Abstract
Accumulating evidence indicates that the serotonin system modulates the behavioral and neurochemical effects of cocaine, but the receptor subtypes mediating these effects remain unknown. Recent studies have demonstrated that pharmacological activation of the serotonin 2C receptor (5-HT2CR) attenuates the behavioral and neurochemical effects of cocaine in rodents, but such compounds have not been systematically evaluated in nonhuman primates. The present experiments sought to determine the impact of pretreatment with the preferential 5-HT2CR agonist m-chlorophenylpiperazine (mCPP) and the selective 5-HT2CR agonist Ro 60-0175 [(α-S)-6-chloro-5-fluoro-α-methyl-1H-indole-1-ethanamine fumarate] on the behavioral and neurochemical effects of cocaine in squirrel monkeys. In subjects trained to lever-press according to a 300-s fixed-interval schedule of stimulus termination, pretreatment with either 5-HT2CR agonist dose-dependently and insurmountably attenuated the behavioral stimulant effects of cocaine. In subjects trained to self-administer cocaine, both compounds dose-dependently and insurmountably attenuated cocaine-induced reinstatement of previously extinguished responding in an antagonist-reversible manner, and the selective agonist Ro 60-0175 also attenuated the reinforcing effects of cocaine during ongoing cocaine self-administration. It is noteworthy that the selective agonist Ro 60-0175 exhibited behavioral specificity because it did not significantly alter nondrug-maintained responding. Finally, in vivo microdialysis studies revealed that pretreatment with Ro 60-0175 caused a reduction of cocaine-induced dopamine increases within the nucleus accumbens, but not the caudate nucleus. These results suggest that 5-HT2CR agonists functionally antagonize the behavioral effects of cocaine in nonhuman primates, possibly via a selective modulation of cocaine-induced dopamine increases within the mesolimbic dopamine system and may therefore represent a novel class of pharmacotherapeutics for the treatment of cocaine abuse.
Introduction
Although cocaine binds with relatively equal potencies to and inhibits the function of each of the monoamine reuptake transporters (Ritz et al., 1990), its abuse-related effects have been largely attributed to the modulation of dopamine (DA) neurotransmission (Ritz et al., 1987; Wise, 2004). For example, the capacity of various psychostimulants to produce increased extracellular levels of DA are highly correlated with their behavioral stimulant effects (Spealman et al., 1989; Howell and Byrd, 1995; Ginsburg et al., 2005), reinforcing effects (Ritz et al., 1987; Bergman et al., 1989), and reinstatement effects (Spealman et al., 1999) in nonhuman primates. The importance of cocaine effects on DA systems extends to humans as well, because the self-reported “high” resulting from cocaine administration is highly correlated with DAT occupancy in the striatum of experienced cocaine users (Volkow et al., 1997). Such studies have indicated a clear role for DA in mediating the behavioral and subjective effects of cocaine and suggest that drugs that functionally inhibit the DA-increasing effects of cocaine may prove useful as pharmacotherapeutics in humans.
Projections of the serotonin (5-hydroxytryptamine; 5-HT) system are advantageously situated to modulate the activity of DA neurotransmission. 5-HT axons arising from neurons localized within the medial and dorsal raphe nuclei terminate not only within the dopamine-producing ventral tegmental area (VTA) and substantia nigra pars compacta, but also within the terminal regions of their respective DA projections, including ventral and dorsal regions of the striatum and the prefrontal cortex (Azmitia and Gannon, 1986; Wallman et al., 2011). Accordingly, studies using indirect 5-HT agonists have revealed a modulatory role for 5-HT for the behavioral and neurochemical effects of cocaine. For example, pharmacological enhancement of extracellular 5-HT levels via administration of serotonin transporter inhibitors or 5-HT releasers has been shown to attenuate the behavioral stimulant effects (Howell and Byrd, 1995), reinforcing effects (Kleven and Woolverton, 1993; Glowa et al., 1997; Czoty et al., 2002; Negus et al., 2007), reinstatement effects (Rüedi-Bettschen et al., 2010), and DA-increasing effects of cocaine (Czoty et al., 2002) in nonhuman primates. Furthermore, administration of these compounds attenuates the subjective effects of cocaine in experienced human cocaine users (Walsh et al., 1994; Walsh and Cunningham, 1997). However, the specific 5-HT receptor subtypes mediating these effects have yet to be determined.
Accumulating evidence suggests that pharmacological activation of the 5-HT2C receptor (5-HT2CR) functionally antagonizes the behavioral and neurochemical effects of cocaine in rodents (Bubar and Cunningham, 2008). Specifically, administration of 5-HT2CR agonists in rodents attenuated cocaine-induced elevations of DA within the nucleus accumbens (NAc) (Navailles et al., 2008), cocaine-induced hyperlocomotion (Grottick et al., 2000; Filip et al., 2004), and the discriminative stimulus effects of cocaine (Callahan and Cunningham, 1995; Frankel and Cunningham, 2004). In addition, 5-HT2CR agonism attenuated the direct reinforcing effects of cocaine as measured by self-administration procedures (Grottick et al., 2000; Fletcher et al., 2008) and reduced reinstatement of previously extinguished cocaine-maintained responding (Neisewander and Acosta, 2007; Burbassi and Cervo, 2008). However, the effects of receptor subtype-selective compounds have yet to be systematically evaluated in nonhuman primates. Previous studies have demonstrated that administration of the nonselective 5-HT2A/2C receptor agonist quipazine insurmountably attenuated the behavioral stimulant, reinforcing, and neurochemical effects of cocaine in squirrel monkeys (Howell and Byrd, 1995; Czoty et al., 2002), but identification of the specific receptors underlying those observed effects was not possible because of a lack of pharmacological specificity.
