Abstract
Macrophages play an integral role in the development of liver fibrosis by releasing mediators, such as platelet-derived growth factor-B (PDGF-B) and transforming growth factor-β1, which stimulate hepatic stellate cell proliferation, chemotaxis, and collagen production. However, the mechanism by which chronic liver injury stimulates macrophages to release these mediators is not completely understood. We tested the hypothesis that chronic liver injury activates hypoxia-inducible factor (HIF) transcription factors in macrophages that regulate the production of mediators that promote fibrosis. To test this hypothesis, Cre/lox technology was used to generate myeloid cell-specific HIF-1α or HIF-1β knockout mice. When these mice were subjected to bile duct ligation (BDL), levels of α-smooth muscle actin and type I collagen in the liver were reduced compared with those of mice with normal levels of HIFs. The deficiency of HIFs in macrophages did not affect liver injury or inflammation after BDL but reduced PDGF-B mRNA and protein, suggesting that HIF activation in macrophages may promote fibrosis by regulating the production of PDGF-B. Consistent with a role for HIFs in liver fibrosis in cholestatic liver disease, nuclear HIF-1α protein was present in macrophages, hepatocytes, and fibroblasts in the livers from patients with primary biliary cirrhosis and primary sclerosing cholangitis. These studies demonstrate that HIFs are important regulators of profibrotic mediator production by macrophages during the development of liver fibrosis and suggest that HIFs may be a novel therapeutic target for the treatment of chronic liver disease in patients.
Introduction
Humans are exposed to a variety of chemicals, pathogens, or conditions that produce chronic liver injury, including alcohol, hepatitis viruses, autoimmune diseases, and genetic disorders. The liver responds to injury by producing soluble mediators, such as growth factors, that facilitate liver repair (Fausto, 2000). This process is essential for the restoration of liver homeostasis after acute injury. During chronic injury, however, persistent activation of these pathways promotes pathological processes, including fibrosis and cancer (Friedman, 2008).
Macrophages play an integral role in the development of liver fibrosis. Studies have shown that the depletion of macrophages slows the progression of fibrosis in animal models (Rivera et al., 2001; Duffield et al., 2005). The mechanism by which macrophages promote fibrosis is not completely understood, although studies have shown that these cells express key mediators of fibrosis, such as platelet-derived growth factor-B (PDGF-B) and transforming growth factor-β (TGF-β) (Nakatsukasa et al., 1990; Faiz Kabir Uddin Ahmed et al., 2000). Accordingly, macrophages may promote fibrosis by releasing profibrotic growth factors that stimulate hepatic stellate cells and peribiliary fibroblasts to become activated and to produce collagen. However, what remains to be identified is the mechanism by which chronic liver injury stimulates macrophages to produce mediators that promote fibrosis. Our recent studies suggest that hepatocellular hypoxia may be important for this process.
Several studies have demonstrated that hepatocellular damage causes regions of hypoxia to develop in the liver (Ji et al., 1982; Rosmorduc et al., 1999; Corpechot et al., 2002; Copple et al., 2004; Moon et al., 2009; Rosmorduc and Housset, 2010). We demonstrated recently that the exposure of Kupffer cells to hypoxia in vitro increased the expression of PDGF-B, a potent mitogen and chemotaxin for hepatic stellate cells, and monocyte chemotactic protein-1 (MCP-1), a mediator of hepatic stellate cell chemotaxis (Copple et al., 2010). This study suggested that hypoxia may be a key driving force for the production of profibrotic mediators by macrophages during fibrosis. These studies further demonstrated that the up-regulation of PDGF-B and MCP-1 in hypoxic Kupffer cells required the transcription factor hypoxia-inducible factor-1α (HIF-1α) (Copple et al., 2010).
HIFs are a group of transcription factors activated in hypoxic cells (Semenza and Wang, 1992; Gaber et al., 2005; Coleman and Ratcliffe, 2007). The functional HIF transcription factor is composed of an α subunit, either HIF-1α or HIF-2α, and a β subunit, HIF-1β. When cells become hypoxic, α subunits of the protein become stabilized and translocate to the nucleus where they heterodimerize with HIF-1β and regulate the expression of genes that allow cells to adapt to a hypoxic environment (Cash et al., 2007). Our recent studies demonstrated that HIF-1α is activated in macrophages in the livers of bile duct ligation (BDL) mice, an animal model of peribiliary fibrosis (Moon et al., 2009). However, whether the activation of HIF-1α in hepatic macrophages in vivo is a key event in the development of liver fibrosis is not known. Accordingly, in the present study, the hypothesis was tested that HIF-1α activation in macrophages is critical for the up-regulation of profibrotic mediators and the development of liver fibrosis in vivo.
Materials and Methods
Animals.
To selectively reduce HIF-1α or HIF-1β levels in myeloid cells, HIF-1αfl/fl and HIF-1βfl/fl mice, described in detail previously (Tomita et al., 2000, 2003), were crossed with mice expressing Cre recombinase under the control of the lysozyme M promoter (LysMCre mice; The Jackson Laboratory, Bar Harbor, ME) (Clausen et al., 1999). In these mice, Cre recombinase is expressed in monocytes, macrophages, and neutrophils (Clausen et al., 1999). The offspring of the HIF-1αfl/fl mouse breeding were HIF-1αfl/flLysMCre+ (i.e., myeloid cells deficient in HIF-1α) or HIF-1αfl/flLysMCre− (i.e., normal HIF-1α levels in myeloid cells). The offspring of the HIF-1βfl/fl mouse breeding were HIF-1βfl/flLysMCre+ (i.e., myeloid cells deficient in HIF-1β) or HIF-1βfl/flLysMCre− (i.e., normal HIF-1β levels in myeloid cells). Polymerase chain reaction (PCR) of genomic DNA was used to detect the floxed HIF-1α gene, floxed HIF-1β gene, and the Cre transgene as described previously (Tomita et al., 2000, 2003; Copple et al., 2009).
All of the mice were maintained on a 12-h light/dark cycle under controlled temperature (18–21°C) and humidity. Food (Rodent Diet; Harlan Teklad, Madison, WI) and tap water were allowed ad libitum. All of the procedures on animals were carried out in accordance with the Guide for the Care and Use of Laboratory Animals promulgated by the National Institutes of Health (Institute of Laboratory Animal Resources, 1996).
BDL.
Mice were subjected to BDL as described previously (Kim et al., 2006). At 10 days after surgery, mice were anesthetized with sodium pentobarbital and euthanized by exsanguination.
Real-Time PCR.
RNA was isolated using TRIzol reagent (Sigma-Aldrich, St. Louis, MO), and contaminating DNA was removed using the TURBO DNA-free kit (Applied Biosystems, Foster City, CA). The RNA then was reverse-transcribed into cDNA as described previously by us (Kim et al., 2006). Real-time PCR was performed on a 7900 real-time PCR instrument (Applied Biosystems) with the SYBR green DNA PCR kit (Applied Biosystems). Real-time PCR was used to quantify mRNA levels on a Prism 7300 real-time PCR instrument (Applied Biosystems) using the SYBR green DNA PCR kit (Applied Biosystems) as described previously (Kim et al., 2006). Primer sequences for real-time PCR are shown in Table 1.
TABLE 1.
