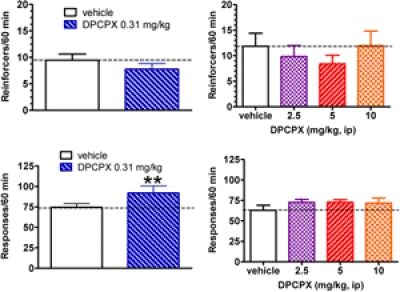
The adenosine A1 receptor antagonist DPCPX (0.31 mg/kg i.p.) alone did not alter the frequency of reinforcers obtained for rats responding on a DRL 72-s schedule compared with vehicle (t12 = 1.46; p > 0.1; Fig. 3) in a second cohort of 13 rats. However, DPCPX (0.31 mg/kg) did increase the frequency of responses compared with vehicle (t12 = 3.64; p < 0.01). The cumulative IRT distribution for the entire group of rats was not altered for the DPCPX treatment compared with the vehicle condition (p > 0.1; K-S test; data not shown). Likewise, the mean (± S.D.) IRT interval was unchanged for the DPCPX treatment (38.7 ± 26.2 s) compared with vehicle (37.6 ± 26.1 s). In a subset (n = 7) of this cohort an extended DPCPX dose range was tested (2.5, 5, and 10 mg/kg versus placebo). There was no effect of DPCPX on the number of reinforcers obtained (F3,18 = 2.22; p = 0.121), although there was a trend for an increased number in the total number of lever presses (F3,18 = 2.74; p = 0.074).
Fig. 3.
Effects of the adenosine A1 receptor antagonist DPCPX (0.31 mg/kg i.p., n = 13, graphs on left; 2.5–10 mg/kg, n = 7, graphs on right) on the number of reinforcers obtained (top) and the total responses (bottom) made by rats performing on a DRL 72-s schedule. Open bars, vehicle; hatched bars, DPCPX. **, p < 0.01 compared with the vehicle condition.
DPCPX Blocks the Antidepressant-Like Effects of CHA on DRL 72-s Behavior.
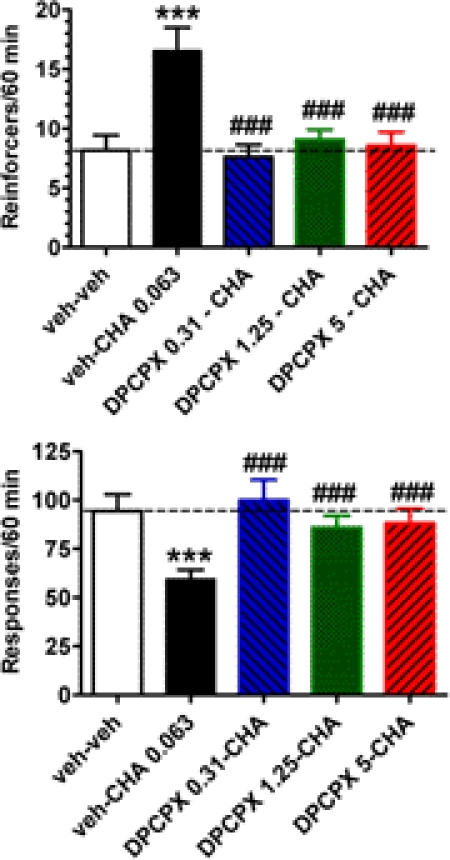
In a third experiment using the second cohort of rats, CHA (0.0625 mg/kg i.p.) increased the frequency of reinforcers obtained for rats performing under a DRL 72-s schedule, and this effect of CHA was blocked by the adenosine A1 receptor antagonist DPCPX (F4,48 = 11.58; p < 0.0001; Fig. 4). CHA significantly increased the reinforcement rate by ∼100% compared with the vehicle–vehicle condition both times the adenosine receptor agonist was administered (p < 0.001; Newman-Keuls test; second vehicle/CHA treatment not shown). In contrast, the reinforcement rate was unchanged compared with the vehicle–vehicle condition when DPCPX was coadministered with CHA at either the 0.31, 1.25, or 5 mg/kg dose. However, the number of reinforcers obtained for each dose of DPCPX administered with CHA was significantly decreased compared with the vehicle–CHA condition (p < 0.001; 0.31, 1.25, or 5 mg/kg DPCPX administered with CHA; Newman-Keuls test).