The present studies therefore sought to determine the impact of selective 5-HT2CR agonism on the behavioral and neurochemical effects of cocaine in squirrel monkeys. We first determined whether pretreatment with the preferential 5-HT2CR agonist m-chlorophenylpiperazine (mCPP) or the selective 5-HT2CR agonist (α-S)-6-chloro-5-fluoro-α-methyl-1H-indole-1-ethanamine fumarate (Ro 60-0175) would modulate the behavioral-stimulant effects of cocaine in subjects trained to lever-press according to a fixed-interval 300-s schedule of stimulus termination. Subsequent experiments assessed the effects of 5-HT2CR activation on the reinforcing and reinstatement effects of cocaine in squirrel monkeys trained to self-administer cocaine according to a second-order schedule of reinforcement. Finally, in vivo microdialysis techniques were used to determine the impact of pretreatment with Ro 60-0175 on the DA-increasing effects of cocaine within both ventral (nucleus accumbens) and dorsal (caudate nucleus) subregions of the striatum. We hypothesized that, in accordance with previous studies in rodents, pharmacological activation of the 5-HT2CR would attenuate the behavioral and neurochemical effects of cocaine in nonhuman primates.
Materials and Methods
Subjects
Nineteen adult male squirrel monkeys (Saimiri sciureus), weighing 850 to 1200 g, served as subjects. Between experimental sessions, animals were individually housed in a climate-controlled room and fed twice daily (LabDiet 5045 High Protein Monkey Chow, PMI Nutrition International, Brentwood, MO; fresh fruit/vegetables; cereal) with ad libitum access to water. Daily enrichment was provided via access to foraging devices, toys, and climbing/swing devices. Each animal had served in previous behavioral studies involving administration of compounds acting on monoaminergic and/or glutamatergic systems (Ginsburg et al., 2005; Kimmel et al., 2005, 2007, 2009; Banks et al., 2009; Bauzo et al., 2009; Fantegrossi et al., 2009). All studies were conducted in strict accordance with the National Institutes of Health's Guide for Care and Use of Laboratory Animals, the American Association for Accreditation of Laboratory Animal Care, and the Institutional Animal Care and Use Committee of Emory University.
Apparatus
During behavioral sessions, animals were comfortably seated in a commercially available Plexiglas chair within a ventilated, sound-attenuating chamber (MED Associates, St. Albans, VT). The chair was equipped with an operant panel consisting of a series of red and white lights, a lever, and a white-noise amplifier that was activated throughout the duration of all behavioral sessions to further reduce the influence of ambient noise. Med-PC IV software (MED Associates) was interfaced with each chamber to allow for automated output control and lever-press recording. For self-administration and reinstatement studies, a motor-driven syringe pump (model PHD2000; Harvard Apparatus Inc., Holliston, MA) was mounted on the outer wall of the operant chamber, which held a 35-cc syringe containing appropriate concentrations of cocaine or its vehicle. Each syringe was connected via stainless-steel adaptors and polyvinyl chloride tubing to the external portion of the subject's catheter.
During microdialysis sessions, subjects were seated in Plexiglas chairs supplemented with an adjustable Lexan barrier that was situated slightly above the level of the animal's shoulders to prevent disturbance to microdialysis probes and connective tubing. A motor-driven syringe pump (model 11Plus Dual-Syringe; Harvard Apparatus Inc.) was mounted on top of the operant chamber for automated delivery of microinfused solutions.
Surgery
For self-administration and reinstatement experiments, subjects were prepared with chronic indwelling venous catheters under aseptic conditions. Animals were initially anesthetized with Telazol (tiletamine HCl and zolazepam HCl, 2.0 mg) and ketamine HCl (20 mg). Anesthesia was maintained throughout the procedure with inhaled isoflurane (0.5–1.5%). A polyvinyl chloride catheter (0.025-inch i.d.; 0.035-inch o.d.) was inserted into either the left or right femoral vein or external jugular vein and allowed to rest near the right atrium. The distal end of the catheter was routed subcutaneously and exited at the interscapular region of the animal's back. A custom-made nylon mesh jacket (Lomir Biomedical Inc., Malone, NY) protected the external portion of the catheter. Animals were allowed to recover for 5 to 7 days before resuming operant-behavioral sessions. When not in use, catheters were filled with heparinized saline and locked by using 25-gauge stainless-steel obturators. To maintain patency, catheters were flushed several days per week with 0.2 ml of saline. If a catheter became occluded or damaged during the course of the study, it was promptly removed, and a new catheter was implanted into the same vessel when possible or into another vessel.
