Abstract
Neuroadaptations underlying sensitization to drugs of abuse seem to influence compulsive drug pursuit and relapse associated with addiction. Our previous data support a role for the corticotropin-releasing factor (CRF) type-1 receptor (CRF1) in ethanol (EtOH)-induced psychomotor sensitization. CRF1 is endogenously activated by CRF and urocortin-1. Because genetic deletion of urocortin-1 did not affect EtOH sensitization, we hypothesized that CRF is the important ligand underlying EtOH sensitization. To test this hypothesis, we used heterozygous and homozygous knockout (KO) mice, which lack one or both copies of the gene coding for CRF, and their respective wild-type controls. EtOH sensitization was normal in heterozygous, but absent in homozygous, CRF KO mice. Corticosterone (CORT) levels were drastically reduced only in CRF KO mice. Because CRF/CRF1 initiate EtOH-induced activation of the hypothalamic-pituitary-adrenal axis, we investigated CORT effects on EtOH sensitization. The CORT synthesis inhibitor metyrapone prevented the acquisition, but not the expression, of EtOH sensitization. Exogenous CORT administration sensitized the locomotor response to a subsequent EtOH challenge; we observed, however, that the exogenous CORT levels necessary to induce sensitization to EtOH were significantly higher than those produced by EtOH treatment. Therefore, participation of CORT seems to be necessary, but not sufficient, to explain the role of CRF/CRF1 in the acquisition of sensitization to EtOH. Extra-hypothalamic CRF/CRF1 mechanisms are suggested to be involved in the expression of EtOH sensitization. The present results are consistent with current theories proposing a key role for CRF and CRF1 in drug-induced neuroplasticity, dependence, and addictive behavior.
Introduction
Repeated exposure to abused drugs can produce enduring changes in the central nervous system that are thought to be responsible for the development and persistence of addictive behavior (Robinson and Berridge, 1993, 2008; Hyman and Malenka, 2001; Koob, 2009). Among other possible key characteristics (e.g., signs of physical dependence), addicted individuals show impairments in cognitive control of behavior and pathological levels of motivation to consume drugs despite detrimental effects (Robinson and Berridge, 1993, 2008; Everitt et al., 2008; Kalivas, 2008; Koob, 2009). Preclinical research suggests that drug-induced neuroplasticity associated with behavioral sensitization underlies amplification of the incentive-motivational properties of drugs and cues paired with drugs (Robinson and Berridge, 1993, 2008; Vanderschuren and Kalivas, 2000). These neural changes might explain drug pursuit, compulsion, and relapse characteristic of addiction (Robinson and Berridge, 1993, 2008; Vezina, 2004; Kalivas et al., 2005). Behavioral sensitization has been defined as a long-lasting progressive increase in a behavioral response (e.g., locomotor activity) that develops upon repeated drug administration and it has been widely used as a measure indicative of neuroplasticity (Robinson and Berridge, 1993, 2008; Sanchis-Segura and Spanagel, 2006).
Behavioral sensitization induced by ethanol (EtOH) has been largely studied in mice (Masur and Boerngen, 1980; Phillips et al., 1997; Broadbent et al., 2003; Pastor et al., 2008), but also demonstrated in rats (Correa et al., 2003) and humans (Newlin and Thomson, 1999). However, the neurobiological determinants of EtOH sensitization remain to be entirely elucidated. Results pertaining to the participation of dopamine (Broadbent et al., 1995; Palmer et al., 2003), opioid (Pastor and Aragon, 2006; Abrahao et al., 2008), γ-aminobutyric acid (Chester and Cunningham, 1999; Meyer et al., 2005), and glutamate (Broadbent et al., 2003; Meyer and Phillips, 2007) systems in ethanol sensitization have not provided consistent support. It is noteworthy that manipulation of hypothalamic-pituitary-adrenal (HPA)-axis components, such as corticotropin-releasing factor (CRF) or glucocorticoids, has been shown to critically modulate EtOH-induced behavioral sensitization (Roberts et al., 1995; Phillips et al., 1997; Fee et al., 2007; Pastor et al., 2008).
EtOH stimulates the HPA axis by activating hypothalamic CRF neurons (Rivier, 1996; Ogilvie et al., 1998). CRF induces the secretion of adrenocorticotropin hormone from the pituitary, which then activates the release of glucocorticoids such as corticosterone (CORT) from the adrenal gland (Rivier, 1996). CRF binds to two G-protein positively coupled receptors, CRF1 and CRF2, and shows greater affinity for CRF1. EtOH sensitization critically depends on CRF1 activation; it is absent in CRF1 knockout (KO) mice (Pastor et al., 2008) and reduced by the CRF1 antagonist N-butyl-N-ethyl-2,5-dimethyl-7-(2,4,6-trimethylphenyl)pyrrolo[3,2-e]pyrimidin-4-amine (CP-154,526) (Fee et al., 2007; Pastor et al., 2008). CRF2 KO mice show normal EtOH sensitization (Pastor et al., 2008). CRF1 is endogenously activated by CRF, but also by urocortin-1. That EtOH sensitization is not absent in urocortin-1 KO animals (Pastor et al., 2008) suggests that CRF is the necessary ligand for EtOH sensitization to develop. However, combined CRF and urocortin-1 actions could still account for the involvement of CRF1. Thus, in the present study we used CRF KO mice to directly assess CRF's role in psychomotor sensitization to EtOH. In addition, we examined whether the lack of CRF altered sensitivity to the sedative effect of EtOH, as a possible explanation for reduced susceptibility to EtOH sensitization.
Our previous results indicate that the influence of CRF1 in the acquisition of EtOH sensitization may be mediated by CORT (Roberts et al., 1995; Pastor et al., 2008). A blunted endocrine response to EtOH has been seen in mice lacking CRF1 (Pastor et al., 2008). Antagonism of CORT-activated glucocorticoid receptors (GRs) blocked acquisition of EtOH-induced sensitization (Roberts et al., 1995). Here, we further investigate the role of CORT by measuring CORT in CRF KO mice and evaluating the effects of the CORT synthesis inhibitor metyrapone on both the acquisition and expression of sensitization to EtOH. We also investigated whether EtOH-induced increases in CORT are sufficient to produce sensitization; a regimen of CORT injections mirroring the EtOH-sensitizing treatment was used to evaluate “cross-sensitization”-like responses between CORT and EtOH. Finally, because CORT activates mineralocorticoid receptors (MRs), in addition to GRs, a MR antagonist (spironolactone) was used to clarify the receptor-specific mechanism underlying the effect of CORT in EtOH sensitization.
Materials and Methods
Animals.