Primer sequences from real-time PCR analysis
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| 18S | 5′-TTGACGGAAGGGCACCACCAG-3′ | 5′-GCACCACCACCCACGGAATCG-3′ |
| α-SMA | 5′-CCACCGCAAATGCTTCTAAGT-3′ | 5′-GGCAGGAATGATTTGGAAAGG-3′ |
| Collagen type Iα1 | 5′-TGTGTTCCCTACTCAGCCGTCT-3′ | 5′-CATCGGTCATGCTCTCTCCAA-3′ |
| MCP-1 | 5′-ACATTCGGCGGTTGCTCTAGA-3′ | 5′-ACATCCTGTATCCACACGGCAG-3′ |
| PAI-1 | 5′-AGTCTTTCCGACCAAGAGCA-3′ | 5′-ATCACTTGCCCCATGAAGAG-3′ |
| PDGF-B | 5′-CCCACAGTGGCTTTTCATTT-3′ | 5′-GTGGAGGAGCAGACTGAAGG-3′ |
| FGF-2 | 5′-AGCGACCCACACGTCAAACTAC-3′ | 5′-CAGCCGTCCATCTTCCTTCATA-3′ |
| KC | 5′-TGGCTGGGATTCACCTCAAG-3′ | 5′-GTGGCTATGACTTCGGTTTGG-3′ |
| MIP-2 | 5′-CTTTGGTTCTTCCGTTGAGG-3′ | 5′-CAAAAAGTTTGCCTTGACCC-3′ |
| TNF-α | 5′-GACCCTCACACTCAGATCATCTTCT-3′ | 5′-CCTCCACTTGGTGGTTTGCT-3′ |
| VEGF-A | 5′-CATCTTCAAGCCGTCCTGTGT-3′ | 5′-CTCCAGGGCTTCATCGTTACA-3′ |
| Ang-1 | 5′-TTTGCATTCTTCGCTGCCA-3′ | 5′-GGCACATTGCCCATGTTGA-3′ |
| Ang-2 | 5′-ACCTTCAGAGACTGTGCGGAAA-3′ | 5′-CGTCCATGTCACAGTAGGCCTT-3′ |
Kupffer Cell Isolation.
Kupffer cells were isolated from mice and exposed to hypoxia as described previously by us (Copple et al., 2010).
Western Blot Analysis.
Nuclear extracts were isolated from Kupffer cells as described previously (Copple et al., 2010). For Western blot analysis, aliquots (15 μg) of nuclear extracts were subjected to 10% SDS-polyacrylamide gel electrophoresis, and proteins were transferred to Immobilon polyvinylidene difluoride transfer membranes (Millipore Corporation, Billerica, MA). The membranes then were probed with rabbit polyclonal anti-HIF-1α antibody (NB100-449; Novus Biologicals, Inc., Littleton, CO) diluted 1:1000 or mouse monoclonal anti-HIF-1β (Millipore Corporation) diluted 1:1000 followed by incubation with goat anti-rabbit antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for HIF-1α or goat anti-mouse antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Inc.) for HIF-1β.
For TGF-β1 Western blot analysis, aliquots of liver extract (50 μg) were separated on a 10% SDS-polyacrylamide gel under nondenaturing conditions. TGF-β1 was detected using a monoclonal anti-TGF-β1 antibody (clone 9016; R&D Systems, Minneapolis, MN).
Assessment of Hepatic Injury and Serum Alkaline Phosphatase, Bilirubin, and Bile Acid Concentrations.
Hepatocyte injury was evaluated by measuring the activity of alanine aminotransferase (ALT) (Pointe Scientific, Inc., Lincoln Park, MI). Serum bile acid concentrations were determined by using a commercially available kit (Colorimetric Total Bile Acids Assay Kit; Bio-Quant, San Diego, CA). Alkaline phosphatase (ALP) and bilirubin were quantified in serum by using commercially available kits (Pointe Scientific, Inc.).
Quantification of Type I Collagen, Cre Recombinase, Macrophages, and Neutrophils in the Liver.
Type I collagen in the liver was detected using immunohistochemistry and quantified morphometrically by analyzing the area of immunohistochemical staining of type I collagen as described previously by us (Kim et al., 2006; Moon et al., 2009). An increase in the area of type I collagen staining in the liver is an indicator of fibrosis. Fluorescent staining in liver sections was visualized on a BX41 microscope (Olympus, Tokyo, Japan). For morphometric analysis of the total area of type I collagen in a liver section, digital images of 10, randomly chosen, low-power (magnification 100×) fields per tissue section were captured using a DP70 camera (Olympus). Samples were coded such that the evaluator was not aware of the treatment, and the same exposure time was used for all of the captured images. Scion Image software (Scion Corporation, Frederick, MD) then was used to quantify the total area of type I collagen (number of positive pixels) using methods described in detail previously (Copple et al., 2002). The staining is expressed as a fraction of the total area. The 10 random fields analyzed for each liver section were averaged and counted as a replicate (i.e., each replicate represents a different mouse).
Neutrophils were detected in liver sections and quantified as described previously (Kim et al., 2006).
For the quantification of macrophages in the liver, sections of frozen liver were fixed in 4% formalin and then incubated with rat anti-mouse CD68 and rat anti-mouse F4/80 antibodies diluted 1:100 (AbD Serotec, Raleigh, NC). The sections then were incubated with goat anti-rat antibody conjugated to Alexa Fluor 488. The area of immunohistochemical staining then was quantified in liver sections as described above for collagen quantification.
For the detection of Cre recombinase in the liver, sections of frozen liver were fixed in 4% formalin and then incubated with rabbit anti-Cre recombinase antibody diluted 1:4000 (Imgenex, San Diego, CA). The sections then were incubated with goat anti-rabbit antibody conjugated to Alexa Fluor 594.
Immunohistochemistry for PDGF-B in Sections of Mouse Liver.
For PDGF-B immunostaining, livers were frozen in isopentane (Sigma-Aldrich) immersed in liquid nitrogen for 8 min. Sections of frozen liver were fixed in acetone for 10 min at −20°C. Sections were incubated with rabbit polyclonal anti-PDGF-B antibody (Bioworld Technology, Inc., St. Louis Park, MN) diluted 1:50 in phosphate-buffered saline (PBS) containing 3% goat serum at room temperature for 3 h. The sections were washed with PBS and then incubated with secondary antibody conjugated to Alexa Fluor 488 (green staining; Invitrogen, Carlsbad, CA). Macrophages were detected in the same sections as described above.
HIF-1α Immunohistochemistry in Sections of Human Liver.