For in vivo microdialysis studies, subjects were implanted with bilateral guide cannulae (CMA/11; CMA/Microdialysis, Acton, MA) using stereotaxic techniques under aseptic conditions as described previously (Czoty et al., 2000). Subjects were initially anesthetized with Telazol (tiletamine HCl and zolazepam HCl, 2.0 mg) and ketamine HCl (20 mg) and maintained with inhaled isoflurane anesthesia (0.5–1.5%) to effect. Guide cannulae targeted the caudate nucleus and nucleus accumbens within the same dorsal-ventral plane by using the following coordinates from the earbar: anterior/posterior +15.0; medial/lateral ±3.0.When not in use, stainless-steel stylets were situated within the cannulae to maintain the integrity and sterility of the tissue site. Subjects were allowed 1 month of recovery before microdialysis experiments commenced.
For all surgical procedures, preoperative and postoperative antibiotics (ceftriaxone) and postoperative analgesics (meloxicam or flunixin) were administered by veterinary staff who closely monitored the animals.
Procedure
Fixed-Interval Stimulus Termination.
Daily sessions were conducted 5 days per week (Monday-Friday) and lasted approximately 90 min. Each session began with the illumination of a pair of red lights. During a 300-s fixed-interval, lever presses were recorded but had no programmed consequences. Once the 300-s interval elapsed, the schedule progressed into a 3-s limited hold. A single response during the limited hold extinguished the red lights and illuminated a white light for 15 s to signal reinforcement, followed by a 60-s timeout during which all lights were extinguished and responses had no scheduled consequences. If the animal failed to press the lever during the limited hold, a mild electrical stimulus (300 ms, 3–6 mA) was delivered to a shaved portion of the distal end of the tail that was secured within an acrylic tail yoke, followed immediately by a 60-s timeout. Each daily session consisted of 15 consecutive fixed-interval components. Lever presses and response rates were recorded for each individual component and then averaged across the session. Experimental sessions involving drug pretreatments were conducted twice per week (Tuesday, Friday). Cocaine (veh, 0.1–3.0 mg/kg) was administered 5-s before the onset of the session while the animal was seated in the operant chair. Before the onset of drug-interaction studies, the maximally effective behavioral-stimulant dose of cocaine (EDMax), i.e., the dose of cocaine that produced maximal increases in responding, was identified for each individual subject. The EDMax dose of cocaine was 0.3 mg/kg in one subject and 1.0 mg/kg in the remaining subjects. For drug interaction studies, the effects of pretreatment with mCPP (veh, 0.1–0.3 mg/kg) or Ro 60-0175 (veh, 0.1–0.3 mg/kg) were tested in combination with three doses of cocaine (EDMax and one-half log-step unit doses above and below the EDMax dose). mCPP or Ro 60-0175 was administered 15 min before administration of cocaine. For antagonist-interaction studies, the selective 5-HT2CR antagonist 6-Chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride (SB 242084) (veh, 0.01–0.3 mg/kg) was administered 15 min before mCPP or Ro 60-0175. Within each experiment, the order of dose combinations was randomized for each individual subject.
Second-Order Cocaine Self-Administration.
Daily sessions were conducted 5 to 7 days per week and lasted approximately 60 min. Animals were allowed to intravenously self-administer cocaine according to a second-order schedule of reinforcement. Each session began with the illumination of a pair of red lights. During a 600-s fixed-interval, a FR20 operant schedule was superimposed such that every 20th lever press extinguished the red lights and briefly illuminated a white light for 2 s, followed immediately by reillumination of the red lights. Responses during the 2-s white light were recorded but did not contribute to the subsequent ratio requirement. Once the 600-s fixed-interval elapsed, the schedule progressed into a 200-s limited hold. The first completed FR20 within the limited hold extinguished the red lights and resulted in an intravenous bolus infusion of cocaine (veh, 0.01–0.3 mg/kg/infusion in 0.5 ml; 25 ml/min flow rate). The cocaine infusion was paired with a 15-s white light, followed by a 60-s timeout during which all lights were extinguished and responses were recorded but had no programmed consequences. If the animal failed to complete an FR20 during the limited hold, the red lights were extinguished and the schedule advanced directly into the timeout. Each daily session consisted of five fixed-interval components. Response rates were calculated for each individual component and then averaged across the session. Responding was deemed stable when response rates for each session varied <20% across 3 consecutive days. Once responding was stable, the unit dose of cocaine was altered and behavior was allowed to stabilize until the maximally effective unit dose of cocaine (EDMax, i.e., the unit dose of cocaine that maintained the highest rates of responding) was identified for each individual subject. The EDMax for most subjects was 0.1 mg/kg/infusion, but ranged from 0.03 to 0.3 mg/kg/infusion.
To assess the effects of 5-HT2CR agonism on the reinforcing effects of cocaine, Ro 60-0175 (veh, 0.1–0.3 mg/kg) was administered 15 min before the onset of cocaine self-administration sessions. The effect of all doses of Ro 60-0175 was tested in combination with three unit doses of cocaine (EDMax and one-half log-step unit doses above and below the EDMax unit dose). Dose combinations were tested for three consecutive sessions (Tuesday, Wednesday, and Thursday). Subjects were allowed to self-administer cocaine without pharmacological pretreatment on the other days of the week (Monday and Friday). All doses of Ro 60-0175 were tested against a given unit dose of cocaine before switching the subject to a different cocaine unit dose. For each subject, the dose order of cocaine self-administration and Ro 60-0175 pretreatment was randomized. Pretreatment studies did not begin until cocaine self-administration response rates had stabilized, varying <20% across three consecutive sessions.
