Abstract
Vagal nerve stimulation (VNS) has been approved for treatment of refractory depression. However, there have been few, if any, studies directly comparing the effects produced by VNS in animals with those caused by antidepressants, particularly using clinically relevant stimulation parameters in nonanesthetized animals. In this study, ΔFosB immunohistochemistry was used to evaluate different brain regions activated by long-term administration of VNS. Effects of VNS were compared with those caused by sertraline or desipramine (DMI). Double-labeling of ΔFosB and serotonin was used to determine whether serotonergic neurons in the dorsal raphe nucleus (DRN) were activated by long-term VNS. VNS significantly increased ΔFosB staining in the nucleus tractus solitarius (NTS), parabrachial nucleus (PBN), locus ceruleus (LC), and DRN, as well as in many cortical and limbic areas of brain including those involved in mood and cognition. Most, but not all, of these effects were seen also upon long-term treatments of rats with sertraline or DMI. Some areas where VNS increased ΔFosB (e.g., the NTS, PBN, LC, and peripeduncular nucleus) were not affected significantly by either drug. Sertraline was similar to VNS in causing an increase in the DRN whereas DMI did not. Double-labeling of the DRN with ΔFosB and an antibody for serotonin revealed that only a small percentage of ΔFosB staining in the DRN colocalized with serotonergic neurons. The effects of VNS were somewhat more widespread than those caused by the antidepressants. The increases in ΔFosB produced by VNS were either equivalent to and/or more robust than those seen with antidepressants.
Introduction
Vagal nerve stimulation (VNS) was approved by the U.S. Food and Drug Administration for treatment-resistant epilepsy (1997) and for treatment-resistant depression (2005). Clinical data show that both response (27–53%) and remission (15–33%) rates over 12 months were significantly higher in patients who received VNS treatment in addition to medications compared with what was reported in another study with similar patients who received only medications (12% response and 4% remission) (Dunner et al., 2006), and such improvement continues for 24 months (Rosa and Lisanby, 2012).
Despite these promising clinical results, there have been relatively few preclinical studies of the effects produced by VNS in animals, particularly using clinically relevant stimulation parameters in nonanesthetized animals. Most of the previous studies examined only short-term effects of VNS in anesthetized animals (Rutherfurd et al., 1992; Yousfi-Malki and Puizillout, 1994). Anesthesia can change the threshold for activation of different types of fibers in the vagal bundle (Woodbury and Woodbury, 1990). In addition, in most of the earlier studies, stimulation was performed using stimulation parameters that resulted in changes in peripheral autonomic function, which would produce reflexes that could activate brain regions and complicate the interpretation of the results.
We found previously (Furmaga et al., 2011) that VNS given as it was in this study and for a similar time period caused anxiolytic-like and antidepressant-like effects. It was of interest then to examine what regions of brain were activated when VNS caused behavioral effects.
Immunohistochemistry of c-Fos, an immediate early gene product, has become the most widely used functional anatomical mapping tool to identify activation of cells (Kovács, 1998). There are four major members of the Fos protein family: c-Fos, FosB, Fra-1, and Fra-2. FosB has a splice variant termed ΔFosB. These proteins respond to stimuli with different time courses. In general, maximal levels of c-Fos protein occur within 1 to 3 h of stimulus exposure and disappear in 4 to 6 h, whereas ΔFosB shows a more delayed activation but persists longer (McClung et al., 2004). Hence, c-Fos has been suggested to be an indicator of short-term neuronal activation, whereas ΔFosB may reflect longer-term neuroadaptations (McClung et al., 2004).
Several studies have examined mRNA for c-fos or c-Fos protein after antidepressant treatments, with the majority studying short-term effects (Beck, 1995; Fraga et al., 2005; Slattery et al., 2005). Data from these studies have been inconsistent with respect to activation in the dorsal raphe nucleus (DRN), locus ceruleus (LC), hippocampus, and cortical areas of brain. In addition, long-term treatment of rats with either paroxetine (Muigg et al., 2007) or citalopram (Kuipers et al., 2006) did not increase c-Fos in any brain region examined. We measured c-Fos and ΔFosB previously and found that long-term (3 weeks) administration of VNS in conscious Sprague-Dawley rats significantly increased ΔFosB staining in several areas of brain (Cunningham et al., 2008). Because of this and the aforementioned literature, it was of interest to compare the activation patterns of long-term VNS in many more regions of brain than those studied previously with those produced by long-term treatment with antidepressants, primarily focusing on ΔFosB. The antidepressants selected for study, sertraline (SERT) and desipramine (DMI), target serotonin and norepinephrine neurons, respectively. This is the first study to compare directly such activation patterns after long-term treatment with VNS and antidepressants in nonanesthetized rats. In light of its effectiveness in treatment-resistant depression, we speculated that VNS might cause a broader pattern of activation than that seen with antidepressants or the extent of activation in certain areas would be greater than that seen with the drugs.
Materials and Methods
Experiments were performed using adult male Sprague-Dawley rats, weighing 250 to 350 g (Harlan, Houston, TX). Rats were group-housed and maintained in a temperature-controlled environment on a 14:10-h light/dark cycle. Rats had ad libitum access to food and water. Experimental protocols were approved by the institutional animal care and use committee in accordance with the guidelines of the U.S. Public Health Service, American Physiological Society, and Society for Neuroscience.