Research involving human livers was reviewed by the University of Kansas Medical Center Human Research Protection Program, and the use of deidentified human liver samples was approved. The specimens used in this study were collected and provided by the KU Liver Center Tissue Bank at The University of Kansas Medical Center. Diseased liver tissue used was from patients with primary biliary cirrhosis (PBC) (2 females, ages 60, 61; 1 male, age 47) and patients with primary sclerosing cholangitis (PSC) (2 males, ages 45, 55; 1 female, age 70). Control liver sections were obtained from liver tissue taken from donor livers before transplantation. Five-micrometer frozen sections were fixed for 10 min in 4% neutral-buffered formalin, washed with PBS, and then blocked for 30 min in 10% goat serum. Sections then were incubated with primary antibodies, rabbit anti-human HIF-1α (NB100-134; Novus Biologicals, Inc.) diluted 1:100 and mouse anti-human CD68 (Ab-3; Thermo Fisher Scientific, Waltham, MA) or mouse anti-human α-smooth muscle actin (α-SMA) (Millipore Bioscience Research Reagents, Temecula, CA) each diluted 1:100 overnight at 4°C. Sections were washed subsequently with PBS and then incubated with secondary Alexa Fluor 594-conjugated rabbit anti-mouse and Alexa Fluor 488-conjuated goat anti-rabbit secondary antibodies, each diluted at 1:500 in blocking buffer for 1 h at room temperature. Sections were washed with PBS and counterstained with 4,6-diamidino-2-phenylindole.
Statistical Analysis.
Results are presented as the mean ± S.E.M. Data were analyzed by an analysis of variance. Data expressed as a fraction were transformed by arcsine square root before the analysis. Comparisons among group means were made using the Student-Newman-Keuls test. The criterion for significance was p < 0.05 for all of the studies.
Results
HIF-1β Deletion in Myeloid Cells Does Not Affect Liver Injury or Hepatic Inflammation after BDL.
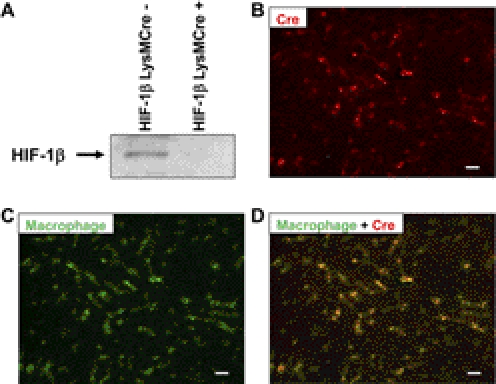
To determine whether HIF-1β in myeloid cells is critical for the development of liver fibrosis, HIF-1βfl/fl mice were crossed with LysMCre mice. To confirm that HIF-1β was deleted in hepatic macrophages (i.e., Kupffer cells), these cells were isolated from HIF-1βLysMCre− and HIF-1βLysMCre+ mice, and HIF-1β was detected by Western blot analysis. HIF-1β was detected in Kupffer cells from HIF-1βLysMCre− mice but not in Kupffer cells from HIF-1βLysMCre+ mice (Fig. 1A). Next, to confirm that Cre recombinase was only expressed in macrophages in the liver, Cre recombinase was detected by immunohistochemistry in liver sections from HIF-1βLysMCre+ mice subjected to BDL (Fig. 1B, red staining). Macrophages then were identified in the same liver section by immunohistochemistry (Fig. 1C, green staining). When Cre recombinase immunostaining and macrophage immunostaining were overlaid, Cre recombinase was only detected in hepatic macrophages (Fig. 1D).
Fig. 1.
Decreased HIF-1β protein in Kupffer cells isolated from HIF-1βLysMCre+ mice. A, Kupffer cells were isolated from HIF-1βLysMCre− and HIF-1βLysMCre+ mice, and HIF-1β was detected by Western blot analysis. HIF-1βLysMCre+ mice were subjected to BDL. B–D, 10 days after surgery, immunohistochemistry was used to detect Cre recombinase (red staining in B and D) and macrophages (green staining in C and D). B, C, and D show the same field. Yellow staining in D demonstrates the colocalization of Cre recombinase and macrophages. Scale bar, 50 μm.
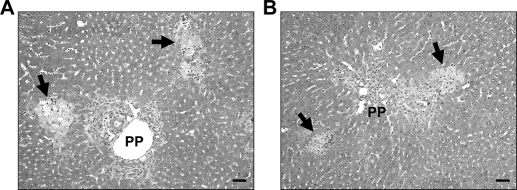
Next, HIF-1βLysMCre− and HIF-1βLysMCre+ mice were subjected to BDL, and liver injury and inflammation were assessed at 10 days after surgery. ALT, bilirubin, ALP, and bile acids in serum were increased to a similar extent in HIF-1βLysMCre− mice and HIF-1βLysMCre+ mice at 10 days after BDL (Table 2). In addition, the extent of necrosis was similar in these mice after BDL (Figs. 2).
TABLE 2.
Serum biomarkers of liver injury and cholestasis in HIF-1βLysMCre− and HIF-1βLysMCre+ mice after BDL
HIF-1βLysMCre− and HIF-1βLysMCre+ mice were subjected to sham operation or BDL. Ten days after surgery, ALT activity, bilirubin concentrations, ALP activity, and bile acid concentrations were quantified in serum. Data are expressed as mean ± S.E.M.; n = 8.
| Biomarker | Sham Operation |
BDL |
||
|---|---|---|---|---|
| HIF-1βLysMCre− | HIF-1βLysMCre+ | HIF-1βLysMCre− | HIF-1βLysMCre+ | |
| ALT, U/l | 5.6 ± 0.9 | 22.0 ± 17.6 | 470.3 ± 62.1a | 463.9 ± 71.6a |
| Bilirubin, mg/dl | 0.2 ± 0.1 | 0.1 ± 0.1 | 15.0 ± 3.4a | 15.9 ± 3.6a |
| ALP, U/l | 124.2 ± 9.8 | 123.1 ± 11.6 | 846.0 ± 261.5a | 970.8 ± 381.1a |
| Bile acids, μM | 5.6 ± 0.9 | 22.0 ± 17.6 | 428.9 ± 25.3a | 414.6 ± 31.8a |
Significantly different (p < 0.05) from sham-operated mice.
Fig. 2.
Liver injury in HIF-1βLysMCre− and HIF-1βLysMCre+ mice after BDL. HIF-1βLysMCre− and HIF-1βLysMCre+ mice were subjected to sham operation or BDL. Ten days after surgery, liver sections were stained with hematoxylin and eosin. A and B, representative photomicrographs from HIF-1βLysMCre− (A) and HIF-1βLysMCre+ (B) mice subjected to BDL. Arrows indicate areas of necrosis. Scale bar, 50 μm.
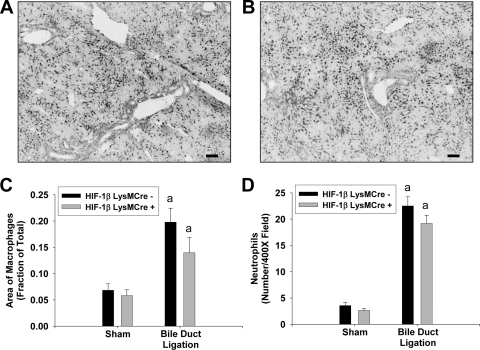
To evaluate the extent of hepatic inflammation after BDL, macrophages and neutrophils were detected in liver sections by immunohistochemistry. In addition, levels of proinflammatory mediators were quantified by real-time PCR. Macrophage and neutrophil numbers were increased to a similar extent in the livers of HIF-1βLysMCre− and HIF-1βLysMCre+ mice after BDL (Fig. 3). In addition, up-regulation of the chemokines keratinocyte-derived chemotactic factor (KC) and macrophage inflammatory protein-2 (MIP-2) and the cytokine tumor necrosis factor-α (TNF-α) were unaffected by the deficiency of HIF-1β in myeloid cells (Table 3).