CRF mutant mice were created from embryonic stem cells that underwent targeted gene inactivation; mice contained a deletion of exon 2 of the Crh gene (Muglia et al., 1995). Mutant mice of a mixed C57BL/6J × 129SV/J background were purchased from The Jackson Laboratory (Bar Harbor, ME) and backcrossed onto the C57BL/6J strain within the Oregon Health & Science University animal facility. After 9 to 10 generations of backcrossing, heterozygous (HT) mating pairs were used to generate all of the littermate KO, heterozygous, and wild-type (WT) CRF mice used in the present work. Sex-balanced groups of male and female mice (50–115 days old at the beginning of experimentation) were used in these studies. Inbred DBA/2J (D2) mice (54–63 days old) purchased from The Jackson Laboratory were used for all pharmacological studies. In this case, female mice were used, which is consistent with the prior use of females to demonstrate EtOH- and stress-induced sensitization to EtOH challenge and the involvement of the HPA axis in sensitization (Roberts et al., 1995; Pastor et al., 2008). However, both male and female D2 mice develop robust sensitization to ethanol (Roberts et al., 1995; Broadbent et al., 2003; Meyer and Phillips, 2007; Pastor et al., 2008). Mice were housed two to five per cage in standard acrylic mouse cages with corncob bedding. Food and water were available ad libitum. On behavioral testing days, animals were transferred in their home cages to the procedure room 45 to 60 min before testing to allow acclimation to the environment. All procedures were approved by the Portland VA or Oregon Health & Science University animal care and use committee and followed the National Institutes of Health's Guidelines for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Drugs.
EtOH (100%; Pharmco Products, Brookfield, CT) was diluted to a 20% (v/v) solution in 0.9% NaCl and injected in an appropriate volume to administer a dose of 1.5, 2.5, or 3.6 g/kg. Two-methyl-1,2-di-3-pyridyl-1-propanone, (metyrapone), 4-pregnen-21-oic acid-17α-ol-3-one-7α-thiol γ-lactone 7-acetate (spironolactone), and CORT (Sigma, St. Louis, MO) were prepared in 20% (w/v) 2-hydroxypropyl-β-cyclodextrin (Sigma) at concentrations of 2.5 or 5 mg/ml for metyrapone, 1.5 or 3 mg/ml for spironolactore, and 1 or 2 mg/ml for CORT and injected at a volume of 10 ml/kg. All injections were administered intraperitoneally except for CORT injections, which were administered subcutaneously.
EtOH-Induced Behavioral Sensitization.
EtOH doses and experimental procedures followed those previously established for measuring EtOH-induced locomotor sensitization (Roberts et al., 1995; Pastor et al., 2008). For the study using CRF KO, HT, and WT mice, animals were treated with EtOH (0 or 2.5 g/kg) once daily for 10 consecutive days in their home cages. No behavioral testing was performed on those days. On day 11, all mice were challenged with 1.5 g/kg EtOH, immediately before placement in automated activity monitors, where locomotor activity was measured for 15 min. On day 12, all mice were tested for activity levels after saline treatment (15 min).
To assess the effects of metyrapone (0, 25, and 50 mg/kg; Marinelli et al., 1997) and spironolactone (0, 15, and 30 mg/kg; Koenig and Olive, 2004) on the acquisition of EtOH sensitization, vehicle or drug was administered to D2 mice 30 min before receiving saline or EtOH (1.5 g/kg) once daily for 10 consecutive days. Locomotor activity was measured after EtOH (1.5 g/kg) or saline on days 11 and 12, respectively, preceded by vehicle treatment (30 min before). This mirrored the injection conditions during the pretreatment phase (days 1–10). The effects of these two compounds were also tested on the expression of EtOH sensitization: two groups of D2 mice were treated for 10 days with vehicle-saline or vehicle-EtOH (1.5 g/kg), respectively (injections spaced 30 min apart). These two groups were then subdivided into three groups each for day-11 treatment and testing; mice were pretreated with vehicle, metyrapone (25 or 50 mg/kg), or spironolactone (15 or 30 mg/kg) 30 min before 1.5 g/kg EtOH. On day 12, all animals were tested for locomotor activity after a vehicle-saline treatment (30 min between injections).
EtOH-Induced Behavior after Repeated CORT Administration.
The study design mirrored that for acquisition of EtOH-induced sensitization to EtOH challenge. Our aim was to investigate whether increasing plasma CORT levels would be sufficient to produce a sensitized response to EtOH, similar to the effects of EtOH itself and the effects of restraint stress (Roberts et al., 1995). Animals received 10 consecutive daily home cage injections of vehicle or CORT (10 or 20 mg/kg; Gregus et al., 2005). On day 11, mice were administered saline or EtOH (1.5 g/kg), and locomotor activity was measured for 15 min. On day 12, all mice were tested for activity levels after saline treatment (15 min).
Locomotor Activity Testing.
Locomotion was tested in clear acrylic plastic boxes (40 × 40 × 30 cm) covered with plastic lids (44 × 44 cm with 0.64-cm holes for ventilation) and placed in AccuScan Instruments, Inc. (Columbus, OH) activity monitors. Consecutive interruptions of two sets of eight intersecting photocell beams, situated 2 cm above the floor, measured horizontal distance traveled (interruptions were recorded and translated by AccuScan Instruments, Inc. software to distance in cm). Activity monitors were set inside individual acrylic chambers (Flair Plastics, Portland, OR), each containing foam for noise insulation, a fluorescent light (15 W) for illumination of the chambers during testing, and a fan for providing ventilation and background noise to mask extraneous laboratory sounds.
Loss of Righting Reflex.
To determine whether CRF influenced sensitivity to the sedative-hypnotic effect of EtOH, CRF KO and WT mice were tested for EtOH-induced loss of righting reflex (LORR). Mice from the EtOH sensitization study were used, partly because experimentally naive animals were in short supply; however, this design also allowed the determination of possible effects of a sensitizing regimen of repeated EtOH exposures on sedative sensitivity. Animals were left undisturbed for 7 days after completion of the sensitization experiment (8 days since their last EtOH exposure); we have found previously that EtOH sensitization can persist for longer than this period (Lessov and Phillips, 1998). Procedures followed those used previously in the Phillips laboratory (Sharpe et al., 2005). At time 0 (T0), mice were injected with 3.6 g/kg EtOH. Then, at 20-s intervals, they were tested for LORR by placing them supine in a V-shaped trough to determine whether they were able to right themselves (rotate to a ventral orientation onto all four paws). The latency to LORR was defined as the time from the EtOH injection (T0) until the time that the mouse was unable to right itself from the supine position for at least 30 s (T1). Mice remained undisturbed in the supine position until they could right themselves. At that time, they were returned to a supine position in the trough and required to right themselves again within 30 s to be scored as having regained the righting reflex (T2). Duration of LORR was calculated by subtraction (T2 − T1).
Determination of Blood EtOH Concentration.