Vagus nerve electrodes were implanted on the left vagus nerve under aseptic conditions. The surgical procedure was similar to that described by Cunningham et al. (2008) except that the anesthetic was a combination of 75 mg/kg ketamine and 0.5 mg/kg medetomidine. In brief, the coil electrode was placed around the left cervical vagus nerve and carotid sinus ventral to the carotid bifurcation. The bipolar stimulating electrode was configured with the cathode at the proximal lead and the anode at the distal lead to preferentially direct action potential propagation toward the central nervous system by creating an anodal block at the distal lead. The electrodes were connected to a stimulator pack (Cyberonics, Inc., Houston, TX) that was sutured in placed in a subcutaneous pouch created on the back of the rat. Rats that received VNS were instrumented with an operational stimulator pack that was programmed by a handheld computer. Controls received a dummy simulator pack that was the same size and weight (48 mm × 33 mm × 7.1 mm; 16 g). Beginning 7 days after surgery, rats received continuous VNS treatment for 14 days. The stimulation paradigm consisted of one burst of 20 Hz, a 250 μs pulse width, and 250 μA current for 30 s every 5 min for 2 weeks. These stimulation parameters are very similar to those used initially in clinical studies (Rush et al., 2005), although parameters may change if patients do not respond. In addition, we found previously that this stimulation protocol does not cause changes in blood pressure, heart rate, respiratory frequency, or locomotor activity in comparison with that measured in rats receiving “dummy” stimulation (Cunningham et al., 2008). However, raising the stimulation current to 500 μA did cause autonomic effects (H. Furmaga, J. T. Cunningham, and A. Frazer, unpublished data). Thirty minutes after the end of continuous VNS for 14 days, rats were perfused, and the brains were removed for subsequent analysis of ΔFosB.
Implantation of Osmotic Minipumps.
One day before surgery, osmotic minipumps delivering 5 μl/h (model 2ML2; Durect Corporation, Cupertino, CA) were filled with drug or vehicle, filtered through 0.9-μm nitrocellulose filters (Millipore Corporation, Billerica, MA) using a sterile technique under an air-filtered hood. Drug solution concentrations were determined on the basis of the mean weight of the rats over the 14 days of treatment. Doses were 7.5 mg/kg per day of sertraline or 10 mg/kg per day of desipramine as these have been shown previously to produce serum concentrations of drug in the therapeutic range (Benmansour et al., 1999). Specific surgical details are given in Furmaga et al. (2011). Rats were perfused at the end of the experiment with the minipumps still in place, and the brains were removed for subsequent immunohistochemical analysis.
Immunohistochemistry of ΔFosB.
The staining procedure was similar to that described by Cunningham et al. (2008). In brief, rats were anesthetized with a cocktail of 75 mg/kg ketamine and 0.5 mg/kg medetomidine and perfused with 0.1 M phosphate-buffered saline (PBS) followed by 500 ml of 4% paraformaldehyde in PBS. Brains were removed and placed in PBS with 30% sucrose for 4 days. Three serial sets of 40-μm coronal sections were cut in a cryostat and placed in cryoprotectant and stored at −20°C until processed for ΔFosB immunohistochemistry.
Sets of serial sections were stained for FosB [goat anti-FosB (102); Santa Cruz Biotechnology, Inc., Santa Cruz, CA]. The primary antibody used in this study does not discriminate between FosB and its splice variant ΔFosB. However, because of the fact that ΔFosB accumulates with long-term stimulation as a result of its long half-life, particularly the 37-kDa isoform (McClung et al., 2004), the antibody chosen for this study can specifically detect this isoform. For this reason, we refer to long-term stimulation increasing ΔFosB levels although a contribution from FosB cannot be excluded. To assess ΔFosB, sections were incubated with FosB antibody (1:5000) for 72 h at 4°C. The sections were then incubated with Alexa Fluor 488 donkey anti-goat IgG (1:1000; Molecular Probes, Carlsbad, CA). Some sets of sections were double-labeled for ΔFosB and serotonin (1:300; Abcam, Cambridge, MA). Sections were then incubated with Alexa Fluor 488 donkey anti-goat IgG (1:1000) for FosB and Alexa Fluor 546 donkey anti-mouse IgG (1:1000) for serotonin at room temperature for 4 h. The sections were washed in PBS and mounted on gelatin-coated slides and coverslipped using ProLong Gold Antifade Reagent (Invitrogen, Carlsbad, CA). No immunoreactivity was detected with controls that were incubated with either primary or secondary antibody alone.
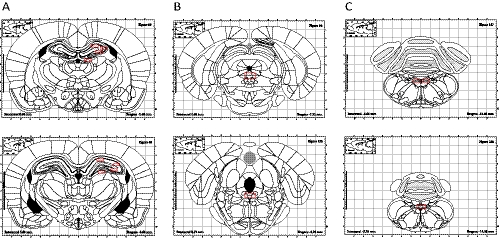
The number of ΔFosB-positive cells per section in selected brain regions was quantified by observers blind to the experimental conditions as described previously (Cunningham et al., 2008). More areas were analyzed than in our previous study and included the nucleus tractus solitaries (NTS), LC, DRN, parabrachial nucleus (PBN), basolateral (BLA) and central (CeA) nuclei of the amygdala, hippocampus, frontal cortex, cingulate cortex, nucleus accumbens (NAc), striatum, bed nucleus stria terminalis (BNST), substantia nigra (SN) or ventral tegmental area (VTA), and peripeduncular area. The areas were defined on the basis of the stereotaxic atlas of Paxinos and Watson (1986). Areas in which cell counts were quantified are shown with red boxes over representative atlas schematic diagrams (Fig. 1). Sections were examined by fluorescence microscopy. For quantitative analysis, at least four representative sections from each brain region were imaged. Digital images were collected using an Olympus BX40 microscope equipped with a DP72 Olympus camera connected to a Pentium computer running imaging software.
Fig. 1.
Representative atlas schematic diagrams of areas in which ΔFosB staining (shown with red boxes) was quantified after either long-term VNS, sertraline (7.5 mg/kg per day i.p.), or desipramine (10 mg/kg per day i.p.) treatments. A, hippocampus from bregma −2.40 mm to bregma −3.60 mm. B, raphe nucleus from bregma −7.32 mm to bregma −8.28 mm. C, nucleus tractus solitarius from bregma −13.68 to bregma −14.28.
Single-Labeling In Situ Hybridization for c-Fos mRNA.