Fig. 3.
Levels of macrophages and neutrophils in HIF-1βLysMCre− and HIF-1βLysMCre+ mice after BDL. HIF-1βLysMCre− and HIF-1βLysMCre+ mice were subjected to sham operation or BDL. Ten days after surgery, macrophages and neutrophils were detected in the liver by immunohistochemistry. A and B, representative photomicrographs of immunohistochemistry for macrophages in liver sections from HIF-1βLysMCre− (A) and HIF-1βLysMCre+ (B) mice subjected to BDL. Positive staining appears black in the photomicrographs. Scale bar, 100 μm. C, the area of macrophage immunostaining was quantified using morphometric techniques. D, neutrophils were quantified in liver sections. Data are expressed as mean ± S.E.M.; n = 8. a, significantly different (p < 0.05) from sham-operated mice.
TABLE 3.
mRNA levels of proinflammatory mediators in HIF-1βLysMCre− and HIF-1βLysMCre+ mice after BDL
HIF-1βLysMCre− and HIF-1βLysMCre+ mice were subjected to sham operation or BDL. Ten days after surgery, mRNA levels were quantified by real-time PCR. Data are expressed as mean ± SEM; n = 8.
| Gene Name/Function | Sham Operation |
BDL |
||
|---|---|---|---|---|
| HIF-1βLysMCre− | HIF-1βLysMCre+ | HIF-1βLysMCre− | HIF-1βLysMCre+ | |
| KC | 1.0 ± 0.2 | 2.4 ± 1.0 | 8.3 ± 1.9a | 8.2 ± 0.8a |
| MIP-2 | 1.0 ± 0.3 | 2.9 ± 1.1 | 14.2 ± 0.3a | 20.6 ± 0.3a |
| TNF-α | 1.2 ± 0.6 | 1.2 ± 0.4 | 4.7 ± 1.0a | 3.4 ± 0.7a |
Significantly different (p < 0.05) from sham-operated mice.
HIF-1β Deletion in Myeloid Cells Reduces Liver Fibrosis after BDL.
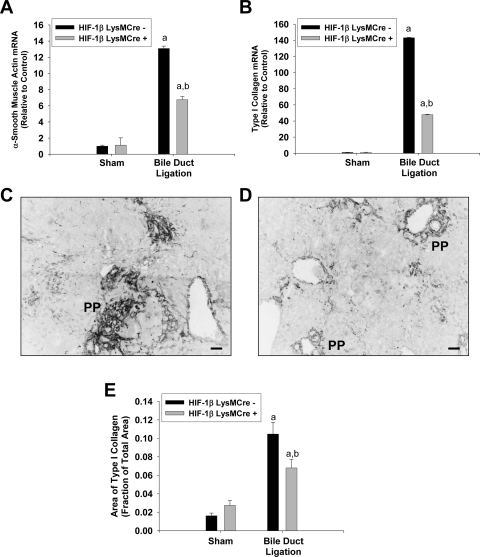
Next, we evaluated biomarkers of liver fibrosis to test the hypothesis that HIF activation in myeloid cells is required for the development of liver fibrosis after BDL. α-SMA mRNA levels were increased to a greater extent in the livers of HIF-1βLysMCre− mice after BDL compared with those of BDL HIF-1βLysMCre+ mice (Fig. 4A). Type I collagen mRNA levels were increased in the livers of HIF-1βLysMCre− mice after BDL (Fig. 4B). Type I collagen mRNA levels were significantly lower in HIF-1βLysMCre+ mice after BDL (Fig. 4B). Extensive deposition of type I collagen protein was observed in periportal (PP) regions of livers from HIF-1βLysMCre− mice after BDL (Fig. 4C). Lower levels of type I collagen were observed in BDL HIF-1βLysMCre+ mice (Fig. 4D). A significant reduction in hepatic type I collagen protein in these mice was confirmed by the quantification of the area of immunohistochemical staining of type I collagen in liver sections (Fig. 4E). These data demonstrate that HIF signaling in myeloid cells is important for the development of liver fibrosis after BDL.
Fig. 4.
Liver fibrosis in HIF-1βLysMCre− and HIF-1βLysMCre+ mice after BDL. HIF-1βLysMCre− and HIF-1βLysMCre+ mice were subjected to sham operation or BDL. A and B, ten days after surgery, mRNA levels of α-SMA (A) and type I collagen (B) were quantified by real-time PCR. C and D, representative photomicrographs of type I collagen immunohistochemistry in liver sections from BDL HIF-1βLysMCre− (C) and HIF-1βLysMCre+ (D) mice. Positive staining appears dark gray in the photomicrographs. Scale bar, 50 μm. E, the area of type I collagen immunostaining was quantified in liver sections. Data are expressed as mean ± S.E.M.; n = 8. a, significantly different (p < 0.05) from sham-operated mice. b, significantly different (p < 0.05) from BDL HIF-1βLysMCre− mice.
HIF-1β in Myeloid Cells Is Required for Up-Regulation of PDGF-B after BDL.
Next, we determined whether HIF-1β deficiency affected the up-regulation of the mediators that are important for the development of fibrosis, such as plasminogen activator inhibitor-1 (PAI-1), fibroblast growth factor-2 (FGF-2), PDGF-B, MCP-1, and TGF-β1. In addition, we determined the impact of HIF deficiency in myeloid cells on the levels of proangiogenic mediators, including vascular endothelial growth factor-A (VEGF-A), angiopoietin-1 (Ang-1), and Ang-2. PAI-1, FGF-2, PDGF-B, and MCP-1 mRNA levels were increased in HIF-1βLysMCre− mice after BDL (Table 4). PDGF-B and MCP-1 mRNA levels were significantly lower in HIF-1βLysMCre+ after BDL compared with those in HIF-1βLysMCre− mice (Table 4). In contrast, VEGF-A, Ang-1, and Ang-2 mRNA levels were not increased significantly in the livers of either HIF-1βLysMCre− or HIF-1βLysMCre+ mice after BDL (Table 4). To confirm this, we evaluated VEGF-A protein levels by Western blot and immunohistochemistry analyses. Consistent with VEGF-A mRNA levels, VEGF-A protein levels were not increased in the liver at 10 days after BDL (data not shown).
TABLE 4.
mRNA levels of profibrotic and proangiogenic mediators in HIF-1βLysMCre− and HIF-1βLysMCre+ mice after BDL
HIF-1βLysMCre− and HIF-1βLysMCre+ mice were subjected to sham operation or BDL. Ten days after surgery, mRNA levels were quantified by real-time PCR. Data are expressed as mean ± SEM; n = 8.
| Gene Name/Function | Sham Operation |
BDL |
||
|---|---|---|---|---|
| HIF-1βLysMCre− | HIF-1βLysMCre+ | HIF-1βLysMCre− | HIF-1βLysMCre+ | |
| PAI-1 | 1.5 ± 1.0 | 1.5 ± 1.0 | 148.1 ± 56.0a | 174.4 ± 43.9a |
| FGF-2 | 1.0 ± 0.1 | 0.7 ± 0.1 | 3.7 ± 1.6a | 1.5 ± 0.3 |
| PDGF-B | 1.0 ± 0.1 | 1.0 ± 0.8 | 14.5 ± 0.3a | 4.7 ± 0.4a,b |
| MCP-1 | 1.0 ± 1.3 | 1.0 ± 1.7 | 12.3 ± 0.3a | 3.8 ± 0.6a,b |
| VEGF-A | 1.0 ± 0.1 | 0.7 ± 0.05a | 0.4 ± 0.01a | 0.4 ± 0.02a |
| Ang-1 | 1.0 ± 0.1 | 1.9 ± 1.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Ang-2 | 1.0 ± 0.2 | 0.7 ± 0.2 | 1.6 ± 0.4 | 1.4 ± 0.3 |
Significantly different (p < 0.05) from sham-operated mice.