Blood samples (20 μl) were collected in calibrated capillary tubes from the tail vein (20 μl) on day 11, immediately after activity testing (15 min after EtOH injection) and upon regaining of the righting reflex to determine whether CRF mutation or pharmacological treatments modified EtOH levels. Blood ethanol concentrations (BECs) were determined by gas chromatography. Samples were added to 50 μl of chilled 5% ZnSO4 and stored on ice. Fifty microliters of 0.3 N Ba(OH)2 and 300 μl of distilled water were later added to each sample. Supernatants were transferred to glass vials and analyzed for EtOH concentration by gas chromatography (Agilent 6890N; Agilent Technologies, Santa Clara, CA) with flame ionization detection. Five pairs of external standards of known EtOH concentrations (0.47–2.96 mg/ml) were used to establish a standard curve.
Determination of Plasma CORT Levels.
Tail blood samples (20 μl) for determination of CORT levels were collected in CRF WT, HT, and KO mice (after sampling for BEC) on day 11, approximately 15 min after EtOH challenge for locomotor activity testing. The effects of the CORT synthesis-inhibitor metyrapone on EtOH-induced CORT levels in D2 mice were also investigated. However, because we were interested in the time course of effect of metyrapone on CORT levels, a different set of experimentally naive animals was used for this evaluation. D2 mice received vehicle or 50 mg/kg of metyrapone (the dose that prevented acquisition of sensitization) 30 min before a saline or EtOH (1.5 g/kg) injection, on 10 consecutive days. On days 1 and 10, blood samples were taken 15, 30, 60, and 120 min after the saline or EtOH challenge. Independent groups of mice were used for each time point, but the same animals were sampled on days 1 and 10. On day 11, all animals received vehicle, followed 30 min later by EtOH (1.5 g/kg), and blood samples were taken at the group-specific time points. This day-11 test was conducted to examine whether the different treatments administered on days 1 to 10 would affect the CORT response to EtOH. Blood CORT levels were also determined after exogenous CORT administration on the first and final CORT treatment days to determine the levels achieved and whether they changed with repeated administration. Vehicle or CORT (10 or 20 mg/kg) was injected on 10 consecutive days, and blood samples were taken on days 1 and 10 (1, 2, 4, and 24 h after CORT administration).
Blood samples were placed into heparinized capillary tubes on ice and later centrifuged to separate the plasma from other blood constituents. Plasma was stored at −20°C until assayed. An ImmuChem 125I CORT Radioimmunoassay from MP Biomedicals, LLC (Orangeburg, NY) was used. All samples were diluted 1:200 with a phosphosaline buffer (provided with the kit), per kit instructions, before being assayed. Counts per minute were normalized and fit to a least-squares regression equation produced by log-logit transformation of the standards (25–1000 ng). Sample concentration was calculated by interpolation of the standards. The detectable range of the assay was from 0.7 to 130 μg of CORT per 100 ml of plasma. Intra-assay and interassay coefficients of variation were less than 10%. Specificity of the assay was 0.34% cross-reactivity to deoxycorticosterone and less than 0.15% cross-reactivity to other endogenous steroids.
Statistical Analysis.
Behavioral sensitization, LORR, and BEC data were analyzed by using two-way analysis of variance (ANOVA), including genotype or pharmacological pretreatment dose and EtOH treatment dose as between-group factors. For all studies with mutant mice, we included sex as a factor in all preliminary analyses. However, no main or interaction effects involving sex were found for any dependent variable; therefore, subsequent analyses examined data for the two sexes combined. Plasma CORT values for the metyrapone and CORT administration studies were analyzed by factorial ANOVA, with metyrapone or CORT dose and time group assignment (and EtOH dose for the metyrapone study only) as factors. Significant two-way interactions were examined for simple main effects, and the Newman-Keuls test was used for mean comparisons. These statistical results are presented in the figures and figure legends. Statistica 6.1 software (StatSoft, Tulsa, OK) was used.
Results
Absence of Psychomotor Sensitization to EtOH in CRF KO Mice.
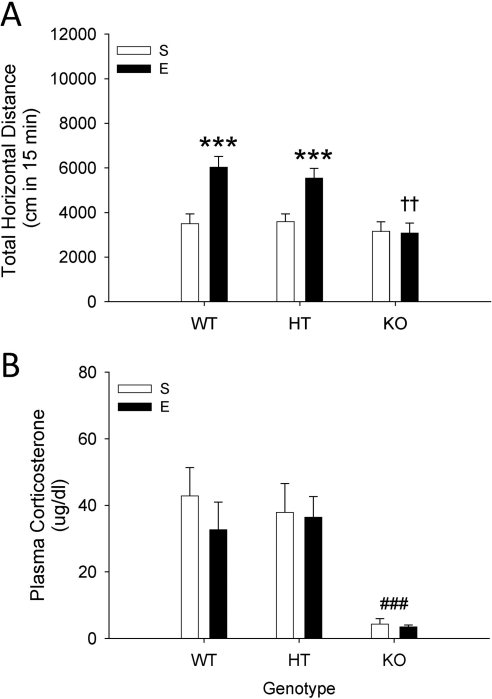
Significant psychomotor sensitization to EtOH was seen in WT and HT mice, but not in CRF KO mice (Fig. 1A). A two-way ANOVA performed on day-11 EtOH challenge activity data revealed a significant genotype × EtOH pretreatment dose interaction (F2,72 = 13.2; p < 0.05). Follow-up simple main-effect analysis identified a significant difference in the level of activity after EtOH challenge between mice with a saline pretreatment history compared with those with an EtOH pretreatment history, indicating the presence of sensitization for WT and HT mice, but not KO mice (see Fig. 1A). Whereas responses to EtOH in saline-pretreated groups were comparable among genotypes, there were significant genotype-associated differences for EtOH-pretreated animals; KO mice showed significantly lower EtOH challenge activity levels compared with WT and HT mice. BEC and levels of locomotion after the day-12 saline treatment (Supplemental Fig. 1, A and B) were not different among groups.
Fig. 1.
A, ethanol (E)-induced psychomotor sensitization is absent in CRF KO mice, but present in WT and HT mice. Shown is total distance traveled (cm; mean ± S.E.M.) after challenge with 1.5 g/kg EtOH in CRH KO, HT, and WT mice (n = 12–16 per group), pretreated for 10 days with saline (S) or 2.5 g/kg EtOH. ***, p < 0.001 for the comparison with the respective repeated saline-treated group. ††, p < 0.01 for the comparison of EtOH-pretreated KO mice with EtOH-pretreated HT and WT mice. B, plasma CORT after EtOH challenge is blunted in CRF KO compared with WT and HT mice (same animals as shown in A). Shown is the concentration of CORT (μg/dl; mean ± S.E.M.) in plasma samples at the end of the 15-min locomotor activity test (15 min after EtOH challenge). ###, p < 0.001 for the comparison of KO collapsed on group with WT and HT.
Blunted CORT Response to EtOH in CRF KO Mice.
CORT levels obtained immediately after the EtOH challenge locomotor activity test on day 11 were significantly lower in KO mice compared with HT and WT mice (Fig. 1B). There was a statistically significant effect of genotype (F2,70 = 11.1; p < 0.001), but no pretreatment group or genotype × pretreatment interaction. Newman-Keuls post hoc comparisons supported the presence of lower CORT levels in CRF KO mice.