The labeling procedure was similar to that described by Liu et al. (2007). Antisense 35S-labeled cRNA probes for rat c-Fos were generated with 35S-UTP and 35S-CTP using a standard transcription system. Brain sections were mounted on polylysine-coated slides, fixed in 4% paraformaldehyde for 1 h, and rinsed in 2× SSC (300 mM NaCl and 30 mM sodium citrate, pH 7.2). Brain sections were acetylated in 0.1 M triethanolamine, pH 8.0, with 0.25% acetic anhydride (for 10 min) and dehydrated through a graded series of alcohol (50–100%) and subsequently air-dried. 35S-labeled cRNA probes were diluted to 3 × 104 cpm/μl in 50% hybridization buffer (50% formamide, 10% dextran sulfate, 3× SSC, 50 mM sodium phosphate buffer, pH 7.4, 1× Denhardt's solution, 0.1 mg/ml yeast tRNA, and 10 mM dithiothreitol). Brain sections were hybridized with 70 μl of the diluted probes and placed in plastic trays moistened with 50% formamide at 55°C overnight. On the following day, coverslips were lifted with 2× SSC, and slides were rinsed three times for 5 min each in 2× SSC and incubated in RNase A buffer containing 200 μg/ml RNase A for 1 h at 37°C followed by a series of washes of increasing stringency (2×, 1×, 0.5×, and 0.1× SSC, for 5 min each at room temperature). The sections were then placed in 0.1× SSC at 65°C for 1 h, rinsed in distilled water, and dehydrated through a graded series of alcohol. Brain sections were exposed to X-ray film (BioMax MR; Eastman Kodak, Rochester, NY) for 7 days.
Levels of mRNA for c-fos were evaluated by analyzing film autoradiography. Films were visualized under a charge-coupled device camera (model XC-77; Sony, Tokyo, Japan), and brain section images were captured and analyzed with the National Institutes of Health ImageJ image analysis system. Signals were expressed as optical density levels above threshold. The threshold level was defined as 3.5 S.D.s above the mean optical density of a region. Results were expressed as integrated optical density, which is the product of the signal intensity and number of pixels above the threshold within the defined brain region. Equivalent planes of coronal brain sections through the DRN were ensured for analysis between animals.
Statistical Analysis.
The mean of cell counts from multiple sections was calculated for each rat, and then the mean of all rats in a treatment group was determined. Data were analyzed by one-way ANOVA followed by Student-Newman-Keuls post hoc tests. To enable direct comparisons between the effects of VNS and the antidepressants, a one-way ANOVA was performed for all groups for each area of brain. P < 0.05 was considered statistically significant. All values are presented as mean ± SEM.
Results
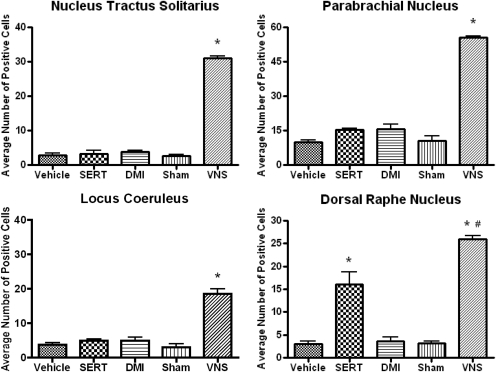
Administration of VNS for 14 days to conscious Sprague-Dawley rats significantly increased ΔFosB staining in the NTS [F(4,19) = 195.6, P < 0.0001] and regions that receive both direct and indirect projections from it such as the PBN [F(4,19) = 69.87, P < 0.0001], LC [F(4,19) = 49.58, P < 0.0001], and DRN [F(4,19) = 32.56, P < 0.0001] (Fig. 2). Because the NTS and PBN are not traditional targets of monoamine-based antidepressants, it was not unexpected that they were not affected significantly by either long-term administration of sertraline or DMI (Fig. 2). Sertraline significantly increased ΔFosB staining in the DRN, although the effect was not as robust as in the VNS. The patterns of activation were similar between VNS and sertraline within the DRN, with ΔFosB staining mainly localized to the lateral wings and little activation in the ventromedial subnucleus. In contrast, noradrenergic neurons in the LC were not activated either by DMI or sertraline (Fig. 2).
Fig. 2.
Effects of long-term VNS, SERT (7.5 mg/kg per day i.p.), or DMI (10 mg/kg per day i.p.) on ΔFosB staining in the NTS, LC, PBN, and DRN. *, significantly different from vehicle or sham control, P < 0.05. #, significantly different from SERT, P < 0.05. One-way ANOVA; Newman-Keuls post hoc test.
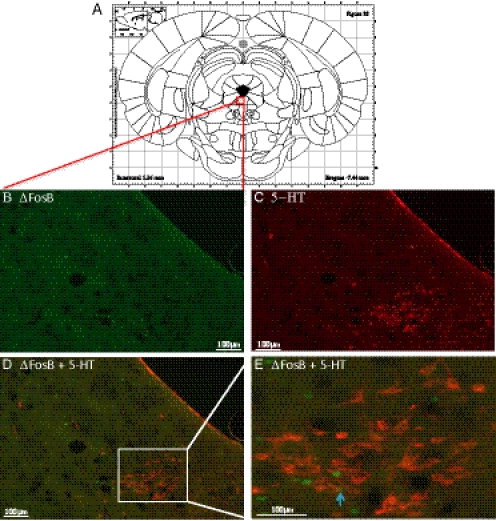
Because essentially all the cells in the LC are noradrenergic (Swanson, 1976), it can be assumed that noradrenergic cells are activated by VNS in the LC. However, only approximately 40% of the cells in the DRN are serotonergic (Nanopoulos et al., 1982). Thus, double-labeling immunohistochemistry was performed in the DRN to see whether the cells activated, i.e., exhibiting an increase in ΔFosB, were serotonergic. To do this, the cells in the DRN were labeled with an antibody for serotonin. As shown in Fig. 3, long-term VNS significantly increased ΔFosB staining in the DRN but only a very small percentage of cells labeled with ΔFosB colocalized with serotonergic cells.