Significantly different (p < 0.05) from BDL HIF-1βLysMCre− mice.
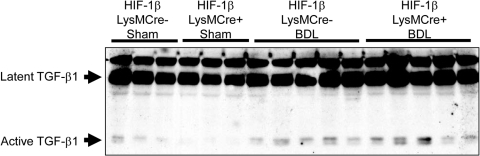
Levels of latent TGF-β1 protein (75 kDa) and active TGF-β1 protein (25 kDa) were increased to a similar extent in the livers of HIF-1βLysMCre− and HIF-1βLysMCre+ mice after BDL (Fig. 5). Consistent with this, the ratio of active TGF-β1 to latent TGF-β1 was not different between HIF-1βLysMCre− and HIF-1βLysMCre+ mice after BDL (HIF-1βLysMCre−, 0.44 ± 0.07, versus HIF-1βLysMCre+, 0.53 ± 0.05).
Fig. 5.
TGF-β1 protein levels in the livers of HIF-1βLysMCre− and HIF-1βLysMCre+ mice after BDL. HIF-1βLysMCre− and HIF-1βLysMCre+ mice were subjected to sham operation or BDL. Ten days after surgery, Western blot analysis was used to detect TGF-β1 in liver homogenates.
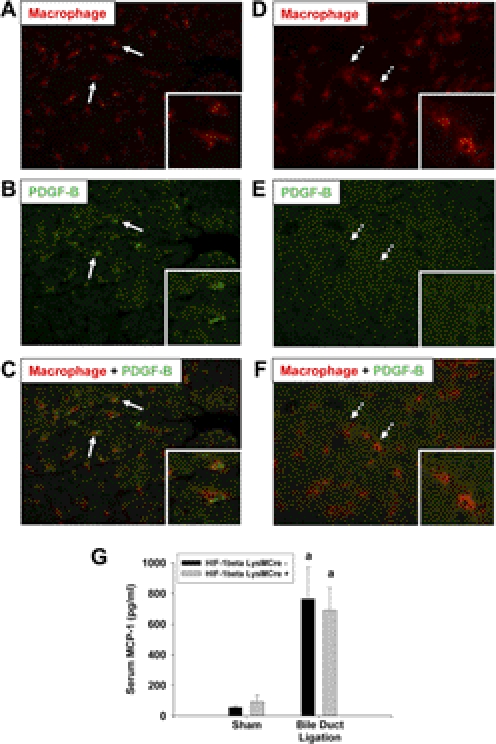
Because myeloid cell-specific HIF-1β deficiency largely prevented the increase in PDGF-B and MCP-1 mRNA levels in the liver, we next evaluated whether PDGF-B and MCP-1 protein levels were increased in a HIF-1β-dependent manner. Immunohistochemistry was used to detect PDGF-B protein in hepatic macrophages. PDGF-B protein was not detected in the livers from sham-operated mice (data not shown). PDGF-B protein levels were increased in the livers of HIF-1βLysMCre− mice after BDL (Fig. 6B, green staining). Furthermore, PDGF-B protein (green staining) colocalized with hepatic macrophages (red staining) in these mice (Fig. 6C). In contrast, PDGF-B protein was not detected in hepatic macrophages from HIF-1βLysMCre+ mice after BDL (Figs. 6, D–F). Next, serum concentrations of MCP-1 protein were quantified. In contrast to MCP-1 mRNA levels (Table 4), MCP-1 protein concentrations were increased to a similar extent in HIF-1βLysMCre− and HIF-1βLysMCre+ mice after BDL (Fig. 6G).
Fig. 6.
Quantification of PDGF-B and MCP-1 protein in HIF-1βLysMCre− and HIF-1βLysMCre+ mice after BDL. HIF-1βLysMCre− and HIF-1βLysMCre+ mice were subjected to sham operation or BDL. A–F, 10 days after surgery, immunohistochemistry was used to detect macrophages (red staining in A, C, D, and F) and PDGF-B (green staining in B, C, E, and F) in liver sections from HIF-1βLysMCre− (A, B, and C) and HIF-1βLysMCre+ (D, E, and F) mice subjected to BDL. A, B, and C show the same field. D, E, and F show the same field. Arrows indicate PDGF-B-positive macrophages from HIF-1βLysMCre− mice. Dashed arrows indicate PDGF-B-negative macrophages from HIF-1βLysMCre+ mice. G, MCP-1 protein was quantified in serum. Data are expressed as mean ± S.E.M.; n = 8. a, significantly different (p < 0.05) from sham-operated mice.
HIF-1α in Myeloid Cells Contributes to the Development of Liver Fibrosis.
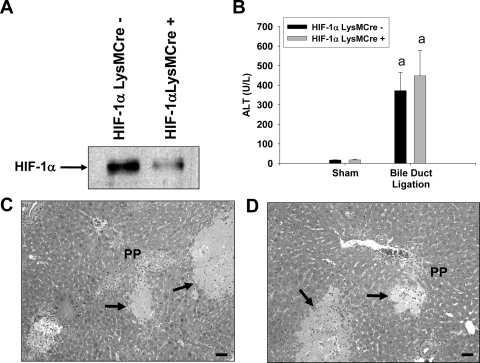
During hypoxia, HIF-1β heterodimerizes with either HIF-1α or HIF-2α to regulate gene expression (Cash et al., 2007). Because our results above demonstrate a role for myeloid HIF-1β in liver fibrosis, we determined whether HIF-1α contributed to liver fibrosis and the up-regulation of profibrotic genes. To determine the role of HIF-1α in myeloid cells, HIF-1αfl/fl mice were crossed with LysMCre mice. Kupffer cells were isolated from HIF-1αLysMCre− mice and HIF-1αLysMCre+ mice and exposed to 1% oxygen, a concentration of oxygen that stimulates nuclear accumulation of HIF-1α in these cells (Copple et al., 2010). HIF-1α protein was detected in nuclear extracts from Kupffer cells isolated from HIF-1αLysMCre− mice (Fig. 7A). HIF-1α protein levels were substantially lower in hypoxic Kupffer cells isolated from HIF-1αLysMCre+ mice (Fig. 7A).
Fig. 7.
Liver injury in HIF-1αLysMCre− and HIF-1αLysMCre+ mice after BDL. A, Kupffer cells were isolated from HIF-1αLysMCre− and HIF-1αLysMCre+ mice and exposed to 1% oxygen for 1 h. HIF-1α was detected in nuclear extracts by Western blot analysis. HIF-1αLysMCre− and HIF-1αLysMCre+ mice were subjected to sham operation or BDL. B, 10 days after surgery, ALT activity was measured in serum. Data are expressed as mean ± S.E.M.; n = 8. a, significantly different (p < 0.05) from sham-operated mice. C and D, representative photomicrographs from HIF-1αLysMCre− (C) and HIF-1αLysMCre+ (D) mice subjected to BDL. Arrows indicates area of necrosis. Scale bar, 50 μm.