Fig. 4.
Effects of the adenosine A1 receptor antagonist DPCPX (0.31, 1.25, and 5 mg/kg) on the antidepressant-like effects of the adenosine A1 receptor agonist CHA (0.0625 mg/kg) in rats performing on a DRL 72-s schedule. Rats were tested using a with-in subject design with drugs administered at weekly intervals in the order shown (n = 13) via an intraperitoneal route of administration. Open bar, vehicle–vehicle; solid black bars, vehicle–CHA (0.0625 mg/kg); blue hatched bars, DPCPX (0.31 mg/kg)–CHA (0.0625 mg/kg).; green cross-hatched bars, DPCPX (1.25 mg/kg)–CHA (0.0625 mg/kg); red hatched bars, DPCPX (5 mg/kg)–CHA (0.0625 mg/kg). ***, p < 0.001 compared with the vehicle–vehicle condition. ###, p ≤ 0.001 compared with the vehicle–CHA (0.0625 mg/kg) condition. The significant effects of the combination of vehicle–CHA (0.0625 mg/kg) administered a second time compared with the vehicle–vehicle condition are not shown.
CHA (0.0625 mg/kg i.p.) decreased the total response frequency, and this CHA effect was blocked by the adenosine A1 receptor antagonist DPCPX (F4,48 = 11.15; p < 0.0001; Fig. 4). CHA significantly decreased the response rate by 24 to 37% compared with the vehicle–vehicle condition both times the adenosine receptor agonist was administered [p < 0.001 and < 0.01; (second administration not shown), respectively, Newman-Keuls test]. In contrast, the response rate was unchanged from the vehicle–vehicle condition when DPCPX was coadministered with CHA. However, the total number of lever press responses for each dose of DPCPX administered with CHA was significantly increased compared with the vehicle–CHA condition (p < 0.001; Newman-Keuls test).
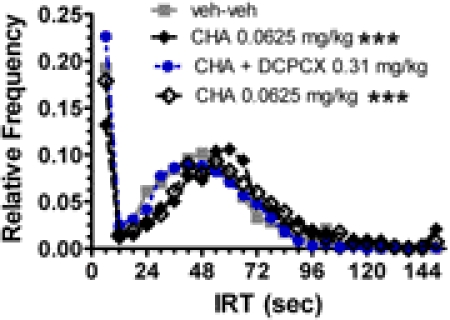
The rightward shift of the IRT distribution induced by CHA (0.0625 mg/kg) was blocked by the adenosine A1 receptor antagonist DPCPX (0.31 mg/kg) (Fig. 5). CHA induced a significant alteration in the cumulative group IRT distribution (n = 13; p < 0.001; K-S test; mean ± S.D. of the IRTs, 52.3 ± 38.5 s) compared with the vehicle condition (mean IRT ± S.D., 37.7 ± 26.1 s). In contrast, the IRT distribution was not significantly altered by administration of both CHA and DPCPX (mean IRT ± S.D., 35.7 ± 26.0 s) compared with the vehicle condition. Rechallenge with CHA (0.0625 mg/kg) again resulted in a significant shift in the cumulative group IRT distribution (p < 0.001; K-S test; mean IRT ± S.D., 47.7 ± 47.0 s) compared with the vehicle–vehicle condition.
Fig. 5.
Effects of the adenosine A1 receptor agonist CHA (0.0625 mg/kg) on IRT distributions for rats performing on a DRL 72-s schedule in both the absence and the presence of the adenosine A1 receptor antagonist DPCPX (0.31 mg/kg). Cumulative IRT distributions for the entire group of 13 rats are plotted for each of the respective dose conditions, including a replication of the CHA-alone effect. Only the two different CHA-alone treatments were significantly different from the vehicle condition. ***, p < 0.001, K-S test.