Fig. 3.
Representative digital images of ΔFosB and 5-HT double-staining in the dorsal raphe nucleus in a long-term VNS-treated rat. A, representative atlas schematic diagram that contains all subnuclei of the DRN. B, ΔFosB staining. C, 5-ΗΤ staining. D, overlay of ΔFosB and 5-HT staining. E, inset at 40× magnification.
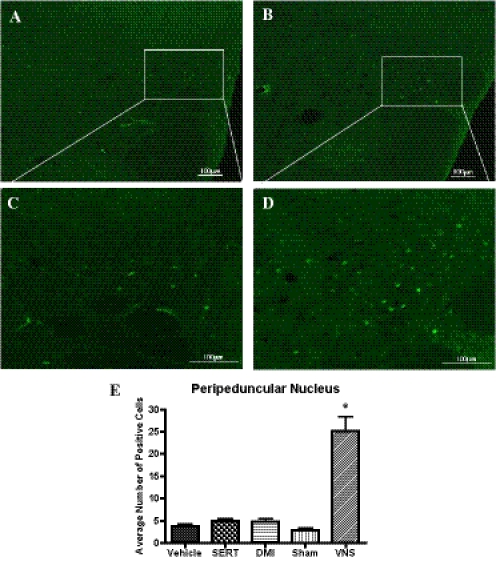
Because dopaminergic cell bodies are not traditional targets for antidepressant drugs, it was of interest to see whether VNS would cause activation in the SN or VTA. Neither VNS nor the drugs did this (data not shown). Of interest, on the sections containing the SN and VTA, it was observed that VNS produced staining in the peripeduncular nucleus [F(4,19) = 44.65, P < 0.0001], but it was subsequently found that the drug treatments did not do this (Fig. 4).
Fig. 4.
Representative digital images of ΔFosB staining in the peripeduncular nucleus at 10× magnification (A and B) and 20× magnification (C and D). A and C, long-term sham-treated rat. B and D, long-term VNS-treated rat. E, effects of long-term VNS, SERT (7.5 mg/kg per day i.p.), or DMI (10 mg/kg per day i.p.) on ΔFosB staining in the peripeduncular nucleus. *, significantly different from vehicle or sham control, P < 0.05. One-way ANOVA, Newman-Keuls post hoc test.
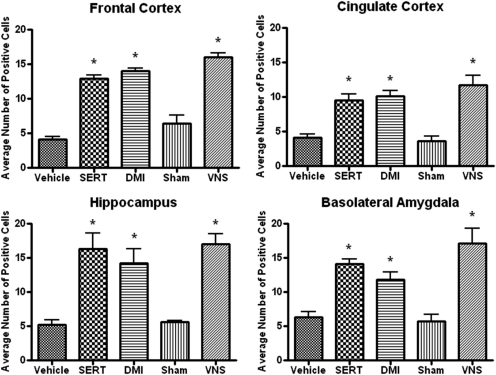
VNS induced widespread increases in ΔFosB in many cortical and limbic areas of brain including regions involved in mood and cognition. In all forebrain areas in which long-term sertraline or desipramine increased staining for ΔFosB, VNS did as well. In general, the increases produced by VNS were either equivalent to (Fig. 5) or more robust than (Fig. 6) those seen with the antidepressants.
Fig. 5.
Effects of long-term VNS, SERT (7.5 mg/kg per day i.p.), or DMI (10 mg/kg per day i.p.) on ΔFosB staining in the frontal cortex [F(4,19) = 85.95, P < 0.0001], cingulate cortex [F(4,19) = 80.38, P < 0.0001], hippocampus [F(4,19) = 8.821, P < 0.001], and basolateral nucleus of the amygdala [F(4,19) = 16.70, P < 0.0001]. *, significantly different from vehicle or sham control, P < 0.05. One-way ANOVA, Newman-Keuls post hoc test.
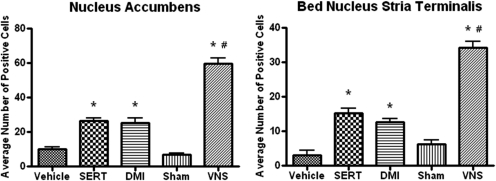
Fig. 6.
Effects of long-term VNS, SERT (7.5 mg/kg per day i.p.), or DMI (10 mg/kg per day i.p.) on ΔFosB staining in the nucleus accumbens [F(4,19) = 82.95, P < 0.0001] and bed nucleus stria terminalis [F(4,19) = 75.08, P < 0.0001]. *, significantly different from vehicle or sham control, P < 0.05. #, significantly different from SERT or DMI, P < 0.05. One-way ANOVA, Newman-Keuls post hoc test.
A comparison of the areas activated by long-term treatment with VNS, sertraline, or DMI is presented in Table 1. VNS caused widespread activation with only four areas, among 15 examined, not showing activation, namely the CeA, SN, VTA, and striatum. The two drug treatments did not activate these areas either (Table 1). Neither sertraline nor DMI elevated ΔFosB in the NTS, PBN, LC, and peripeduncular area, whereas sertraline elevated ΔFosB in the DRN and desipramine did not.
TABLE 1.
Summary data for the effects of long-term VNS, SERT, or DMI on ΔFosB staining in the rat brain
| NTS | LC | PBN | DRN | Hip | BLA | CeA | BNST | NAc | ST | Fr | Cg | SN | VTA | PP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VNS | + | + | + | ++ | + | + | 0 | ++ | ++ | 0 | + | + | 0 | 0 | + |
| SERT | 0 | 0 | 0 | + | + | + | 0 | + | + | 0 | + | + | 0 | 0 | 0 |
| DMI | 0 | 0 | 0 | 0 | + | + | 0 | + | + | 0 | + | + | 0 | 0 | 0 |
Hip, hippocampus; ST, striatum; Fr, frontal cortex; Cg, cingulate cortex; PP, peripeduncular nucleus.