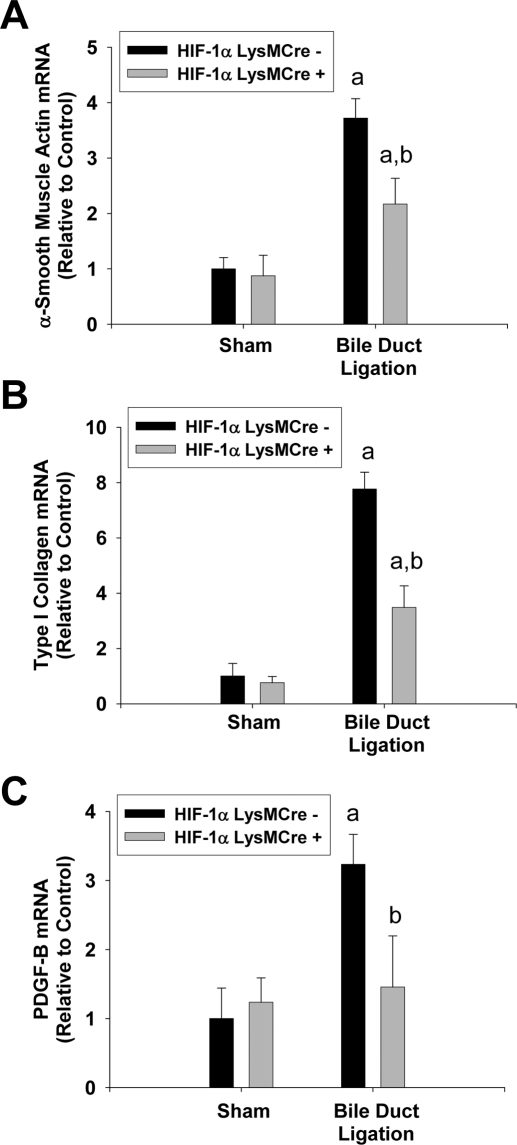
ALT activity was increased to a similar extent in HIF-1αLysMCre− and HIF-1αLysMCre+ mice after BDL (Fig. 7B). In addition, pathological changes that occurred in the liver were similar (Fig. 7, C and D). mRNA levels of α-SMA, type I collagen, and PDGF-B were increased in HIF-1αLysMCre− mice after BDL (Fig. 8). mRNA levels of all of these genes were significantly lower in BDL HIF-1αLysMCre+ mice (Fig. 8).
Fig. 8.
Liver fibrosis in HIF-1αLysMCre− and HIF-1αLysMCre+ mice after BDL. HIF-1αLysMCre− and HIF-1αLysMCre+ mice were subjected to sham operation or BDL. A–C, 10 days after surgery, mRNA levels of α-SMA (A), type I collagen (B), and PDGF-B (C) were quantified by real-time PCR. Data are expressed as mean ± S.E.M.; n = 8. a, significantly different (p < 0.05) from sham-operated mice. b, significantly different (p < 0.05) from BDL HIF-1αLysMCre− mice.
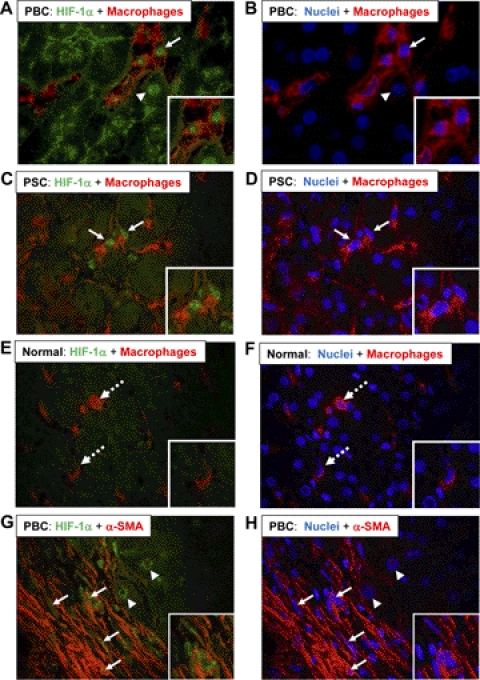
Activation of HIF-1α in Macrophages, Hepatocytes, and Fibroblasts in the Livers of Patients with PBC and PSC.
Immunohistochemistry was used to detect HIF-1α in macrophages and fibroblasts in the livers from three patients with PBC, three patients with PSC, and three normal human livers. Nuclear accumulation of HIF-1α was observed in macrophages in the liver of a patient with PBC (Fig. 9A). In addition, nuclear HIF-1α was observed in hepatocytes adjacent to HIF-1α-positive macrophages (Fig. 9A). HIF-1α was detected in macrophages in the livers from all three patients with PBC. Likewise, nuclear HIF-1α was observed in macrophages in all of the livers from patients with PSC (Fig. 9C). In contrast, HIF-1α protein was not detected in the nuclei of macrophages in normal human livers (Fig. 9E). We also observed nuclear HIF-1α protein in cells within fibrotic regions of livers from patients with PBC and PSC; therefore, we determined whether fibroblasts, identified by α-SMA immunostaining, were positive for HIF-1α. As shown in Fig. 9G, HIF-1α protein was detected in the nuclei of α-SMA-positive cells in all of the livers from patients with PBC and PSC. In addition, HIF-1α protein was detected in the nuclei of hepatocytes adjacent to regions of bridging fibrosis (Fig. 9G).
Fig. 9.
HIF-1α activation in the livers of patients with PBC and PSC. A–H, sections of frozen liver from patients with PBC (A, B, G, and H), patients with PSC (C and D), and normal human livers (E and F) were stained for HIF-1α (green staining in A, C, E, and G) and either CD68 (red staining in A–F) or α-SMA (red staining in G and H). Nuclei were stained with 4,6-diamidino-2-phenylindole (blue staining in B, D, F, and H). The same fields in A, C, E, and G are shown in B, D, F, and H. Insets show higher-power fields. Arrows indicate HIF-1α-positive nuclei in macrophages (A and C) or α-SMA-positive cells (G). Arrowheads indicate HIF-1α-positive nuclei in hepatocytes. Dashed arrows indicate HIF-1α-negative macrophages (E and F).
Discussion
Our results demonstrate that HIF-1β and HIF-1α in myeloid cells contribute to the development of liver fibrosis after BDL. In LysMCre mice, Cre recombinase is expressed in macrophages and neutrophils (Clausen et al., 1999). Accordingly, it is possible that HIF-1β and HIF-1α deletion in macrophages and/or neutrophils was responsible for the reduction of fibrosis observed in our studies. However, a recent study showed that neutrophil depletion does not affect the development of liver fibrosis after BDL (Saito et al., 2003). Accordingly, it is unlikely that HIF activation in neutrophils contributes to the development of fibrosis after BDL. Therefore, our results indicate HIF-1β and HIF-1α in macrophages is important for liver fibrosis.