CRF Deficit Does Not Modify EtOH-Induced Loss of Righting Reflex.
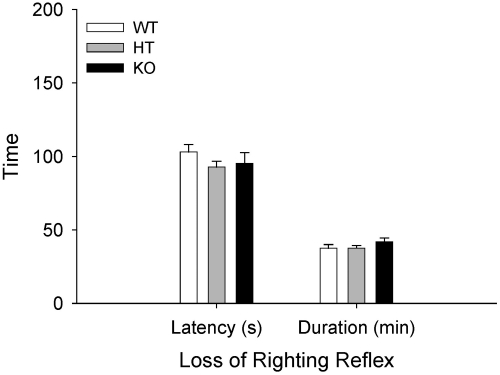
There were no effects of genotype on latency to LORR or LORR duration (Fig. 2). Likewise, there were no effects of prior EtOH administration; therefore, data are presented collapsed on EtOH pretreatment group. Thus, differences in susceptibility to EtOH sensitization were not reflected in differences in sensitivity to the sedative-hypnotic effects of EtOH. Analysis of BEC data from samples taken upon recovery of LORR also indicated no differences (Supplemental Fig. 1C).
Fig. 2.
Sensitivity to EtOH-induced LORR is not affected by CRF. Shown are mean (± S.E.M.) LORR latency (s) and duration (min). CRF KO, HT, and WT mice were treated with 3.6 g/kg EtOH 8 days after their final EtOH exposure in the EtOH sensitization study (Fig. 1). Latency to LORR was the time from EtOH injection until the time that the mouse was unable to right itself from the supine position for at least 30 s. Duration of LORR was the time from LORR until the time that the mouse could right itself onto all four paws twice within 30 s.
The CORT Synthesis Inhibitor Metyrapone Prevents Acquisition but Does Not Affect Expression of EtOH Sensitization.
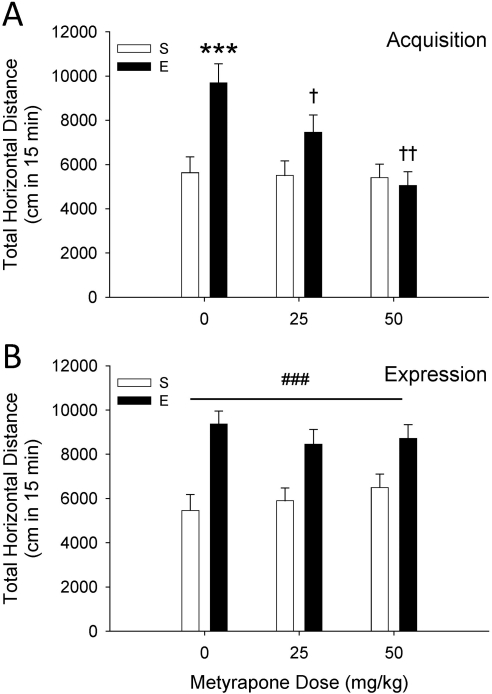
D2 animals showed significant sensitization to the locomotor-activating effects of EtOH that was dose-dependently prevented by metyrapone (Fig. 3A). ANOVA of EtOH challenge day-11 data identified a statistically significant metyrapone pretreatment dose × EtOH pretreatment dose interaction (F2,72 = 4.9; p < 0.05). A significant sensitized response to EtOH was present only in nonmetyrapone-treated mice (saline versus EtOH group mice in the 0 mg/kg groups). Metyrapone (25 and 50 mg/kg) given during the repeated EtOH exposure period significantly reduced EtOH sensitization compared with vehicle. There were no differences in BEC among groups, and when all groups were tested with saline 1 day after the EtOH challenge no differences among groups were found (Supplemental Fig. 2, A and B).
Fig. 3.
Acquisition, but not expression, of EtOH (E)-induced behavioral sensitization is prevented by the CORT synthesis inhibitor metyrapone. A, results for the acquisition study. Shown is total distance traveled (cm; mean ± S.E.M.) after 1.5 g/kg EtOH treatment in D2 mice (n = 12–14 per group), treated for 10 days with metyrapone (0, 25, or 50 mg/kg), then saline (S) or 1.5 g/kg EtOH, with a 30-min separation between treatments. ***, p < 0.001 for the comparison with the respective repeated saline-treated group. †, p < 0.05 and ††, p < 0.01 for the comparison with the 0 metyrapone-EtOH group. B, results for the expression study. Shown is total distance traveled (cm; mean ± S.E.M.) in D2 mice (n = 12 per group) treated with 0, 25, or 50 mg/kg metyrapone, and then saline or EtOH (1.5 g/kg) 30 min later. During days 1 to 10, mice were treated with saline or EtOH (1.5 g/kg) without metyrapone pretreatment. ###, p < 0.01 for the main effect of repeated EtOH exposure (sensitization).
Figure 3B shows the effect of metyrapone on the expression of sensitization to EtOH. In this case, metyrapone was given only on the EtOH challenge day, rather than during the period of repeated saline or EtOH exposure, as was the case in the acquisition study. A two-way ANOVA revealed a main effect of EtOH pretreatment dose (F1,66 = 39.1; p < 0.001), indicating the presence of sensitization, but no effect of metyrapone dose or metyrapone dose by EtOH pretreatment dose interaction. Thus, metyrapone given just before EtOH challenge did not prevent acute stimulation or the expression of a sensitized locomotor response to EtOH. Analysis of blood sample BEC data and day-12 baseline activity data revealed no differences among groups (Supplemental Fig. 2, C and D).
Reduction in EtOH-Elevated CORT Levels in Metyrapone-Pretreated Mice.