+, significant increases in ΔFosB staining; ++, increase induced by VNS is significantly more than those of antidepressants; 0, no significant changes.
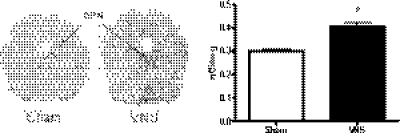
As stated previously, it is widely accepted that the maximal levels of c-Fos protein occur within 1 to 3 h of stimulus exposure and disappear in 4 to 6 h, whereas ΔFosB shows a more delayed activation that persists longer. Hence, increases in c-Fos have been suggested to be an indicator of short-term neuronal activation, whereas ΔFosB reflects long-term activation (McClung et al., 2004). Of interest, we observed increases in c-Fos after long-term VNS or antidepressant drug administration in the same brain regions as seen with ΔFosB (data not shown). Because of this somewhat surprising result, quantitative analysis of mRNA levels for c-fos using in situ hybridization was performed in rats given VNS. Expression of c-Fos increased in the DRN with VNS treatment, with the increase present throughout the nucleus (Fig. 7). This result implies that the increases in c-Fos measured by immunohistochemistry reflected increases in c-Fos.
Fig. 7.
Effects of VNS on c-fos mRNA levels in the DRN. *, significantly different from vehicle or sham control, P < 0.05 (Student's t test).
Discussion
VNS significantly increased ΔFosB staining in the NTS and regions that receive either direct and/or indirect projections from it such as the PBN, LC, and DRN (Peyron et al., 1996; Berthoud and Neuhuber, 2000). In addition, VNS induced widespread increases in ΔFosB in many cortical and limbic areas of brain including regions involved in mood and cognition. Most, but not all, of these effects were seen also upon repeated treatments of rats with sertraline or DMI. Neither drug treatment caused an increase in ΔFosB in the LC, whereas sertraline was similar to VNS in causing an increase in the DRN. In addition, neither drug treatment increased ΔFosB in the peripeduncular nucleus, whereas VNS did. In general, the increases produced by VNS were either equivalent to or more robust than those caused with antidepressants.
There is an anatomical rationale for VNS having effects on many cortical and limbic areas of brain involved in mood and cognition. Approximately 80% of vagal fibers carry afferent sensory information to the central nervous system (Foley and DuBois, 1937), with the initial projection area in brain being the NTS (Kalia and Sullivan, 1982). One set of projections from the NTS contains ascending projections from the NTS to the midbrain, hypothalamic, and cortical regions involved in central autonomic control; included in this ascending system are direct/and or indirect projections to the LC and DRN (Peyron et al., 1996; Van Bockstaele et al., 1999; Berthoud and Neuhuber, 2000) containing cell bodies for noradrenergic or serotonergic neurons, respectively, and it is well established that such neurons are targets for antidepressants. The NTS also sends projections throughout the brain involving areas thought to be involved in mood and emotion (Berthoud and Neuhuber, 2000).
The stimulation parameters used here are similar to those used initially in clinical studies (e.g., Rush et al., 2005). In addition, when VNS was given to rats using these stimulation parameters for time periods similar to those in the current study, both anxiolytic-like and antidepressant-like effects were observed (Furmaga et al., 2011). Thus, effects seen in this study are probably occurring in rats when VNS is effective behaviorally. If ΔFosB, then, can be used as an index of long-term cellular activation, some of the brain regions demonstrated to be activated in this study may be involved in the behavioral effects of VNS.
In agreement with our previous study (Cunningham et al., 2008), repeated VNS administration significantly increased ΔFosB staining in the NTS, PBN, LC, and DRN. Moreover, the present study revealed that VNS induced widespread ΔFosB activation in many cortical and limbic areas such as the frontal cortex, cingulate cortex, hippocampus, BNST, and NAc. Results of imaging studies in patients with epilepsy or depression who are treated with VNS also show widespread effects on subcortical and cortical regions, with short-term VNS producing increases in blood flow in the hypothalamus, thalamus, and insular cortex but decreases in the hippocampus and posterior cingulate gyrus (Chae et al., 2003). Long-term VNS produced both increased (Kosel et al., 2011) and decreased (Nahas et al., 2007) changes in blood flow in cortical regions although subcortical regions were activated (Henry et al., 2004). Inconsistent results were also obtained in the amygdala (Zobel et al., 2005; Conway et al., 2006). VNS causes short-term limbic hyperperfusion and long-term thalamic hypoperfusion in patients with refractory epilepsy, and these changes correlate with clinical efficacy (Vonck et al., 2008).
Data from previous studies that examined mRNA for c-fos or c-Fos protein after short-term antidepressant administration have been inconsistent with respect to the LC, DRN, and many cortical areas. Somewhat surprisingly, administration of fluoxetine (Fraga et al., 2005; Slattery et al., 2005) but not other types of antidepressants, with one exception (Kovács, 1998), was found to increase c-Fos in the LC. In general, increases have not been reported in the DRN although Fraga et al. (2005) found an increase after short-term administration of fluoxetine. Most studies show increases in c-fos or c-Fos in the CeA and BNST after short-term administration of different classes of antidepressants (Beck, 1995; Fraga et al., 2005; Slattery et al., 2005; Bechtholt et al., 2008). Long-term treatment of rats with either paroxetine (Muigg et al., 2007) or citalopram (Kuipers et al., 2006) did not increase c-Fos in any brain region examined, including the CeA. In contrast, Bechtholt et al. (2008) showed that long-term fluoxetine increased c-Fos in multiple brain areas including the BNST, cingulate cortex, anterior NAc, and hippocampus and long-term mirtazapine treatment increased c-Fos in the CeA and dentate gyrus (Gerrits et al., 2006).