Discussion
This is the first known demonstration that the activation of adenosine A1 receptors, potentially by suppressing glutamate release in the medial PFC and associated limbic macrocircuitry, results in effects similar to antidepressant drugs by using the rat DRL 72-s schedule of reinforcement. The known pharmacological selectivity of both CHA and DPCPX for adenosine A1 receptors indicates that the effects of these drugs are mediated via activation and blockade of adenosine A1 receptors. Although CHA is only modestly selective for rat adenosine A1 compared with rat adenosine A3 receptors (>200-fold), this agonist is nearly 500-fold and more than 10,000-fold selective for rat A2A and human A2B receptors (Bruns, 1980; Daly et al., 1993; van Galen et al., 1994) Although DPCPX is only modestly selective for adenosine A1 compared with A2B receptors (∼400-fold), this antagonist is nearly 300- to 1000-fold and 10,000-fold selective compared with rodent A2A and A3 receptors, respectively (Bruns, 1980; Auchampach et al., 1997; Weyler et al., 2006). Thus, the potent antagonism exerted by DPCPX against CHA is mediated by their most potent shared site of action, adenosine A1 receptors.
Furthermore, previous in vivo experiments suggest that the doses of CHA and DPCPX chosen for the present experiments do specifically interact with adenosine A1 receptors. First, complete displacement of the binding for a different adenosine A1 receptor radiotracer was obtained with a 1 mg/kg i.p. dose of DPCPX (Paul et al., 2011). Second, CHA and DPCPX (each 0.03–0.30 mg/kg doses) altered locomotor activity through a specific interaction with brain adenosine A1 receptors (Marston et al., 1998). Third, although adenosine A1 and adenosine A2A receptors easily are the most abundant adenosine receptor subtypes found in the brain (Ribeiro et al., 2002), DPCPX (0.375–1.5 mg/kg i.p.) failed to attenuate the locomotor effects of the dopamine D2 receptor antagonist eticlopride unlike an adenosine A2A receptor antagonist (Collins et al., 2010). Unfortunately, the 0.3 mg/kg DPCPX dose completely suppressed the antidepressant-like effect of CHA. A more complete understanding of CHA and DPCPX pharmacological potency that was available after the present studies (Collins et al., 2010; Paul et al., 2011) were conducted suggests that the 0.3 mg/kg DPCPX dose results in maximal effects at blocking adenosine A1 receptors. Furthermore, similar antidepressant-like effects of CHA alone were observed in this experiment both times the agonist was administered. Phosphodiesterase-4 inhibition also results in antidepressant-like effects on DRL behavior that may be mediated by increases in cAMP (reviewed in O'Donnell et al., 2005), raising the question as to whether adenosine A1 receptors with transduction pathways through Gs proteins and increased cAMP (adenosine A2A or A2B receptors) might play a role in the effects of CHA and DPCPX. However, the common in vitro and in vivo pharmacological potency of both CHA and DPCPX only for adenosine A1 receptors suggests that this adenosine receptor subtype mediates the antidepressant-like action of CHA on DRL behavior.
The prominent presynaptic localization of adenosine A1 receptors throughout the limbic forebrain is consistent with the hypothesis that the suppression of glutamate release in forebrain circuits may be responsible for the antidepressant-like effects of CHA on DRL behavior. Synapses where adenosine A1 receptors suppress glutamate release include thalamocortical (Fontanez and Porter, 2006), thalamostriatal (Flagmeyer et al., 1997), corticostriatal (Flagmeyer et al., 1997), the hippocampal formation including the perforant pathway (Dragunow et al., 1988), and the subthalamic nucleus (Shen and Johnson, 2003). Adenosine A1 receptors also seem to modulate corpus callosal axon physiology (Swanson et al., 1998). However, these considerations do not rule out a role for postsynaptic adenosine A1 receptors.
A significant overlap exists between forebrain distributions of mGlu2 and adenosine A1 receptors in thalamocortical pathways (regulated by 5-HT2A receptor activation) from midline/intralaminar thalamic nuclei (Marek et al., 2001; Stutzmann et al., 2001; Benneyworth et al., 2007), the medial perforant pathways in the hippocampal formation (Dragunow et al., 1988; Shigemoto et al., 1997), and corticostriatal pathways (Flagmeyer et al., 1997; Conn et al., 2005; Johnson et al., 2005) where both receptors play autoreceptor or heteroreceptor functions. Given the prominent autoreceptor and heteroreceptor effects of mGlu2 and adenosine A1 receptors in the central nervous system it is parsimonious to propose the working hypothesis that the antidepressant-like effects observed with mGlu2 receptor PAMs (Nikiforuk et al., 2010; Fell et al., 2011) and the adenosine A1 receptor agonist CHA on DRL 72-s behavior is attributable to a suppression of glutamate release at limbic synapses.