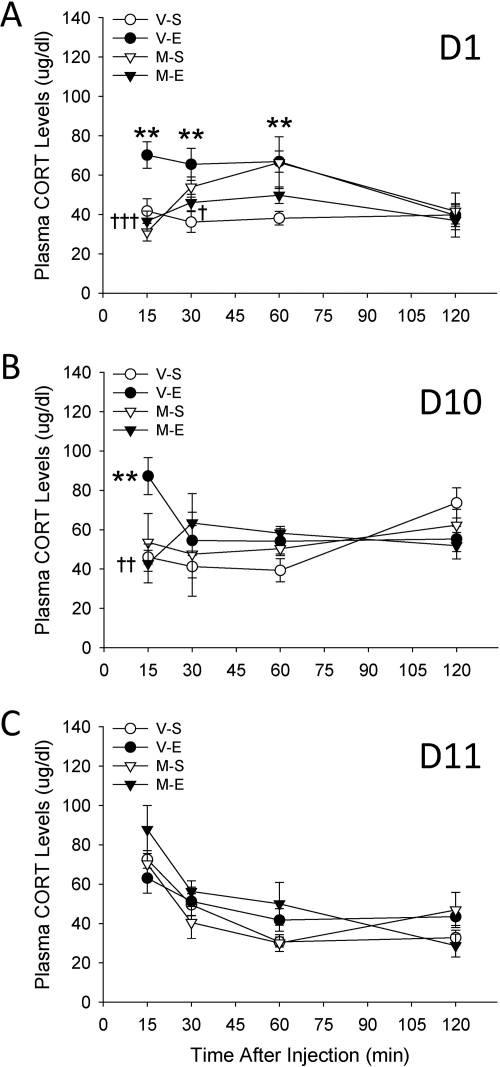
Levels of plasma CORT obtained after EtOH in animals pretreated with metyrapone are shown in Fig. 4. A three-way ANOVA performed on data from day 1 (Fig. 4A) revealed significant interactions of metyrapone and EtOH (F1,102 = 5.5; p < 0.05) and metyrapone and time (F3,102 = 3.1; p < 0.05). Follow-up analysis of these interactions showed that CORT levels were elevated in vehicle-EtOH versus vehicle-saline groups at 15, 30, and 60 min after EtOH injection. However, metyrapone blocked increases in CORT levels. Metyrapone had no effect on CORT levels in saline-treated mice except at the 60-min time point. Analysis of day-10 data (Fig. 4B) identified a significant metyrapone pretreatment × EtOH treatment × time interaction (F3,102 = 3.9; p < 0.05). Generally, the data suggested the presence of tolerance to some effects: EtOH increased CORT levels, but only at the first 15-min time point. Metyrapone blocked this EtOH-induced increase and had no effect on CORT levels after saline. Analysis of ethanol challenge day-11 data (Fig. 4C), when all mice received a vehicle injection followed by EtOH, only identified a significant effect of time (F3,102 = 15.1; p < 0.001), indicating that previous metyrapone treatment did not alter the CORT response to EtOH. Furthermore, CORT levels were similar across groups whether they were receiving EtOH for the first or 11th time.
Fig. 4.
Metyrapone reduces EtOH (E)-induced increases in CORT levels. Shown are plasma CORT values (μg/dl; mean ± S.E.M) on days 1 (A), 10 (B), and 11 (C) (n = 7–10 per group). D2 mice were pretreated with vehicle (V) or 50 mg/kg of metyrapone (M) 30 min before receiving saline (S) or EtOH (1.5 g/kg) on 10 consecutive days. Then, on day 11, all animals received a vehicle-EtOH (1.5 g/kg) treatment. Each animal contributed a blood sample on days 1, 10, and 11, but blood samples were collected at 15, 30, 60, or 120 min after saline or EtOH from independent groups of mice. **, p < 0.01 for the comparison with the respective vehicle-saline group. †, p < 0.05, ††, p < 0.01, and †††, p < 0.001 for the comparison between metyrapone-EtOH and vehicle-EtOH groups.
Repeated Injections of CORT Induce Sensitization to the Locomotor-Activating Effects of EtOH.
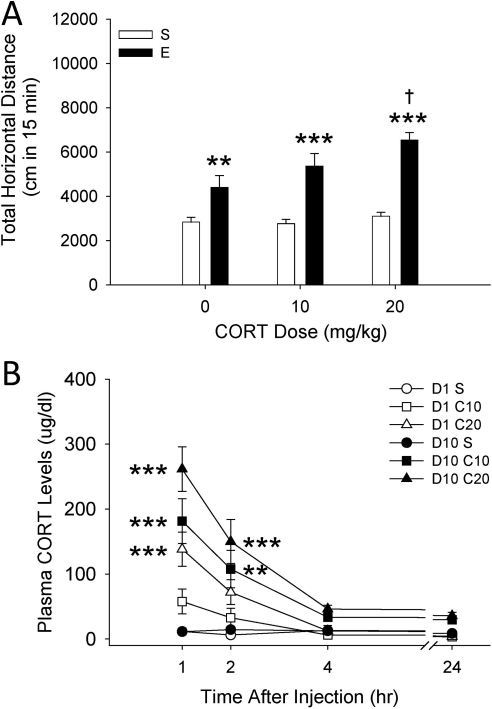
Figure 5A shows the locomotor response to saline or EtOH on day 11 in mice treated for 10 previous days with vehicle or 10 or 20 mg/kg of CORT. Results from a two-way ANOVA indicated that the EtOH response depended on previous CORT dose (F2,74 = 2.2; p < 0.05 for the CORT dose by EtOH dose interaction). EtOH produced significant activation in all pretreatment groups; however, animals that had received 20 mg/kg of CORT for 10 days were significantly more activated by EtOH compared with those that had received vehicle. This indicates a sensitized response to the EtOH challenge in the 20 mg/kg CORT-treated group. No differences among groups were found in BEC after testing on day 11, and locomotion after saline injection on day 12 was not different among groups (Supplemental Fig. 3, A and B).
Fig. 5.
Repeated CORT injections induce a sensitized response to the locomotor-stimulating effects of EtOH (E). A, total distance traveled (cm; mean ± S.E.M.) after treatment with saline (S) or 1.5 g/kg EtOH in D2 mice (n = 13–14 per group) that were pretreated on 10 days with CORT (0, 10, or 20 mg/kg). **, p < 0.01 and ***, p < 0.001 for the comparison with the respective saline-treated group. †, p < 0.05 for the comparison with the 0 mg/kg CORT-EtOH group. B, plasma CORT levels (μg/dl; mean ± S.E.M.) obtained on days 1 and 10 of CORT administration (different animals than those shown in A; n = 10 per group). Blood samples were collected from separate groups of animals at 1, 2, 4, or 24 h after CORT administration, but the same animals contributed samples on both days 1 and 10. **, p < 0.01 and ***, p < 0.001 for the comparison with the respective saline-treated control on the same day and at the same time.
Exogenous CORT Administration Increases Plasma CORT Levels.
Analysis of blood CORT data (Fig. 5B) from day 1 indicated that changes in CORT levels across time depended on CORT dose (F6,108 = 6.6; p < 0.001 for the CORT dose by time interaction). CORT levels found at the 1-h time point were dose-dependent, and a marginally significant elevation in CORT remained for the 20 mg/kg CORT group at 2 h (p = 0.057); lower levels of CORT were found across dose groups at the 4- and 24-h time points. Analysis of data from day 10 indicated that repeated CORT treatment increased blood CORT levels beyond those seen after the first CORT treatment. Initial three-way ANOVA confirmed a main effect of day (F1,108 = 54.1; p < 0.001). Analysis of CORT-level data for day 10 indicated only that changes in CORT levels across time depended on CORT dose (F6,108 = 7.7; p < 0.001 for the CORT dose by time interaction). Similar to day-1 data, the 20 mg/kg CORT dose increased CORT levels compared with vehicle-treated animals. However, in addition, the 10 mg/kg CORT dose produced a significant elevation. For both doses, CORT levels were significantly elevated at the 1- and 2-h time points, but not at 4 or 24 h.