There are very few studies that examined the effects of antidepressant drug treatments on ΔFosB. In a recent study, Vialou et al. (2010) showed that long-term fluoxetine treatment produced an accumulation of ΔFosB in the NAc shell. In addition, they showed that virus-mediated overexpression of ΔFosB in the rat NAc produced a significant antidepressant-like effect in the forced swim test.
Comparing such results with those seen after long-term sertraline and DMI in the present study reveals both similarities and differences. This is probably because most of the previous work involved short-term antidepressant treatment and measured c-Fos, whereas we report on ΔFosB. However, although the data are not shown, we measured c-Fos as well and found results with it that were comparable to those found with ΔFosB. Of interest, we found that sertraline increased c-Fos staining in the DRN, whereas this was not seen with or seen inconsistently with short-term antidepressant treatments other than fluoxetine (Fraga et al., 2005). Even though ΔFosB staining increased in the DRN, this was not primarily in serotonergic soma (Fig. 4). In agreement with most previous studies, we found that neither drug treatment caused an increase in ΔFosB in the LC. This finding is not surprising because activation of α2-autoreceptors in the LC persists in restraining norepinephrine neurotransmission in the face of tonically elevated basal norepinephrine levels after long-term reuptake blockade (Garcia et al., 2004). The increase in ΔFosB staining in the LC after VNS stimulation is probably a consequence of efferents from the NTS, the initial projection area of vagal afferents in brain, innervating noradrenergic dendrites of LC neurons with synaptic contacts characteristic of both excitatory- and inhibitory-type transmitters (Van Bockstaele et al., 1999).
Similar to the results found with long-term treatment of rats with paroxetine (Muigg et al., 2007) and citalopram (Kuipers et al., 2006), none of the treatments in this study increased ΔFosB in the CeA. Vialou et al. (2010) hypothesized that ΔFosB induction in NAc is required for the antidepressant action of fluoxetine. Whether this is true of sertraline and DMI remains to be seen although our data show that they, as well as VNS, also increase ΔFosB in the NAc.
Long-term VNS and antidepressants increased ΔFosB in many cortical areas including the frontal cortex, cingulate cortex, and NAc. As mentioned, antidepressant drug-induced increases in these areas have not consistently been observed by others. To the best of our knowledge, though, we are the first to examine this upon long-term administration of antidepressants administered using osmotic minipumps to obtain steady-state plasma concentrations of drugs in the therapeutic range. VNS increased ΔFosB in the BLA, although it did not in the CeA. The absence of an effect in the CeA is different from results reported by others studying effects of antidepressants, as mentioned above. However, we also did not see any effect of antidepressants on ΔFosB in the CeA. This difference could be due to the anesthetic used in the previous studies. It is well known that c-Fos expression can be greatly affected by anesthesia. For example, barbiturates may interfere with c-Fos expression, whereas other anesthetics such as urethane and chloralose cause a high level of baseline expression (Miura et al., 1994; Dampney et al., 1995; Rocha and Herbert, 1997).
Little, if any, overlap was found between the cells showing ΔFosB and those staining for 5-HT in the DRN. This is unexpected because Dorr and Debonnel (2006) showed that repeated administration of VNS to nonanesthetized rats, using stimulation parameters identical to ours, raised the firing rate of noradrenergic neurons in the LC and serotonergic neurons in the DRN. This discrepancy could be due to limitations of using Fos proteins as markers for neuronal activation. Neurons may differ in their capacity to produce Fos, the time course of Fos induction and decay varies with different inducing stimuli, and some brain regions do not express Fos regardless of stimuli (Dampney and Horiuchi, 2003). Herdegen et al. (1991) reported that the onset of Fos production in somatic motor neurons is considerably delayed (by several hours) compared with that in most other neurons (typically 30–60 min). The temporal pattern of activation is of great significance given that Dorr and Debonnel (2006) showed that stimulation of the vagus nerve for as little as 1 h produced increases in the firing rate of noradrenergic neurons, whereas it took 14 days for the firing rate of serotonergic neurons to increase. Moreover, Dragunow et al. (1989) reported that high-frequency stimulation protocols that produced good long-term potentiation do not lead to c-Fos induction. Hence, the absence of ΔFosB in serotonergic neurons in the DRN after long-term VNS does not necessarily mean that they were not activated, especially in light of the data of Dorr and Debonnel (2006).
As shown in Fig. 4, the majority of VNS-induced ΔFosB expression in the DRN was observed in its lateral margins and comprised an area corresponding to the ventrolateral periaqueductal gray (vLPAG). This distinctive pattern of ΔFosB induction is interesting because the vLPAG has been characterized previously as an important neural substrate for passive coping responses (Bandler and Shipley, 1994). Berton et al. (2007) show that the strongest ΔFosB induction in the vLPAG was observed in animals that were most resilient to behavioral despair. They hypothesized that expression of ΔFosB is part of an adaptive mechanism that promotes resilience to stress by inhibiting the stress-induced activation of substance P neurotransmission to the forebrain. This is in good agreement with our behavioral data showing that repeated administration of VNS produces anxiolytic-like and antidepressant-like effects in the novelty suppressed feeding test and the forced swim test, respectively (Furmaga et al., 2011).
The in situ hybridization signal for c-Fos occurs throughout the DRN, whereas ΔFosB immunohistochemistry was localized primarily to the lateral wings. The most likely reason for this discrepancy is that the increase in these two immediate early genes is occurring in different cells or at least the increase in ΔFosB is occurring in only a subset of cells in which c-Fos is elevated. As mentioned previously, these proteins respond to stimuli with different time courses with c-Fos increasing more rapidly than ΔFosB (McClung et al., 2004). Consistent with this, we found previously that 2 h of VNS increased c-Fos in many brain areas, whereas ΔFosB was not increased in any region at this time (Cunningham et al., 2008).