Regarding a triangulation of 5-HT2A, mGlu2, and adenosine A1 receptors on operant behavior, the highly selective 5-HT2A receptor antagonist M100907 exerts effects similar to most antidepressant drugs with DRL 72-s behavior (Marek et al., 2005; Ardayfio et al., 2008). The present demonstration that CHA exerted effects on DRL 72-s behavior similar to most antidepressant drugs is interesting given the previously documented antidepressant-like effects for several mGlu2 receptor PAMs (Nikiforuk et al., 2010; Fell et al., 2011). Thus, the hypothesis that both mGlu2 receptor PAMs and adenosine A1 receptor agonists induce antidepressant-like effects on DRL 72-s behavior by virtue of attenuating glutamate release in limbic synapses is driven by 1) overlapping anatomical distribution of mGlu2 and adenosine A1 receptors, 2) activation of both receptors suppressing glutamate release, 3) activation of both receptors suppressing excitatory synaptic currents induced by 5-HT2A receptor activation, and 4) mGlu2 receptor activation suppressing hallucinogen-induced immediate early gene expression in the medial PFC.
How might CHA, mGlu2 receptor PAMs, and selective 5-HT2A receptor antagonists exert antidepressant-like effects on DRL behavior? Alteration of sedation is one potential explanation for the effects of CHA and DPCPX on DRL 72-s behavior. Although this could contribute to the behavioral effects of CHA, numerous drugs that decrease the response rate and are known to have sedative action in both animals and humans such as typical antipsychotic drugs, alcohol, antihistamines, α1-adrenergic receptor antagonists, 5-HT1B/1C receptor agonists, and the peripheral administration of 5-HT generally do not induce an antidepressant-like effect on DRL 72-s as reviewed elsewhere (O'Donnell et al., 2005).
Thus, a different explanation is required to understand how 5-HT2A receptor antagonists, CHA, and mGlu2 receptor PAMs induce a constellation of effects similar to most antidepressant drugs on DRL behavior independent of simply a sedative-like decrease in the response rate. Alterations of motoric impulsivity (behavioral inhibition) may be a shared explanatory construct (Pattij et al., 2003; Navarra et al., 2008; Robinson et al., 2008) for these drugs and a range of other drugs including tricyclic antidepressants and norepinephrine transporter inhibitors (Winstanley et al., 2004; Greco et al., 2005; Carli et al., 2006; Blondeau and Dellu-Hagedorn, 2007; Paine et al., 2007; Wischhof and Koch, 2012). This potential involvement of impulsivity in mediating the antidepressant-like effects of norepinephrine transporter inhibitors, tricyclic antidepressants, 5-HT2A receptor antagonists, mGlu2 receptor PAMs, and adenosine A1 receptor agonists on DRL behavior highlights an important differential feature compared with the FST, for which adenosine A1 receptor agonists and mGlu2 receptor agonists also test similarly to antidepressants. Rather than potentially modulating impulsivity on DRL behavior, the FST measures the ability of drugs to modulate an escape behavior. Learned helplessness provides a well known theoretical background for the FST.