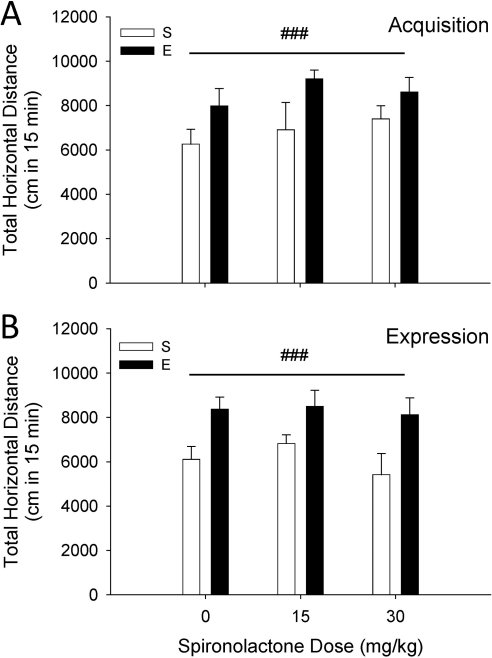
The MR Antagonist Spironolactone Does Not Prevent the Acquisition or Expression of EtOH Sensitization.
Figure 6 shows the effects of spironolactone on the acquisition (A) and expression (B) of EtOH sensitization. Both sets of data were analyzed by two-way ANOVA. No effect of spironolactone given before each EtOH treatment during the 10-day repeated-treatment phase was found (Fig. 6A). Likewise, no effect of sprinonolactone given only before EtOH challenge on day 11 was found (Fig. 6B). For both studies, higher levels of activity were found on EtOH challenge day 11 in mice that had received repeated EtOH treatments on days 1 to 10, compared with those that had received saline (F1,61 = 25.9, p < 0.001 for the main effect of EtOH dose in the acquisition study; F1,57 = 18.9, p < 0.001 for the main effect of EtOH dose in the expression study). There were no differences among groups for BEC or day-12 locomotor activity level after saline treatment (Supplemental Fig. 4).
Fig. 6.
Acquisition and expression of EtOH (E)-induced behavioral sensitization are not prevented by the mineralocorticoid receptor antagonist spironolactone. A, results for the acquisition study. Total 15-min locomotor activity (mean cm ± S.E.M.) response to 1.5 g/kg EtOH in D2 mice (n = 8–13 per group) pretreated for 10 days with spironolactone (0, 10, or 30 mg/kg) 30 min before receiving saline (S) or 1.5 g/kg EtOH. B, results for the expression study. Total 15-min locomotor activity (mean cm ± S.E.M.) in D2 mice (n = 7–9 per group) that received vehicle-saline or vehicle-EtOH during days 1 to 10. On day 11, they were pretreated with spironolactone (0, 10, or 30 mg/kg) 30 min before receiving EtOH. For both acquisition and expression no effects of spironolactone were found. A main effect of repeated EtOH versus saline treatment, supporting the presence of sensitization, was found in both studies (###, p < 0.001).
Discussion
In the present article, the use of a combination of genetic and pharmacological approaches allowed us to identify the specific role of CRF and CORT in EtOH-induced behavioral sensitization. Our findings demonstrated that deletion of CRF was able to prevent sensitization to the locomotor-activating effects of EtOH. It is noteworthy that CRF deficiency did not alter spontaneous locomotion, the stimulant response to acute EtOH, or sensitivity to the sedative-hypnotic effect of EtOH. This suggests that CRF is involved in the neuroadaptive processes induced by repeated EtOH administration but does not affect general neurosensitivity to EtOH. These data are consistent with our previous results in CRF1 KO mice (Pastor et al., 2008). CRF1 KO and WT mice did not differ in their locomotor activity when tested after saline or acute EtOH. However, after repeated administration of EtOH psychomotor sensitization was seen only in WT mice. These results are consistent with those obtained by using the selective CRF1 antagonist CP-154,526, which addresses possible alternative interpretations associated with the use of KO mice (e.g., passenger gene effects; compensatory effects during development). Together with previous results showing no involvement of CRF2 or urocortin-1 in EtOH sensitization (Pastor et al., 2008), our data strongly suggest that CRF signaling via CRF1 is a critical mechanism underlying neural changes associated with behavioral sensitization induced by repeated EtOH treatment.
Central CRF activity via CRF1 has been implicated in a number of behavioral effects of EtOH that involve neuroadaptation. Excessive EtOH consumption in EtOH-dependent animals and withdrawal- and stress-induced reinstatement of EtOH drinking have been associated with changes in central CRF1 signaling activity (Heilig and Koob, 2007; Lowery and Thiele, 2010). EtOH has been shown to sensitize the response to the motor-activating effects of centrally administered CRF (Ehlers and Chaplin, 1987), and the selective CRF1 antagonist CP-154,526 attenuated both the acquisition and expression of locomotor sensitization to EtOH (Fee et al., 2007; Pastor et al., 2008). In addition, the CRF1 antagonist 2-(N-(2-methylthio-4-isopropylphenyl)-N-ethylamino-4-(4-(3-fluorophenyl)-1,2,3,6-tetrahydropyridin-1-yl)-6-methylpyrimidine) (CRA-1000) minimized multiple-stressor/withdrawal-induced sensitization of anxiety-like behavior (Breese et al., 2004). In the case of EtOH drinking, studies using CRF1 manipulations have found that these receptors are involved in EtOH effects, especially when tested in postdependent animals, in lines of rodents bred for elevated EtOH consumption or under the effects of stress (Heilig and Koob, 2007; Pastor et al., 2011). Recent data also suggest that CRF1 can play a role in EtOH drinking even in nondependent or nonstressed animals if they are tested under conditions that facilitate elevated or binge-like drinking (Lowery and Thiele, 2010; Pastor et al., 2011; Kaur et al., 2012). Thus, as proposed previously (Breese et al., 2004; Heilig and Koob, 2007; Lowery and Thiele, 2010; Kaur et al., 2012), CRF1 manipulations might be excellent candidates for the development of pharmaceutical interventions for excessive EtOH drinking or EtOH abuse in dependent, genetically susceptible, or stress-prone individuals.