Cocaine-Induced Reinstatement.
For reinstatement experiments, the EDMax unit dose for cocaine self-administration was assessed for each individual animal as described earlier. The reinstatement procedure consisted of three phases. During maintenance, animals were allowed to self-administer their respective EDMax of cocaine. Response rates were considered stable when responses varied by <20% across three consecutive self-administration sessions. Once response rates were stable, subjects progressed to the extinction phase during which completed fixed ratios within the 600-s fixed interval or the limited hold were recorded but did not produce conditioned reinforcement (i.e., the white light was withheld) and saline infusions were substituted for cocaine. Under extinction conditions, response rates for all subjects rapidly decreased across sessions. Responding was deemed extinguished when the overall response rate within a single session reached ≤20% of the mean response rate of the three maintenance sessions. Reinstatement tests occurred on the day immediately after successful extinction of responding. Five minutes before the onset of the session, animals were administered a noncontingent intravenous bolus infusion (prime) of cocaine (veh, 0.03–1.0 mg/kg). The white light was reintroduced as per a maintenance self-administration session, but importantly, saline was still substituted for cocaine infusions throughout the duration of the session. Therefore, all responding during a reinstatement test depended on the dose of the noncontingent prime and the reintroduction of conditioned reinforcement, but not on cocaine reinforcement during the session.
For each subject, the dose of cocaine prime that induced maximal rates of responding was deemed the EDPeak. The EDPeak for each individual subject was typically one-half log-step above the EDMax unit dose for maintenance cocaine self-administration sessions. Each pharmacological pretreatment was tested across a range of cocaine prime doses, relative to the EDPeak, within each individual subject. For drug interaction studies, mCPP (0.1–0.3 mg/kg) or Ro 60-0175 (0.1–0.3 mg/kg) was administered 15 min before the cocaine prime, and SB 242084 (0.03 mg/kg) was administered 30 min before the cocaine prime. Within each drug-interaction experiment, reinstatement tests for each drug dose were separated by the re-establishment of maintenance cocaine self-administration and subsequent extinction. The dose order of drug combinations for reinstatement tests was randomized within subjects.
Second-Order Stimulus Termination.
Daily sessions were conducted 5 days per week and lasted approximately 60 min. Each session began with the illumination of a pair of red lights. During a 600-s fixed interval a FR20 operant schedule was superimposed such that every 20th lever press extinguished the red lights and briefly illuminated a white light for 2 s, followed immediately by reillumination of the red lights. Responses during the 2-s white light were recorded but did not contribute to the subsequent ratio requirement. Once the 600-s fixed interval elapsed, the schedule progressed into a 20-s limited hold. A completed FR20 during the limited hold extinguished the red lights and illuminated a white light for 15 s to signal reinforcement. If the animal failed to complete a FR20 during the limited hold, a mild electrical stimulus (300 ms, 3–6 mA) was delivered to a shaved portion of the distal end of the tail that was secured within an acrylic tail yoke. Each daily session consisted of five consecutive components separated by 60-s timeout periods during which all lights were extinguished. Response rates were recorded for each individual component and then averaged across the session. mCPP (veh, 0.1–0.3 mg/kg) or Ro 60-0175 (veh, 0.1–0.3 mg/kg) was administered 15 min before session onset. The order of dose administration for each drug was randomized within subjects.
In Vivo Microdialysis.
The microdialysis protocols used in the present study were similar to those described previously (Czoty et al., 2000, 2002; Kimmel et al., 2005, 2007; Bauzo et al., 2009). CMA/11 dialysis probes (CMA Microdialysis) with a shaft length of 14 mm (caudate nucleus access) or 20 mm (nucleus accumbens access) and active dialysis membrane measuring 4 × 0.24 mm (caudate nucleus) or 2 × 0.24 mm (nucleus accumbens) were used for all studies. Separate groups of animals were used to assess drug effects in either the caudate nucleus (n = 4) or nucleus accumbens (n = 3). The probe inlet was connected via FEP Teflon tubing to a microinfusion syringe mounted on a motor-driven syringe pump. Probes were flushed with artificial cerebrospinal fluid (aCSF; 1.0 mM Na2HPO4, 150 mM NaCl, 3 mM KCl, 1.3 mM CaCl2, 1.0 mM MgSO4, and 0.15 mM ascorbic acid) for 30 min before insertion into guide cannulae. FEP Teflon tubing was connected to the probe outlet and terminated outside the experimental chamber to allow for sample collection within microcentrifuge tubes.
During experiments, aCSF was perfused through the probe at a flow rate of 0.2 μl/min. Once probes were inserted into the guide cannula, a 60-min equilibration period was followed by acquisition of three baseline samples collected at 10-min intervals before drug treatment for determination of basal DA concentrations. After baseline sample collection, additional 10-min samples were taken after drug administration according to the following conditions: Ro 60-0175 (veh, 0.3 mg/kg) administered 15 min before cocaine (1.0 mg/kg). After cocaine administration, additional 10-min samples were collected for a total duration of 2 h. The interval between pretreatments and cocaine administration and the doses of all drugs were chosen based on results from previous behavioral studies. All samples were refrigerated or frozen until immediately before analysis. Probes were tested in vitro both before and immediately after each session to determine probe viability and percentage of recovery. To confirm integrity of the site, the KCl concentration within the perfused aCSF was increased to 100 mM after the final experimental samples had been collected within each session to induce voltage-dependent DA release, and a final 10-min sample was collected. A robust increase in extracellular DA levels confirmed site viability. We have previously demonstrated the validity of repeated microdialysis accesses without a resultant loss of site viability (Czoty et al., 2000). Each experimental session was conducted in a single brain hemisphere. For each subject, all drug combinations within a given experiment were acquired from the same ipsilateral hemisphere. Accesses at each brain site were separated by at least 2 weeks. The order of drug dose combinations was randomized within subjects.