The mechanism by which the activation of HIFs in macrophages promoted fibrosis may be in part due to HIF-dependent production of PDGF-B by hepatic macrophages during chronic injury. PDGF-B is a potent mitogen and chemotaxin for hepatic stellate cells (Friedman and Arthur, 1989; Carloni et al., 1997; Marra et al., 1997). Furthermore, PDGF-B increases the expression of α-SMA in peribiliary fibroblasts, and the inhibition of PDGF-B signaling reduces fibrosis after BDL (Kinnman et al., 2003). Consistent with our results (Fig. 6), others have demonstrated that hepatic macrophages express PDGF-B in the liver during chronic injury (Pinzani et al., 1996). However, the mechanism by which chronic injury increases the expression of PDGF-B in hepatic macrophages has remained largely unknown. We demonstrated previously that HIF-1α is activated in hepatic macrophages after BDL (Moon et al., 2009). We also demonstrated that the exposure of Kupffer cells in vitro to hypoxia up-regulates PDGF-B in a HIF-1α-dependent manner (Copple et al., 2010). Collectively, these studies suggested that HIF-1α may be an important regulator of PDGF-B in the liver during the development of fibrosis. Consistent with this, our present study demonstrated that the deletion of either HIF-1α or HIF-1β in myeloid cells attenuated the increase in PDGF-B mRNA (Fig. 8; Table 4) and prevented the increase in PDGF-B protein in hepatic macrophages during the development of fibrosis in BDL mice (Fig. 6). This was associated with a marked reduction in α-SMA and type I collagen (Figs. 4 and 8). HIF-1β deletion did not affect hepatic macrophage accumulation (Fig. 3), indicating that the decrease in PDGF-B was not simply due to a reduction in the numbers of hepatic macrophages. It is likely that HIF-1α and HIF-1β directly regulate PDGF-B in macrophages, because studies have shown that these HIFs directly regulate this gene in other cell types (Yoshida et al., 2006). Collectively, this suggests that, during chronic injury induced by cholestasis, hepatic macrophages accumulate in hypoxic regions of liver, where hypoxia stimulates the activation of HIF-1α. HIF-1α then heterodimerizes with HIF-1β and regulates the production of PDGF-B that promotes fibrosis. Of importance to human disease, our studies demonstrated further that HIF-1α is activated in hepatic macrophages in PBC and PSC patients with fibrosis (Fig. 9), suggesting that this mechanism also may be important for the regulation of PDGF-B and the progression of fibrosis in humans with cholestatic liver disease.
An interesting observation from these studies is that although a deficiency of either HIF-1α or HIF-1β in myeloid cells reduced liver fibrosis after BDL, it did not affect hepatocyte injury. This suggests that liver fibrosis after BDL is not dependent upon hepatocyte injury. Consistent with this observation, we demonstrated previously that early growth response factor-1 knockout mice had reduced liver injury and neutrophil accumulation after BDL compared with those of wild-type mice but had similar levels of fibrosis (Kim et al., 2006). Likewise, Fickert et al. (2009) demonstrated that farnesoid X receptor knockout mice have reduced liver fibrosis after BDL but have similar levels of liver injury compared with those of wild-type mice. Collectively, these studies indicate that hepatocyte injury after BDL is not an important stimulus for hepatic fibrosis. However, it remains possible that HIFs may contribute to the development of liver injury at earlier times after BDL. Our study focused on a late time point after BDL (i.e., 10 days). Much of the liver injury after BDL occurs within the first few days, and it is possible that the deletion of HIFs delayed the development of liver injury. This may have been sufficient to delay the development of liver fibrosis. Further studies are needed to evaluate this possibility, however.
Studies indicate that angiogenesis is important for the development of hepatic fibrosis (Rosmorduc et al., 1999; Corpechot et al., 2002; Taura et al., 2008). Furthermore, a key function of HIFs is to regulate the production of proangiogenic mediators (Forsythe et al., 1996; Kelly et al., 2003; Yamakawa et al., 2003). Surprisingly, however, although HIF-1α was activated in the liver after BDL, levels of proangiogenic mediators, such as VEGF-A and Angs, remained unchanged. We reported previously that hypoxia increases the expression of VEGF-A in primary mouse hepatocytes, Kupffer cells, and hepatic stellate cells in a HIF-1α-dependent manner (Copple et al., 2009, 2010, 2011), demonstrating that HIF-1α regulates the production of these mediators in these cell types. One possible explanation for the lack of induction of proangiogenic mediators may be that they are transiently produced and the time point that we chose did not coincide with the up-regulation of VEGF-A and Angs. In addition, it is possible that the up-regulation of VEGF-A occurs at a later time point in this model. Consistent with this, it was shown previously that the up-regulation of VEGF in the livers of rats after BDL required several weeks (Rosmorduc et al., 1999).
In addition to detecting nuclear HIF-1α protein in macrophages in the livers of patients with PBC and PSC, we also detected nuclear HIF-1α protein in α-SMA-positive cells (Fig. 9). We demonstrated recently that the exposure of primary mouse hepatic stellate cells to hypoxia increased the expression of a number of genes involved in the genesis of liver fibrosis, including the chemokine receptors Ccr1 and Ccr5, prolyl-4-hydroxylase-α1, prolyl-4-hydroxylase-α2, and the proangiogenic mediators placental growth factor and Ang-like 4 (Copple et al., 2011). Many of these were increased in hepatic stellate cells in a HIF-dependent manner (Copple et al., 2011). Accordingly, it is possible that, in addition to macrophages, the activation of HIF-1α in hepatic stellate cells may be important for the development of liver fibrosis.
Collectively, these studies demonstrate that HIF-1α and HIF-1β are important regulators of profibrotic mediator production by macrophages during the development of liver fibrosis in cholestatic mice. Furthermore, these studies indicate that this mechanism may be important for fibrosis development in humans because HIF-1α was activated in macrophages in the livers of patients with PBC and PSC. Accordingly, these studies suggest that therapeutic targeting of HIF-1α may be effective at preventing the progression of liver fibrosis in patients with cholestatic liver disease and potentially in patients with liver fibrosis produced by other hepatic insults.
Acknowledgments
We thank Dr. James P. Luyendyk for a critical reading of the manuscript; Drs. Erik Schadde, Richard Gilroy, Bashar Abdulkarim, Jameson Forster, Mojtaba Olyaee, and Atta M. Nawabi for assistance in collecting the human liver specimens used in this study; and Natali Navarro-Cazarez, Marsha Danley, Dr. Yvonne Wan, and Dr. Ossama Tawfik for help with the procurement and preparation of frozen liver sections.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK073566]; the National Institutes of Health National Center for Research Resources [Grant P20-RR016475]; and the National Institutes of Health, Center of Biomedical Research Excellence [Grant P20-RR021940] as well as the Molecular Biology Core and the Histology Core supported by the Center of Biomedical Research Excellence grant.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- PDGF-B
- platelet-derived growth factor-B
- α-SMA
- α-smooth muscle actin
- ALT
- alanine aminotransferase
- Ang
- angiopoietin
- BDL
- bile duct ligation
- FGF-2
- fibroblast growth factor-2
- HIF
- hypoxia-inducible factor
- KC
- keratinocyte-derived chemotactic factor
- MCP-1
- monocyte chemotactic protein-1
- MIP-2
- macrophage inflammatory protein-2
- PAI-1
- plasminogen activator inhibitor-1
- PBC
- primary biliary cirrhosis
- PBS
- phosphate-buffered saline
- PCR
- polymerase chain reaction
- PP
- periportal
- PSC
- primary sclerosing cholangitis
- TGF-β
- transforming growth factor-β
- TNF-α
- tumor necrosis factor-α
- VEGF-A
- vascular endothelial growth factor-A.