It is well established that HPA-axis activation is initiated by hypothalamic CRF (Rivier, 1996). To date, direct actions of EtOH on CRF receptors have not been described. Rather, EtOH is thought to initiate its HPA-axis actions by up-regulating CRF expression (Lee et al., 2008) and secretion (Li et al., 2005) at the level of the paraventricular nucleus of the hypothalamus. The molecular mechanisms by which EtOH activates CRF gene expression and release remain to be completely elucidated, but results suggest that EtOH up-regulates CRF expression through cAMP/protein kinase A-dependent pathways that might also involve the participation of nitric oxide (Lee et al., 2008). Our present results indicate that the activating effects of EtOH on the HPA axis are blunted in CRF KO mice, because they do not show EtOH-induced CORT elevations such as those seen in WT mice. These results are consistent with the absent EtOH-induced CORT response seen in CRF1 KO animals compared with WT mice (Pastor et al., 2008). On the other hand, EtOH-induced CORT increases were found to be normal in CRF2 and urocortin-1 KO mice (Pastor et al., 2008), and EtOH administration has been found to increase the expression of hypothalamic CRF1, but not CRF2, mRNA (Lee and Rivier, 1997). Previous data indicate that the HPA axis participates in drug-induced sensitization, and GR receptor-mediated effects have been implicated in EtOH sensitization (Roberts et al., 1995; Pastor et al., 2008). Thus, we asked whether EtOH-induced changes in CORT are required for EtOH-induced psychomotor sensitization. Using the CORT synthesis inhibitor metyrapone, which reduced EtOH-increased CORT levels, we found that normal CORT responses to EtOH (i.e., increased CORT levels) are required for the acquisition but not the expression of EtOH sensitization. That is, once animals acquired sensitization to EtOH metyrapone was unable to alter the locomotor response to EtOH challenge. One might argue that we did not see an effect of metyrapone on the expression of sensitization because of a lack of efficiency of this treatment in animals that received repeated EtOH injections or because the doses used were not high enough. Adaptations in EtOH-induced CORT levels (progressive tolerance to EtOH-induced increases in CORT) have been, indeed, seen previously (Roberts et al., 1995). To explore this, we tested a higher dose (100 mg/kg) of metyrapone, and we obtained the same result (data not shown). There was no effect of this higher dose of metyrapone on the expression of EtOH sensitization; however, there was a trend for a reduction in baseline activity, indicating nonspecific effects on activity. Thus, existing data indicate that CORT synthesis inhibition affects the acquisition, but not the expression, of EtOH sensitization. Antagonism of GR has been found to prevent the acquisition, but not the expression, of sensitization to EtOH (Roberts et al., 1995; Pastor et al., 2008). This may be because extra-hypothalamic, CRF-dependent mechanisms are more critically involved in the expression of EtOH sensitization. In the present study we also ruled out the involvement of MRs in the acquisition of EtOH sensitization; the MR antagonist spironolactone (even when administered at doses that had effects on spontaneous locomotion) did not alter either the acquisition or expression of sensitization.
That CORT was necessary for induction of EtOH sensitization begged the question of whether CORT elevations alone are sufficient to explain sensitization to EtOH. To respond to this question, we administered exogenous CORT to induce systemic elevations in the absence of HPA axis-independent effects of EtOH. We found that, consistent with previous data obtained for psychostimulants (Deroche et al., 1992), repeated injections of CORT produced a sensitized response to the locomotor-activating effects of EtOH. CORT-level data confirmed significant increases in plasma levels produced by the two CORT doses used. It is noteworthy that we observed that the lower 10 mg/kg dose of CORT produced plasma levels (approximately 60 μg/dl) that were similar to those induced by EtOH (compared 60 min after CORT or EtOH administration; data shown in Figs. 4 and 5, respectively). However, repeated injections of 10 mg/kg of CORT did not sensitize mice to EtOH, whereas 20 mg/kg of CORT, which resulted in levels of plasma CORT that were almost three times higher than those induced by EtOH, did induce sensitization. We also explored CORT levels after 10 days of injections; we wondered whether CORT levels would be reduced after repeated administration, perhaps because of alterations in exogenous HPA axis-related mechanisms, which could explain the lack of an effect of the 10 mg/kg dose. However, rather than tolerance-like levels we found that CORT levels were elevated on day 10, compared with day 1. This could have been caused by accumulation across days. Therefore, unless there are pharmacokinetic differences between CORT increases derived from EtOH versus CORT injections, it seems that EtOH requires elevations in CORT to induce neural changes associated with the acquisition of sensitization. However, CORT increases alone may not be sufficient to explain the behavioral sensitization induced by repeated EtOH treatment.
Together with previous research, the current findings indicate a dissociated neurobiology underlying the acquisition and expression of EtOH-induced behavioral sensitization to EtOH. These two phases are temporally as well as neuroanatomically distinct (Robinson and Berridge, 1993, 2008). It is important to point out that the mechanisms involved in the expression of EtOH sensitization, although endocrine-independent, still seem to involve CRF and CRF1; CRF1 antagonism blocked the expression of EtOH sensitization (Fee et al., 2007; Pastor et al., 2008). Therefore, the present data have important clinical implications, because blockade of mechanisms associated with the expression of drug-induced sensitization could contribute to the management of addictive behavior. Current research shows that other neuroadaptive changes associated with alcoholism are also related to CRF and CRF1. CRF1 antagonists can block excessive alcohol drinking and the increases in anxiety-like symptoms after a history of EtOH dependence (Breese et al., 2004; Heilig and Koob, 2007; Lowery and Thiele, 2010; Kaur et al., 2012). In addition, they can prevent stress-induced reinstatement of EtOH-seeking in postdependent rodents (Heilig and Koob, 2007; Lowery and Thiele, 2010). CRF and CRF1 are emerging as critical components of the HPA-axis system that could be targeted for the treatment of excessive EtOH pursuit and consumption.
Supplementary Material
Acknowledgments
We thank Lauren Brown, Ju Li, and Dawn M. Cote for technical assistance; and Dr. Suzanne Mitchell for the use of test equipment.
This work was supported by the Department of Veterans Affairs and the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants R01-AA13331, P60-AA010760, R01-AA013738, R24-AA020245, U01016647].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- EtOH
- ethanol
- ANOVA
- analysis of variance
- BEC
- blood ethanol concentration
- CORT
- corticosterone
- CRF
- corticotropin-releasing factor
- CRF1
- CRF type-1 receptor
- CRF2
- CRF type-2 receptor
- D2
- DBA/2J
- GR
- glucocorticoid receptor
- HT
- heterozygous
- HPA
- hypothalamic-pituitary-adrenal
- KO
- knockout
- LORR
- loss of righting reflex
- MR
- mineralocorticoid receptor
- WT
- wild type
- CP-154,526
- N-butyl-N-ethyl-2,5-dimethyl-7-(2,4,6-trimethylphenyl)pyrrolo[3,2-e]pyrimidin-4-amine
- CRA-1000
- 2-(N-(2-methylthio-4-isopropylphenyl)-N-ethylamino-4-(4-(3-fluorophenyl)-1,2,3,6-tetrahydropyridin-1-yl)-6-methylpyrimidine).
Authorship Contributions
Participated in research design: Pastor, Ryabinin, and Phillips.
Conducted experiments: Pastor, Reed, Meyer, and McKinnon.
Contributed new reagents or analytic tools: Ryabinin.
Performed data analysis: Pastor, Reed, and Meyer.
Wrote or contributed to the writing of the manuscript: Pastor, Ryabinin, and Phillips.