Caveats regarding the present study include 1) the use of a single adenosine A1 receptor agonist and antagonist to examine DRL behavior and 2) the failure to demonstrate a no-effect dose for the adenosine A1 receptor antagonist in blocking the effects of CHA. However, the novel antidepressant-like action reported here for CHA agrees with an earlier study suggesting that activation of adenosine A1 receptors mediates antidepressant-like effects on the FST and the tail suspension test (Kaster et al., 2004). The convergence of these different antidepressant screens is intriguing because they would seem to measure different behavioral constructs. Adenosine A1 receptor agonists also decrease rapid eye movement sleep, consistent with the preclinical/clinical action of most antidepressants (Schwierin et al., 1996). This confluence of positive results on DRL 72-s behavior, both the FST and tail suspension test, and suppression of rapid eye movement sleep increases the probability of these divergent behavioral screens correctly predicting effects in patients. Adenosine A1 receptor agonists are not available to test the general hypothesis that decreasing glutamate release in limbic circuitry will result in antidepressant effects in depressed patients because of the peripheral on-target cardiovascular toxicity. However, administration of mGlu2 receptor PAMs could test this hypothesis. Although one mGlu2 receptor PAM has been tested for antipsychotic effects by AstraZeneca Pharmaceuticals LP (Wilmington, DE) and another Johnson & Johnson (New Brunswick, NJ) phase 2 schizophrenia study is currently recruiting subjects to test JNJ-40411813 (www.clinicaltrials.gov), testing of these novel glutamatergic drugs in depressed patients should be pursued (Fell et al., 2011).
Acknowledgments
I thank Allyson Abo, Angela Keene, Andy Kwak, and Kim Reiss for technical assistance in carrying out the experiments. These experiments were performed in the Department of Psychiatry at the Yale University School of Medicine (New Haven, CT) and the Ribicoff Research Facilities of the Connecticut Medical Health Center (New Haven, CT).
This work was supported by the National Institutes of Health National Institute of Mental Health [Grants K08-MH0151, R01-MH62186], a National Alliance for Research on Schizophrenia and Depression Young Investigator Award, and the State of Connecticut.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- PFC
- prefrontal cortex
- DPCPX
- 8-cyclopentyl-1,3-dipropylxanthine
- DRL
- differential reinforcement of low rate
- FST
- forced swim test
- mGlu
- metabotropic glutamate
- CHA
- N6-cyclohexyladenosine
- PAM
- positive allosteric modulator
- IRT
- inter-response time
- MK-801
- (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine
- 5-HT
- 5-hydroxytryptamine
- K-S
- Kolmogorov-Smirnov.
Authorship Contributions
Participated in research design: Marek.
Conducted experiments: Marek.
Performed data analysis: Marek.
Wrote or contributed to the writing of the manuscript: Marek.
References
- Ardayfio PA, Benvenga MJ, Chaney SF, Love PL, Catlow J, Swanson SP, Marek GJ. (2008) The 5-hydroxytryptamine2A receptor antagonist R-(+)-α-(2,3-dimethoxyphenyl)-1–2-(4-fluorophenyl)ethyl-4-piperidinemethanol (M100907) attenuates impulsivity after both drug-induced disruption (dizocilpine) and enhancement (antidepressant drugs) of differential-reinforcement-of-low-rate 72-s behavior in the rat. J Pharmacol Exp Ther 327:891–897 [DOI] [PubMed] [Google Scholar]
- Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. (1997) Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranualation is mediated by the A2B receptor. Mol Pharmacol 52:846–860 [DOI] [PubMed] [Google Scholar]
- Bauer A, Holschbach MH, Cremer M, Weber S, Boy C, Shah NJ, Olsson RA, Halling H, Coenen HH, Zilles K. (2003) Evaluation of 18F-CPFPX, a novel adenosine A1 receptor ligand: in vitro autoradiography and high-resolution small animal PET. J Nucl Med 44:1682–1689 [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. (2007) A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol 72:477–484 [DOI] [PubMed] [Google Scholar]
- Blondeau C, Dellu-Hagedorn F. (2007) Dimensional analysis of ADHD subtypes in rats. Biol Psychiatry 61:1340–1350 [DOI] [PubMed] [Google Scholar]
- Bruns RF. (1980) Adenosine receptor activation in human fibroblasts: nucleoside agonists and antagonists. Can J Physiol Pharmacol 58:673–691 [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, Balducci C. (2006) Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology 31:757–767 [DOI] [PubMed] [Google Scholar]