Levels of DA were quantified within each sample by using high-performance liquid chromatography with electrochemical detection as described previously (Czoty et al., 2000; Kimmel et al., 2005; Bauzo et al., 2009; Murnane et al., 2010). The high-performance liquid chromatography system consisted of a small-bore (3.2 × 150 mm, 3 μm) column (ESA Inc., Chelmsford, MA) with a commercially available mobile phase (MD-TM; ESA Inc.). Microcentrifuge vials containing experimental samples (20 μl) were loaded into a refrigerated ESA model 542 autosampler. Each sample was mixed with 3 μl of ascorbate oxidase, and 5 μl of the mixture was injected into the high-performance liquid chromatography system via an ESA 582 solvent delivery pump at a flow rate of 0.6 ml/min. Electrochemical analyses were performed using an ESA dual-channel analytical cell (model 5040) and guard cell (model 5020) and an ESA Coulochem II or III detector. Potentials were set as follows: channel 1, −150 mV (oxidation); channel 2, +275 mV (reduction); guard cell, 350 mV. EZChrome Elite v. 3.1 software (Scientific Software, Pleasanton, CA) was used to generate chromatograms for each sample analyzed. A set of DA standards containing experimenter-prepared concentrations of DA (0.5–25 nM) were analyzed before and after each set of experimental samples. Area under the curve was calculated for each standard and used to generate a standard plot (area under the curve × estimated DA concentration) from which the estimated DA concentration for each experimental sample could be extrapolated.
Drugs
Cocaine HCl (National Institute on Drug Abuse, Bethesda, MD), mCPP (Sigma-Aldrich, St. Louis, MO), and Ro 60-0175 (Tocris Bioscience, Ellisville, MO) were dissolved in 0.9% saline. SB 242084 (Tocris Bioscience) was initially dissolved at a concentration of 1.0 mg/ml in a 20:20:60 mixture of 95% ethanol, Tween 80 (Sigma-Aldrich), and 0.9% saline and further diluted to appropriate concentrations by using 0.9% saline. All drug solutions were passed through a 0.2-μm pore polysulfone filter before use and administered intramuscularly unless otherwise specified. Doses were calculated from the salt weights.
Data Analysis
For fixed-interval stimulus termination experiments, only response rates obtained from the first 10 components (approximately 60 min) of each test session were used for analyses because the behavioral stimulant effects of lower doses of cocaine returned to baseline values by this time. For each subject, rates of responding after each drug combination test were normalized as a percentage of the overall response rate after vehicle treatments of all drugs. The effects of pretreatment with mCPP or Ro 60-0175 before administration of multiple doses of cocaine were analyzed by using a two-way repeated-measures analysis of variance (ANOVA) with post hoc Tukey's tests. The effects of pretreatment with mCPP, Ro 60-0175, or SB 242084 alone (before saline administration) on responding were analyzed by using a one-way repeated-measures ANOVA with post hoc Tukey's tests. For antagonist-interaction studies, data were analyzed by using a one-way repeated-measures ANOVA with post hoc Dunnett's tests to compare the effects of all drug combinations against the behavioral stimulant effects of EDMax cocaine alone.
For self-administration and reinstatement experiments, response rates were normalized to the percentage of responding maintained during maintenance cocaine self-administration sessions when the EDMax unit cocaine dose was available. For second-order stimulus termination experiments, response rates after pharmacological pretreatments were normalized to the percentage of responding after vehicle drug pretreatment. The effects of pretreatment with mCPP or Ro 60-0175 were analyzed by using repeated-measures ANOVAs with post hoc Tukey's tests. For antagonist-interaction studies, data were analyzed by using repeated-measures ANOVA with post hoc Dunnett's tests to compare each drug combination against the reinstatement effects of EDPeak cocaine alone. The effects of mCPP or Ro 60-0175 pretreatments on the number of reinforcers earned during second-order drug self-administration or stimulus termination sessions was analyzed with repeated-measures ANOVA and post hoc Tukey's tests.
For in vivo microdialysis studies, DA levels within each test session were normalized as the percentage of the mean of three baseline values acquired before drug administration. Because the effects of cocaine typically returned to near-baseline levels within 60 min after cocaine administration, samples collected after this time point were excluded from analyses. Data were analyzed by using repeated-measures ANOVA. Tukey's post hoc tests corrected with a Bonferroni adjustment then determined at each time point whether DA levels were affected by pretreatment with Ro 60-0175 compared with vehicle pretreatment.
Data were graphically plotted by using Prism version 5.01 (GraphPad Software Inc., San Diego, CA) and analyzed by using SigmaStat version 3.0 software (Systat Software, Inc., San Jose, CA). With the exception of antagonist-interaction studies, statistical analyses were followed by post hoc Tukey's tests as the number of means to be compared (≤3) was insufficient for Dunnett's tests. For all statistical analyses, significance was accepted at the 95% level of confidence (α = 0.05).