References
- Abrahao KP, Quadros IM, Souza-Formigoni ML. (2008) Morphine attenuates the expression of sensitization to ethanol, but opioid antagonists do not. Neuroscience 156:857–864 [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. (2004) Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology 29:470–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent J, Grahame NJ, Cunningham CL. (1995) Haloperidol prevents ethanol-stimulated locomotor activity but fails to block sensitization. Psychopharmacology 120:475–482 [DOI] [PubMed] [Google Scholar]
- Broadbent J, Kampmueller KM, Koonse SA. (2003) Expression of behavioral sensitization to ethanol by DBA/2J mice: the role of NMDA and non-NMDA glutamate receptors. Psychopharmacology 167:225–234 [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. (1999) Baclofen alters ethanol-stimulated activity but not conditioned place preference or taste aversion in mice. Pharmacol Biochem Behav 63:325–331 [DOI] [PubMed] [Google Scholar]
- Correa M, Arizzi MN, Betz A, Mingote S, Salamone JD. (2003) Locomotor stimulant effects of intraventricular injections of low doses of ethanol in rats: acute and repeated administration. Psychopharmacology 170:368–375 [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Maccari S, Le Moal M, Simon H. (1992) Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res 598:343–348 [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI. (1987) Chronic ethanol exposure potentiates the locomotor-activating effects of corticotropin-releasing factor (CRF) in rats. Regul Pept 19:345–353 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. (2008) Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci 363:3125–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee JR, Sparta DR, Picker MJ, Thiele TE. (2007) Corticotropin releasing factor-1 receptor antagonist, CP-154,526, blocks the expression of ethanol-induced behavioral sensitization in DBA/2J mice. Neuroscience 150:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. (2005) Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res 156:105–114 [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. (2007) A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci 30:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. (2001) Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2:695–703 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Kalivas PW. (2008) Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res 14:185–189 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. (2005) Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45:647–650 [DOI] [PubMed] [Google Scholar]
- Kaur S, Li J, Stenzel-Poore MP, Ryabinin AE. (2012) Corticotropin-releasing factor acting on corticotropin-releasing factor receptor type 1 is critical for binge alcohol drinking in mice. Alcohol Clin Exp Res 36:369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig HN, Olive MF. (2004) The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology 29:999–1003 [DOI] [PubMed] [Google Scholar]
- Koob GF. (2009) Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry 42 (Suppl 1):S32–S41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi I, Kang S, Rivier C. (2008) Role of various neurotransmitters in mediating the long-term endocrine consequences of prenatal alcohol exposure. Ann NY Acad Sci 1144:176–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Rivier C. (1997) Alcohol increases the expression of type 1, but not type 2 α corticotropin-releasing factor (CRF) receptor messenger ribonucleic acid in the rat hypothalamus. Brain Res Mol Brain Res 52:78–89 [DOI] [PubMed] [Google Scholar]
- Lessov CN, Phillips TJ. (1998) Duration of sensitization to the locomotor stimulant effects of ethanol in mice. Psychopharmacology 135:374–382 [DOI] [PubMed] [Google Scholar]
- Li Z, Kang SS, Lee S, Rivier C. (2005) Effect of ethanol on the regulation of corticotrophin-releasing factor (CRF) gene expression. Mol Cell Neurosci 29:345–354 [DOI] [PubMed] [Google Scholar]
- Lowery EG, Thiele TE. (2010) Pre-clinical evidence that corticotropin-releasing factor (CRF) receptor antagonists are promising targets for pharmacological treatment of alcoholism. CNS Neurol Disord Drug Targets 9:77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Rougé-Pont F, De Jesus-Oliveira C, Le Moal M, Piazza PV. (1997) Acute blockade of corticosterone secretion decreases the psychomotor stimulant effects of cocaine. Neuropsychopharmacology 16:156–161 [DOI] [PubMed] [Google Scholar]
- Masur J, Boerngen R. (1980) The excitatory component of ethanol in mice: a chronic study. Pharmacol Biochem Behav 13:777–780 [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Palmer AA, McKinnon CS, Phillips TJ. (2005) Behavioral sensitization to ethanol is modulated by environmental conditions, but is not associated with cross-sensitization to allopregnanolone or pentobarbital in DBA/2J mice. Neuroscience 131:263–273 [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Phillips TJ. (2007) Behavioral sensitization to ethanol does not result in cross-sensitization to NMDA receptor antagonists. Psychopharmacology 195:103–115 [DOI] [PubMed] [Google Scholar]
- Muglia L, Jacobson L, Dikkes P, Majzoub JA. (1995) Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature 373:427–432 [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. (1999) Chronic tolerance and sensitization to alcohol in sons of alcoholics: II. Replication and reanalysis. Exp Clin Psychopharmacol 7:234–243 [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Weiss B, Rivier C. (1998) Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. Alcohol Clin Exp Res 22 (5 Suppl):243S–247S [DOI] [PubMed] [Google Scholar]
- Palmer AA, Low MJ, Grandy DK, Phillips TJ. (2003) Effects of a Drd2 deletion mutation on ethanol-induced locomotor stimulation and sensitization suggest a role for epistasis. Behav Genet 33:311–324 [DOI] [PubMed] [Google Scholar]
- Pastor R, Aragon CM. (2006) The role of opioid receptor subtypes in the development of behavioral sensitization to ethanol. Neuropsychopharmacology 31:1489–1499 [DOI] [PubMed] [Google Scholar]
- Pastor R, McKinnon CS, Scibelli AC, Burkhart-Kasch S, Reed C, Ryabinin AE, Coste SC, Stenzel-Poore MP, Phillips TJ. (2008) Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc Natl Acad Sci U S A 105:9070–9075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor R, Reed C, Burkhart-Kasch S, Li N, Sharpe AL, Coste SC, Stenzel-Poore MP, Phillips TJ. (2011) Ethanol concentration-dependent effects and the role of stress on ethanol drinking in corticotropin-releasing factor type 1 and double type 1 and 2 receptor knockout mice. Psychopharmacology 218:169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Roberts AJ, Lessov CN. (1997) Behavioral sensitization to ethanol: genetics and the effects of stress. Pharmacol Biochem Behav 57:487–493 [DOI] [PubMed] [Google Scholar]
- Rivier C. (1996) Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res 20:240–254 [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Lessov CN, Phillips TJ. (1995) Critical role for glucocorticoid receptors in stress- and ethanol-induced locomotor sensitization. J Pharmacol Exp Ther 275:790–797 [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. (2008) Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci 363:3137–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. (2006) Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol 11:2–38 [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Coste SC, Burkhart-Kasch S, Li N, Stenzel-Poore MP, Phillips TJ. (2005) Mice deficient in corticotropin-releasing factor receptor type 2 exhibit normal ethanol-associated behaviors. Alcohol Clin Exp Res 29:1601–1609 [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology 151:99–120 [DOI] [PubMed] [Google Scholar]
- Vezina P. (2004) Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev 27:827–839 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.