- Collins LE, Galtieri DJ, Collins P, Jones SK, Port RG, Paul NE, Hockemeyer J, Müller CE, Salamone JD. (2010) Interactions between adenosine and dopamine receptor antagonists with different selectivity profiles: effects on locomotor activity. Behav Brain Res 211:148–155 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Battaglia G, Marino MJ, Nicoletti F. (2005) Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci 6:787–798 [DOI] [PubMed] [Google Scholar]
- Daly JW, Padgett WL, Secunda SI, Thompson RD, Olsson RA. (1993) Structure-activity relationships for 2-substituted adenosines at A1 and A2 receptors. Pharmacology 46:91–100 [DOI] [PubMed] [Google Scholar]
- Dragunow M, Murphy K, Leslie RA, Robertson HA. (1988) Localization of adenosine A1-receptors to the terminals of the perforant path. Brain Res 462:252–257 [DOI] [PubMed] [Google Scholar]
- Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. (2008) Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology 33:1603–1610 [DOI] [PubMed] [Google Scholar]
- Fell MJ, Witkin JM, Falcone JF, Katner JS, Perry KW, Hart J, Rorick-Kehn L, Overshiner CD, Rasmussen K, Chaney SF, et al. (2011) N-(4-((2-trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1-methyl-1H-imidazole-4-carboxamide (THIIC), a novel metabotropic glutamate 2 potentiator with potential anxiolytic/antidepressant properties: in vivo profiling suggests a link between behavioral and central nervous system neurochemical changes. J Pharmacol Exp Ther 336:165–177 [DOI] [PubMed] [Google Scholar]
- Flagmeyer I, Haas HL, Stevens DR. (1997) Adenosine A1 receptor-mediated depression of corticostriatal and thalamostriatal glutamatergic synaptic potentials in vitro. Brain Res 778:178–185 [DOI] [PubMed] [Google Scholar]
- Fontanez DE, Porter JT. (2006) Adenosine A1 receptors decrease thalamic excitation of inhibitory and excitatory neurons in the barrel cortex. Neuroscience 137:1177–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh L, Kawanami N, Nakahara T, Hondo H, Motomura K, Ohta E, Kanchiku I, Kuroki T, Hirano M, Uchimura H. (2002) Effects of the adenosine A1 receptor agonist N6-cyclopentyladenosine on phencyclidine-induced behavior and expression of the immediate early genes in the discrete brain regions of rats. Mol Brain Res 100:1–12 [DOI] [PubMed] [Google Scholar]
- Greco B, Invernizzi RW, Carli M. (2005) Phencyclidine-induced impairment in attention and response control depends on the background genotype of mice: reversal by the mGLU(2/3) receptor agonist LY379268. Psychopharmacology 179:68–76 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE, McKinzie DL, Nisenbaum ES, Tizzano JP, Schoepp DD. (2005) Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s). Psychopharmacology 179:271–283 [DOI] [PubMed] [Google Scholar]
- Kaster MP, Rosa AO, Rosso MM, Goulart EC, Santos AR, Rodrigues AL. (2004) Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors. Neurosci Lett 355:21–24 [DOI] [PubMed] [Google Scholar]
- Marek GJ. (2009) Activation of adenosine1 (A1) receptors suppresses head shakes induced by a serotonergic hallucinogen in rats. Neuropharmacology 56:1082–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Martin-Ruiz R, Abo A, Artigas F. (2005) The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology 30:2205–2215 [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. (2001) A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience 105:379–392 [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. (2000) Physiological antagonism between 5-hydroxytryptamine2A and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther 292:76–87 [PubMed] [Google Scholar]
- Marston HM, Finlayson K, Maemoto T, Olverman HJ, Akahane A, Sharkey J, Butcher SP. (1998) Pharmacological characterization of a simple behavioral response mediated selectively by central adenosine A1 receptors, using in vivo and in vitro techniques. J Pharmacol Exp Ther 285:1023–1030 [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. (2008) Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry 32:34–41 [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Popik P, Drescher KU, van Gaalen M, Relo AL, Mezler M, Marek G, Schoemaker H, Gross G, Bespalov A. (2010) Effects of a positive allosteric modulator of group II metabotropic glutamate receptors, LY487379, on cognitive flexibility and impulsive-like responding in rats. J Pharmacol Exp Ther 335:665–673 [DOI] [PubMed] [Google Scholar]