In conclusion, the present study identified potential sites in the brain activated by VNS. In general, the effects of VNS were somewhat more widespread than those caused by the antidepressants, and in some areas, the extent of activation was greater with VNS than with the drugs. Whether such differences in effects between VNS and antidepressants contribute to the utility of VNS in treatment-resistant depression remains to be determined.
Acknowledgments
We acknowledge the technical assistance of Aparna Shah and Dr. Milena Gerotti and thank Dr. David Morilak for insightful suggestions throughout the course of the experiment.
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant MH082933]. The electrodes, VNS stimulators, and dummy stimulators were gifts from Cyberonics, Inc. (Houston, TX).
Dr. Frazer has served on advisory boards for Lundbeck and for Takeda in the last 3 years. Previously, Dr. Frazer had received financial compensation as a consultant for Cyberonics Inc. and had also obtained grant support from them for a preclinical study.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- VNS
- vagal nerve stimulation
- DRN
- dorsal raphe nucleus
- LC
- locus ceruleus
- SERT
- sertraline
- DMI
- desipramine
- PBS
- phosphate-buffered saline
- NTS
- nucleus tractus solitarius
- PBN
- parabrachial nucleus
- BLA
- basolateral amygdala
- CeA
- central amygdala
- NAc
- nucleus accumbens
- BNST
- bed nucleus stria terminalis
- SN
- substantia nigra
- VTA
- ventral tegmental area
- SCC
- standard saline citrate
- ANOVA
- analysis of variance
- 5-HT
- serotonin
- vLPAG
- ventrolateral periaqueductal gray.
Authorship Contributions
Participated in research design: Furmaga and Frazer.
Conducted experiments: Furmaga and Sadhu.
Contributed new reagents or analytic tools: Furmaga and Sadhu.
Performed data analysis: Furmaga and Frazer.
Wrote or contributed to the writing of the manuscript: Furmaga, Sadhu, and Frazer.
References
- Bandler R, Shipley MT. (1994) Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci 17:379–389 [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Valentino RJ, Lucki I. (2008) Overlapping and distinct brain regions associated with the anxiolytic effects of chlordiazepoxide and chronic fluoxetine. Neuropsychopharmacology 33:2117–2130 [DOI] [PubMed] [Google Scholar]
- Beck CH. (1995) Acute treatment with antidepressant drugs selectively increases the expression of c-fos in the rat brain. J Psychiatry Neurosci 20:25–32 [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, Frazer A. (1999) Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci 19:10494–10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. (2000) Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85:1–17 [DOI] [PubMed] [Google Scholar]
- Berton O, Covington HE, 3rd, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, et al. (2007) Induction of ΔFosB in the periaqueductal gray by stress promotes active coping responses. Neuron 55:289–300 [DOI] [PubMed] [Google Scholar]
- Chae JH, Nahas Z, Lomarev M, Denslow S, Lorberbaum JP, Bohning DE, George MS. (2003) A review of functional neuroimaging studies of vagus nerve stimulation (VNS). J Psychiatr Res 37:443–455 [DOI] [PubMed] [Google Scholar]
- Conway CR, Sheline YI, Chibnall JT, George MS, Fletcher JW, Mintun MA. (2006) Cerebral blood flow changes during vagus nerve stimulation for depression. Psychiatry Res 146:179–184 [DOI] [PubMed] [Google Scholar]
- Cunningham JT, Mifflin SW, Gould GG, Frazer A. (2008) Induction of c-Fos and ΔFosB immunoreactivity in rat brain by vagal nerve stimulation. Neuropsychopharmacology 33:1884–1895 [DOI] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J. (2003) Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol 71:359–384 [DOI] [PubMed] [Google Scholar]
- Dampney RA, Li YW, Hirooka Y, Potts P, Polson JW. (1995) Use of c-fos functional mapping to identify the central baroreceptor reflex pathway: advantages and limitations. Clin Exp Hypertens 17:197–208 [DOI] [PubMed] [Google Scholar]
- Dorr AE, Debonnel G. (2006) Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther 318:890–898 [DOI] [PubMed] [Google Scholar]
- Dragunow M, Abraham WC, Goulding M, Mason SE, Robertson HA, Faull RL. (1989) Long-term potentiation and the induction of c-fos mRNA and proteins in the dentate gyrus of unanesthetized rats. Neurosci Lett 101:274–280 [DOI] [PubMed] [Google Scholar]
- Dunner DL, Rush AJ, Russell JM, Burke M, Woodard S, Wingard P, Allen J. (2006) Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J Clin Psychiatry 67:688–695 [DOI] [PubMed] [Google Scholar]
- Foley JO, DuBois F. (1937) Quantitative studies of the vagus nerve in the cat. I. The ratio of sensory motor studies. J Comp Neurol 67:49–67 [Google Scholar]
- Fraga IC, Fregoneze JB, Carvalho FL, Dantas KB, Azevedo CS, Pinho CB, de Castro E, Silva E. (2005) Acute fluoxetine administration differentially affects brain C-Fos expression in fasted and refed rats. Neuroscience 134:327–334 [DOI] [PubMed] [Google Scholar]
- Furmaga H, Shah A, Frazer A. (2011) Serotonergic and noradrenergic pathways are required for the anxiolytic-like and antidepressant-like behavioral effects of repeated vagal nerve stimulation in rats. Biol Psychiatry 70:937–945 [DOI] [PubMed] [Google Scholar]
- Garcia AS, Barrera G, Burke TF, Ma S, Hensler JG, Morilak DA. (2004) Autoreceptor-mediated inhibition of norepinephrine release in rat medial prefrontal cortex is maintained after chronic desipramine treatment. J Neurochem 91:683–693 [DOI] [PubMed] [Google Scholar]