Results
Behavioral Stimulant Effects of Cocaine
Effects of Pretreatment with mCPP.
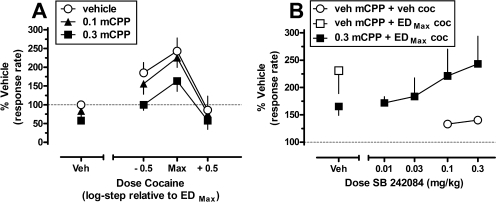
The effects of pretreatment with the preferential 5-HT2CR agonist mCPP alone and in combination with cocaine are shown in Fig. 1A. The mean response rate (± S.E.M.) after vehicle pretreatments of both mCPP and cocaine was 0.41 ± 0.06 responses/s. Administration of mCPP induced a dose-dependent downward shift of the ascending limb of the cocaine dose-response function. Two-way repeated-measures ANOVA indicated significant main effects for cocaine (F2,6 = 8.50; p = 0.018) and mCPP (F2,6 = 11.47; p = 0.009) but not a significant interaction (F4,12 = 0.79, p = 0.55). Post hoc Tukey's tests indicated that, averaged across all cocaine doses, pretreatment with 0.3 mg/kg mCPP attenuated the behavioral stimulant effects of cocaine (p < 0.05). Specifically, pretreatment with 0.3 mg/kg mCPP reduced the behavioral stimulant effect of the maximally effective dose of cocaine (∼163% versus ∼243% baseline) and abolished the behavioral stimulant effects of the submaximal dose of cocaine (∼100% versus ∼185%, respectively). The 0.3 mg/kg dose of mCPP also suppressed responding significantly when administered alone to approximately 57% of baseline responding (F2,6 = 5.23; p = 0.048; Tukey's test, p < 0.05). Pretreatment with 0.1 mg/kg mCPP did not affect responding when administered before either cocaine or saline (p > 0.05).
Fig. 1.
Modulation of the behavioral stimulant effects of cocaine after pretreatment with mCPP alone and in combination with SB 242084 in squirrel monkeys (n = 4) trained to lever-press on a fixed-interval 300-s schedule of stimulus termination. A, effects of pretreatment with mCPP (veh, 0.1–0.3 mg/kg) before administration of saline or multiple doses of cocaine. B, effects of pretreatment with SB 242084 (veh, 0.01–0.3 mg/kg) on the mCPP-induced attenuation of behavioral stimulant effects induced by the maximally effective dose of cocaine (coc). Data (mean ± S.E.M.) are expressed as a percentage of responding after administration of the vehicles for all drugs. The dotted lines represent baseline response rate after vehicle treatments (100%). Abscissae, dose of cocaine, relative to EDMax dose determined in each individual subject (A) or dose of SB 242084 (B). Ordinates, normalized response rate.
To confirm that the actions of mCPP were mediated through the 5-HT2CR, the dose combination of 0.3 mg/kg mCPP and EDMax cocaine was retested after pretreatment with several doses of the highly selective 5-HT2CR antagonist SB 242084 (Fig. 1B). One-way repeated-measures ANOVA with post hoc Tukey's tests indicated that pretreatment with 0.1 or 0.3 mg/kg SB 242084 alone produced modest, but significant, behavioral stimulant effects (F2,6 = 18.83; p = 0.003; Tukey's test, p < 0.05) as response rates were increased to ∼132 and ∼140% of baseline, respectively. When administered before combined testing with mCPP and EDMax cocaine, one-way repeated-measures ANOVA detected a main effect of treatment condition (F5,15 = 3.08; p = 0.042). Post hoc Dunnett's tests failed to identify significant differences among any of the treatments, likely because of the low power of the performed analysis (0.520). However, visual inspection of the data suggests that SB 242084, at doses that produced modest behavioral stimulant effects alone, dose-dependently blocked the attenuating effects of mCPP (Fig. 1B).
Effects of Pretreatment with Ro 60-0175.
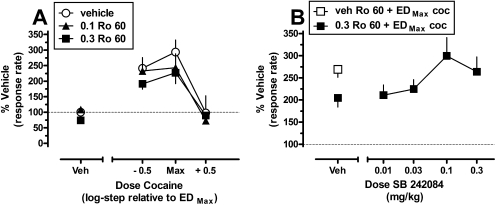
The effects of pretreatment with the selective 5-HT2CR agonist Ro 60-0175 alone and in combination with cocaine are shown in Fig. 2A. The mean response rate (± S.E.M.) after vehicle pretreatments of both Ro 60-0175 and cocaine was 0.44 ± 0.14 responses/s. Two-way repeated-measures ANOVA indicated a significant main effect for cocaine (F2,6 = 10.10; p = 0.012), although the main effect for Ro 60-0175 only trended toward significance (F2,6 = 4.49; p = 0.064). The cocaine × Ro 60-0175 interaction was not significant (F4,12 = 1.12; p = 0.393). Although the main effect of Ro 60-0175 did not reach significance, the results suggested that the behavioral stimulant effect of EDMax cocaine (∼293%) was blunted after pretreatment with 0.3 mg/kg Ro 60-0175 to ∼226% baseline responding, and a similar effect was observed at the submaximal cocaine dose (∼241% versus ∼191%). Furthermore, and in contrast to mCPP, one-way repeated-measures ANOVA with Tukey's post hoc tests indicated that neither dose of Ro 60-0175 significantly altered response rates when administered alone (p > 0.05, compared with vehicle pretreatment). The highest dose of Ro 60-0175 (0.3 mg/kg) reduced response rates to approximately 75% of baseline responding, nearly half the magnitude of change in responding induced by the highest (0.3 mg/kg) dose of mCPP tested in the previous experiment (Fig. 1A).