- O'Donnell JM, Marek GJ, Seiden LS. (2005) Antidepressant effects assessed using behavior maintained under a differential-reinforcement-of-low-rate (DRL) operant schedule. Neurosci Biobehav Rev 29:785–798 [DOI] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA., Jr (2007) Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biol Psychiatry 62:687–693 [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, et al. (2007) Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized phase 2 clinical trial. Nat Med 13:1102–1107 [DOI] [PubMed] [Google Scholar]
- Pattij T, Broersen LM, van der Linde J, Groenink L, van der Gugten J, Maes RA, Olivier B. (2003) Operant learning and differential-reinforcement-of-low-rate 36-s responding in 5-HT1A and 5-HT1B receptor knockout mice. Behav Brain Res 141:137–145 [DOI] [PubMed] [Google Scholar]
- Paul S, Khanapur S, Rybczynska AA, Kwizera C, Sijbesma JW, Ishiwata K, Willemsen AT, Elsinga PH, Dierckx RA, van Waarde A. (2011) Small-animal PET study of adenosine A1 receptors in rat brain: blocking receptors and raising extracellular adenosine. J Nucl Med 52:1293–1300 [DOI] [PubMed] [Google Scholar]
- Pei Q, Tordera R, Sprakes M, Sharp T. (2004) Glutamate receptor activation is involved in 5-HT2 agonist-induced Arc gene expression in the rat cortex. Neuropharmacology 46:331–339 [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. (2010) Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastião AM, de Mendonça A. (2002) Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol 68:377–392 [DOI] [PubMed] [Google Scholar]
- Richards G, Messer J, Malherbe P, Pink R, Brockhaus M, Stadler H, Wichmann J, Schaffhauser H, Mutel V. (2005) Distribution and abundance of metabotropic glutamate receptor subtype 2 in rat brain revealed by [3H]LY354740 binding in vitro and quantitative radioautoradiography: correlation with the sites of synthesis, expression, and agonist stimulation of [35S]GTPγS binding. J Comp Neurol 487:15–27 [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. (2008) Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology 33:1028–1037 [DOI] [PubMed] [Google Scholar]
- Schoepp DD. (2001) Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299:12–20 [PubMed] [Google Scholar]
- Schwierin B, Borbély AA, Tobler I. (1996) Effects of N6-cyclopentyladenosine and caffeine on sleep regulation in the rat. Eur J Pharmacol 300:163–171 [DOI] [PubMed] [Google Scholar]
- Scruggs JL, Patel S, Bubser M, Deutch AY. (2000) DOI-induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J Neurosci 20:8846–8852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. (2003) Presynaptic inhibition of synaptic transmission by adenosine in rat subthalamic nucleus in vitro. Neuroscience 116:99–106 [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, et al. (1997) Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci 17:7503–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, Marek GJ, Aghajanian GK. (2001) Adenosine preferentially suppresses serotonin2A receptor-enhanced excitatory postsynaptic currents in layer V neurons of the rat medial prefrontal cortex. Neuroscience 105:55–69 [DOI] [PubMed] [Google Scholar]
- Swanson TH, Krahl SE, Liu YZ, Drazba JA, Rivkees SA. (1998) Evidence for physiologically active axonal adenosine receptors in the rat corpus callosum. Brain Res 784:188–198 [DOI] [PubMed] [Google Scholar]
- van Galen PJ, van Bergen AH, Gallo-Rodriguez C, Melman N, Olah ME, IJzerman AP, Stiles GL, Jacobson KA. (1994) A binding site model and structure-activity relationships for the rat A3 receptor. Mol Pharmacol 45:1101–1111 [PMC free article] [PubMed] [Google Scholar]
- Weyler S, Fülle F, Diekmann M, Schumacher B, Hinz S, Klotz KN, Müller CE. (2006) Improving potency, selectivity, and water solubility of adenosine A1 receptor antagonists: xanthines modified at position 3 and related pyrimido[1,2,3-cd]purinediones. ChemMedChem 1:891–902 [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. (2004) 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology 176:376–385 [DOI] [PubMed] [Google Scholar]
- Wischhof L, Koch M. (2012) Pretreatment with the mGlu2/3 receptor agonist LY379268 attenuates DOI-induced impulsive responding and regional c-Fos protein expression. Psychopharmacology 219:387–400 [DOI] [PubMed] [Google Scholar]