- Gerrits M, Bakker PL, Koch T, Ter Horst GJ. (2006) Stress-induced sensitization of the limbic system in ovariectomized rats is partly restored by cyclic 17beta-estradiol administration. Eur J Neurosci 23:1747–1756 [DOI] [PubMed] [Google Scholar]
- Henry TR, Bakay RA, Pennell PB, Epstein CM, Votaw JR. (2004) Brain blood-flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: II. Prolonged effects at high and low levels of stimulation. Epilepsia 45:1064–1070 [DOI] [PubMed] [Google Scholar]
- Herdegen T, Kovary K, Leah J, Bravo R. (1991) Specific temporal and spatial distribution of JUN, FOS, and KROX-24 proteins in spinal neurons following noxious transsynaptic stimulation. J Comp Neurol 313:178–191 [DOI] [PubMed] [Google Scholar]
- Kalia M, Sullivan JM. (1982) Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol 211:248–265 [DOI] [PubMed] [Google Scholar]
- Kosel M, Brockmann H, Frick C, Zobel A, Schlaepfer TE. (2011) Chronic vagus nerve stimulation for treatment-resistant depression increases regional cerebral blood flow in the dorsolateral prefrontal cortex. Psychiatry Res 191:153–159 [DOI] [PubMed] [Google Scholar]
- Kovács KJ. (1998) c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int 33:287–297 [DOI] [PubMed] [Google Scholar]
- Kuipers SD, Trentani A, Westenbroek C, Bramham CR, Korf J, Kema IP, Ter Horst GJ, Den Boer JA. (2006) Unique patterns of FOS, phospho-CREB and BrdU immunoreactivity in the female rat brain following chronic stress and citalopram treatment. Neuropharmacology 50:428–440 [DOI] [PubMed] [Google Scholar]
- Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY. (2007) The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology 148:5531–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. (2004) ΔFosB: a molecular switch for long-term adaptation in the brain. Brain res Mol Brain Res 132:146–154 [DOI] [PubMed] [Google Scholar]
- Miura M, Takayama K, Okada J. (1994) Neuronal expression of Fos protein in the rat brain after baroreceptor stimulation. J Auton Nerv Syst 50:31–43 [DOI] [PubMed] [Google Scholar]
- Muigg P, Hoelzl U, Palfrader K, Neumann I, Wigger A, Landgraf R, Singewald N. (2007) Altered brain activation pattern associated with drug-induced attenuation of enhanced depression-like behavior in rats bred for high anxiety. Biol Psychiatry 61:782–796 [DOI] [PubMed] [Google Scholar]
- Nahas Z, Teneback C, Chae JH, Mu Q, Molnar C, Kozel FA, Walker J, Anderson B, Koola J, Kose S, et al. (2007) Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology 32:1649–1660 [DOI] [PubMed] [Google Scholar]
- Nanopoulos D, Belin MF, Maitre M, Vincendon G, Pujol JF. (1982) Immunocytochemical evidence for the existence of GABAergic neurons in the nucleus raphe dorsalis. Possible existence of neurons containing serotonin and GABA. Brain Res 232:375–389 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1986) The Rat Brain in Stereotaxic Coordinates, Academic Press, New York [Google Scholar]
- Peyron C, Luppi PH, Fort P, Rampon C, Jouvet M. (1996) Lower brainstem catecholamine afferents to the rat dorsal raphe nucleus. J Comp Neurol 364:402–413 [DOI] [PubMed] [Google Scholar]
- Rocha MJ, Herbert H. (1997) Effects of anesthetics on Fos protein expression in autonomic brain nuclei related to cardiovascular regulation. Neuropharmacology 36:1779–1781 [DOI] [PubMed] [Google Scholar]
- Rosa MA, Lisanby SH. (2012) Somatic treatments for mood disorders. Neuropsychopharmacology 37:102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, Howland R, Kling MA, Rittberg BR, Burke WJ, et al. (2005) Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry 58:347–354 [DOI] [PubMed] [Google Scholar]
- Rutherfurd SD, Widdop RE, Sannajust F, Louis WJ, Gundlach AL. (1992) Expression of c-fos and NGFI-A messenger RNA in the medulla oblongata of the anaesthetized rat following stimulation of vagal and cardiovascular afferents. Brain Res Mol Brain Res 13:301–312 [DOI] [PubMed] [Google Scholar]
- Slattery DA, Morrow JA, Hudson AL, Hill DR, Nutt DJ, Henry B. (2005) Comparison of alterations in c-fos and Egr-1 (zif268) expression throughout the rat brain following acute administration of different classes of antidepressant compounds. Neuropsychopharmacology 30:1278–1287 [DOI] [PubMed] [Google Scholar]
- Swanson LW. (1976) The locus coeruleus: a cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res 110:39–56 [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Telegan P. (1999) Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J Comp Neurol 412:410–428 [DOI] [PubMed] [Google Scholar]
- Vialou V, Maze I, Renthal W, LaPlant QC, Watts EL, Mouzon E, Ghose S, Tamminga CA, Nestler EJ. (2010) Serum response factor promotes resilience to chronic social stress through the induction of ΔFosB. J Neurosci 30:14585–14592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonck K, De Herdt V, Bosman T, Dedeurwaerdere S, Van Laere K, Boon P. (2008) Thalamic and limbic involvement in the mechanism of action of vagus nerve stimulation, a SPECT study. Seizure 17:699–706 [DOI] [PubMed] [Google Scholar]
- Woodbury DM, Woodbury JW. (1990) Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia 31 (Suppl 2):S7–S19 [DOI] [PubMed] [Google Scholar]
- Yousfi-Malki M, Puizillout JJ. (1994) Induction of Fos-like protein in neurons of the medulla oblongata after electrical stimulation of the vagus nerve in anesthetized rabbit. Brain Res 635:317–322 [DOI] [PubMed] [Google Scholar]
- Zobel A, Joe A, Freymann N, Clusmann H, Schramm J, Reinhardt M, Biersack HJ, Maier W, Broich K. (2005) Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: an exploratory approach. Psychiatry Res 139:165–179 [DOI] [PubMed] [Google Scholar]