Fig. 2.
Modulation of the behavioral stimulant effects of cocaine after pretreatment with Ro 60-0175 alone and in combination with SB 242084 in squirrel monkeys (n = 4) trained to lever-press on a fixed-interval 300-s schedule of stimulus termination. A, effects of pretreatment with Ro 60-0175 (veh, 0.1–0.3 mg/kg) before administration of saline or multiple doses of cocaine. B, effects of pretreatment with SB 242084 (veh, 0.01–0.3 mg/kg) on the Ro 60-0175-induced attenuation of behavioral-stimulant effects induced by the maximally effective dose of cocaine (coc). Otherwise, as in Fig. 1.
Although pretreatment with Ro 60-0175 failed to produce a significant modulation of the behavioral stimulant effects of cocaine, the pattern of its effects, i.e., a downward shift of the ascending limb of the cocaine dose-response function, seemed consistent with that observed after mCPP pretreatment. We therefore replicated the earlier antagonist-interaction experiment with SB 242084 to confirm that the effects of Ro 60-0175, although modest, were mediated via the 5-HT2CR. The effects of pretreatment with several doses of SB 242084 before combined administration of 0.3 mg/kg Ro 60-0175 and EDMax cocaine are shown in Fig. 2B. One-way repeated-measures ANOVA detected a main effect for treatment condition (F5,15 = 3.11; p = 0.040). However, similar to results obtained earlier with mCPP pretreatments, post hoc Dunnett's tests failed to identify significant differences among any of the treatments, again likely because of the low power of the performed analysis (0.527). Nevertheless, visual inspection of the data suggests that the effects of Ro 60-0175 on the behavioral stimulant effects of EDMax cocaine were antagonized by pretreatment with SB 242084 at doses similar to those that seemed to antagonize the effects of mCPP (Fig. 1B).
Cocaine-Induced Reinstatement and Self-Administration
Cocaine-Induced Reinstatement: Effects of mCPP.
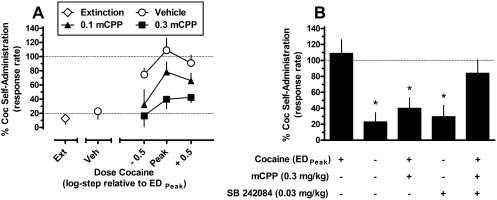
The effects of pretreatment with the preferential 5-HT2CR agonist mCPP on cocaine-induced reinstatement are shown in Fig. 4A. The mean response rate (± S.E.M.) during maintenance EDMax cocaine self-administration sessions was 1.35 ± 0.28 responses/s. After pretreatment with the mCPP vehicle, cocaine-induced reinstatement across a range of cocaine doses produced an inverted U-shaped dose-response function, with the maximally effective priming dose of cocaine (EDPeak) producing responding that was ∼109% of the response rate maintained during maintenance cocaine self-administration. Pretreatment with mCPP induced a dose-dependent downward shift in the cocaine dose-response function. Two-way repeated-measures ANOVA indicated a significant main effect for mCPP dose (F2,4 = 21.89; p = 0.007) but not for cocaine dose (F2,4 = 2.00; p = 0.25) or their interaction (F4,8 = 1.88; p = 0.21). Post hoc Tukey's tests revealed that, averaged across all cocaine doses, pretreatment with either 0.1 or 0.3 mg/kg mCPP significantly attenuated cocaine-induced reinstatement compared with vehicle pretreatment (p < 0.05).
Fig. 4.
Effects of pretreatment with Ro 60-0175 (veh, 0.1–0.3 mg/kg) alone or in combination with SB 242084 (veh, 0.03 mg/kg) on cocaine (Coc)-induced reinstatement in squirrel monkeys (n = 3). A, effects of Ro 60-0175 pretreatment on reinstatement induced by several doses of cocaine priming infusions. ♢, responding on the last day of extinction sessions. ○, cocaine-induced reinstatement after pretreatment with the Ro 60-0175 vehicle. Filled symbols, cocaine-induced reinstatement after pretreatment with Ro 60-0175. B, pretreatment with 0.03 mg/kg SB 242084 did not induce reinstatement alone, but antagonized the effects of Ro 60-0175. Otherwise, as in Fig. 3.
To confirm that the observed effects of mCPP were mediated via actions at the 5-HT2CR, we redetermined the effect of 0.3 mg/kg mCPP on cocaine-induced reinstatement after pretreatment with the selective 5-HT2CR antagonist SB 242084 (Fig. 3B). One-way repeated-measures ANOVA indicated a significant effect of treatment condition (F4,8 = 8.53; p = 0.006). Post hoc Dunnett's tests revealed that 0.3 mg/kg mCPP significantly attenuated the reinstatement effects of EDPeak cocaine to ∼40% of the cocaine self-administration response rate (p < 0.05). Administration of 0.03 mg/kg SB 242084 did not appreciably induce reinstatement alone (30%). However, this same dose of SB 2424084 given before mCPP resulted in a magnitude of cocaine-induced reinstatement that was not significantly different from cocaine alone (∼84% compared with ∼108%).