Authorship Contributions
Participated in research design: Copple, Kaska, and Wentling.
Conducted experiments: Copple, Kaska, and Wentling.
Performed data analysis: Copple and Wentling.
Wrote or contributed to the writing of the manuscript: Copple and Kaska.
References
- Carloni V, Romanelli RG, Pinzani M, Laffi G, Gentilini P. (1997) Focal adhesion kinase and phospholipase C gamma involvement in adhesion and migration of human hepatic stellate cells. Gastroenterology 112:522–531 [DOI] [PubMed] [Google Scholar]
- Cash TP, Pan Y, Simon MC. (2007) Reactive oxygen species and cellular oxygen sensing. Free Radic Biol Med 43:1219–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. (1999) Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8:265–277 [DOI] [PubMed] [Google Scholar]
- Coleman ML, Ratcliffe PJ. (2007) Oxygen sensing and hypoxia-induced responses. Essays Biochem 43:1–15 [DOI] [PubMed] [Google Scholar]
- Copple BL, Bai S, Burgoon LD, Moon JO. (2011) Hypoxia-inducible factor-1α regulates the expression of genes in hypoxic hepatic stellate cells important for collagen deposition and angiogenesis. Liver Int 31:230–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copple BL, Bai S, Moon JO. (2010) Hypoxia-inducible factor-dependent production of profibrotic mediators by hypoxic Kupffer cells. Hepatol Res 40:530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copple BL, Banes A, Ganey PE, Roth RA. (2002) Endothelial cell injury and fibrin deposition in rat liver after monocrotaline exposure. Toxicol Sci 65:309–318 [DOI] [PubMed] [Google Scholar]
- Copple BL, Bustamante JJ, Welch TP, Kim ND, Moon JO. (2009) Hypoxia-inducible factor-dependent production of profibrotic mediators by hypoxic hepatocytes. Liver Int 29:1010–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copple BL, Rondelli CM, Maddox JF, Hoglen NC, Ganey PE, Roth RA. (2004) Modes of cell death in rat liver after monocrotaline exposure. Toxicol Sci 77:172–182 [DOI] [PubMed] [Google Scholar]
- Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. (2002) Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology 35:1010–1021 [DOI] [PubMed] [Google Scholar]
- Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. (2005) Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 115:56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiz Kabir Uddin Ahmed A, Ohtani H, Nio M, Funaki N, Iwami D, Kumagai S, Sato E, Nagura H, Ohi R. (2000) In situ expression of fibrogenic growth factors and their receptors in biliary atresia: comparison between early and late stages. J Pathol 192:73–80 [DOI] [PubMed] [Google Scholar]
- Fausto N. (2000) Liver regeneration. J Hepatol 32 (1 Suppl):19–31 [DOI] [PubMed] [Google Scholar]
- Fickert P, Fuchsbichler A, Moustafa T, Wagner M, Zollner G, Halilbasic E, Stöger U, Arrese M, Pizarro M, Solís N, et al. (2009) Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am J Pathol 175:2392–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL. (2008) Mechanisms of hepatic fibrogenesis. Gastroenterology 134:1655–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL, Arthur MJ. (1989) Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest 84:1780–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F. (2005) Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann Rheum Dis 64:971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Ji S, Lemasters JJ, Christenson V, Thurman RG. (1982) Periportal and pericentral pyridine nucleotide fluorescence from the surface of the perfused liver: evaluation of the hypothesis that chronic treatment with ethanol produces pericentral hypoxia. Proc Natl Acad Sci USA 79:5415–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. (2003) Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 93:1074–1081 [DOI] [PubMed] [Google Scholar]
- Kim ND, Moon JO, Slitt AL, Copple BL. (2006) Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci 90:586–595 [DOI] [PubMed] [Google Scholar]
- Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, Housset C. (2003) The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest 83:163–173 [DOI] [PubMed] [Google Scholar]
- Marra F, Gentilini A, Pinzani M, Choudhury GG, Parola M, Herbst H, Dianzani MU, Laffi G, Abboud HE, Gentilini P. (1997) Phosphatidylinositol 3-kinase is required for platelet-derived growth factor's actions on hepatic stellate cells. Gastroenterology 112:1297–1306 [DOI] [PubMed] [Google Scholar]
- Moon JO, Welch TP, Gonzalez FJ, Copple BL. (2009) Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am J Physiol Gastrointest Liver Physiol 296:G582–G592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsukasa H, Nagy P, Evarts RP, Hsia CC, Marsden E, Thorgeirsson SS. (1990) Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest 85:1833–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani M, Milani S, Herbst H, DeFranco R, Grappone C, Gentilini A, Caligiuri A, Pellegrini G, Ngo DV, Romanelli RG, et al. (1996) Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am J Pathol 148:785–800 [PMC free article] [PubMed] [Google Scholar]
- Rivera CA, Bradford BU, Hunt KJ, Adachi Y, Schrum LW, Koop DR, Burchardt ER, Rippe RA, Thurman RG. (2001) Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol 281:G200–G207 [DOI] [PubMed] [Google Scholar]
- Rosmorduc O, Housset C. (2010) Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin Liver Dis 30:258–270 [DOI] [PubMed] [Google Scholar]
- Rosmorduc O, Wendum D, Corpechot C, Galy B, Sebbagh N, Raleigh J, Housset C, Poupon R. (1999) Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary cirrhosis. Am J Pathol 155:1065–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito JM, Bostick MK, Campe CB, Xu J, Maher JJ. (2003) Infiltrating neutrophils in bile duct-ligated livers do not promote hepatic fibrosis. Hepatol Res 25:180–191 [DOI] [PubMed] [Google Scholar]
- Semenza GL, Wang GL. (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12:5447–5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, Kodama Y, Miura K, Ikai I, Uemoto S, et al. (2008) Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology 135:1729–1738 [DOI] [PubMed] [Google Scholar]
- Tomita S, Sinal CJ, Yim SH, Gonzalez FJ. (2000) Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1alpha. Mol Endocrinol 14:1674–1681 [DOI] [PubMed] [Google Scholar]
- Tomita S, Ueno M, Sakamoto M, Kitahama Y, Ueki M, Maekawa N, Sakamoto H, Gassmann M, Kageyama R, Ueda N, et al. (2003) Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Mol Cell Biol 23:6739–6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, Kuriyama T, Cheng SH, Gregory RJ, Jiang C. (2003) Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res 93:664–673 [DOI] [PubMed] [Google Scholar]
- Yoshida D, Kim K, Noha M, Teramoto A. (2006) Hypoxia inducible factor 1-alpha regulates of platelet derived growth factor-B in human glioblastoma cells. J Neurooncol 76:13–21 [DOI] [PubMed] [Google Scholar]