Fig. 3.
Effects of pretreatment with mCPP (veh, 0.1–0.3 mg/kg) alone or in combination with SB 242084 (veh, 0.03 mg/kg) on cocaine (Coc)-induced reinstatement in squirrel monkeys (n = 3). A, effects of mCPP pretreatment on reinstatement induced by several doses of cocaine priming infusions. ♢, responding on the last day of extinction sessions. ○, cocaine-induced reinstatement after pretreatment with the mCPP vehicle. Filled symbols, cocaine-induced reinstatement after pretreatment with mCPP. B, pretreatment with 0.03 mg/kg SB 242084 did not induce reinstatement alone, but antagonized the effects of mCPP. Data (mean ± S.E.M.) are expressed as the percentage of responding maintained during EDMax cocaine self-administration sessions. The dotted lines represent baseline self-administration rate (100%, A and B) and extinction criterion (20%, A). *, significant difference (p < 0.05) for a given data point compared with the reinstatement effect induced by EDPeak cocaine prime alone (B). Abscissae, dose of cocaine prime (A) or drug combination (B). Ordinates, normalized response rate.
Cocaine-Induced Reinstatement: Effects of Ro 60-0175.
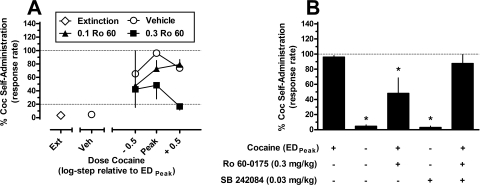
The effects of pretreatment with the selective 5-HT2CR agonist Ro 60-0175 on cocaine-induced reinstatement are shown in Fig. 4A. The mean response rate (± S.E.M.) during maintenance EDMax cocaine self-administration sessions was 1.14 ± 0.16 responses/s. As was observed in the previous experiment, cocaine-induced reinstatement across a range of cocaine doses resulted in an inverted U-shaped dose-response function after vehicle Ro 60-0175 pretreatment. The maximally effective priming dose of cocaine (EDPeak) produced responding that was ∼96% of the response rate maintained during maintenance cocaine self-administration. Similar to the effects of mCPP, pretreatment with Ro 60-0175 induced a dose-dependent downward shift in the cocaine dose-response function. Two-way repeated-measures ANOVA indicated a significant main effect of Ro 60-0175 dose (F2,4 = 59.04; p = 0.001) but not for cocaine dose (F2,4 = 0.43; p = 0.68) or their interaction (F4,8 = 1.96; p = 0.194). Subsequent post hoc Tukey's tests revealed that, averaged across all cocaine doses, 0.3 mg/kg Ro 60-0175 significantly attenuated the reinstatement effects of cocaine compared with vehicle (p < 0.05).
We next redetermined the effect of 0.3 mg/kg Ro 60-0175 on cocaine-induced reinstatement after pretreatment with the selective 5-HT2CR antagonist SB 242084 (Fig. 4B). One-way repeated-measures ANOVA indicated a significant effect of treatment condition (F4,8 = 21.35; p < 0.001). Post hoc Dunnett's tests further revealed that 0.3 mg/kg Ro 60-0175 significantly attenuated the reinstatement effects of EDPeak cocaine (p < 0.05). In addition, and in accordance with the previous mCPP experiment, administration of 0.03 mg/kg SB 242084 did not induce any measurable reinstatement alone (∼3%), but antagonized the effects of Ro 60-0175 on cocaine-induced reinstatement because their combined administration resulted in responding at a level (∼88%) that was not significantly different from the effect of cocaine alone (p > 0.05).
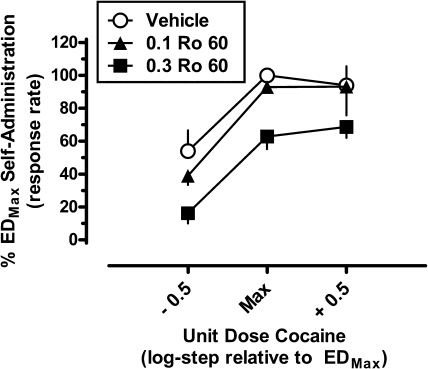
Cocaine Self-Administration: Effects of Ro 60-0175.
To determine whether 5-HT2CR activation alters the direct reinforcing effects of cocaine, subjects were pretreated with the selective 5-HT2CR agonist Ro 60-0175 before maintenance cocaine self-administration sessions (Fig. 5). The mean response rate (± S.E.M.) during maintenance EDMax cocaine self-administration sessions was 1.56 ± 0.41 responses/s. Varying the unit dose of cocaine produced a broad inverted U-shaped dose-response function typical of second-order schedules of drug self-administration (Schindler et al., 2002). Similar to its effects on the behavioral stimulant and reinstatement effects of cocaine, pretreatment with Ro 60-0175 caused a dose-dependent downward shift of the dose-response function, indicating an attenuation of the direct reinforcing effects of cocaine. Two-way repeated-measures ANOVA indicated significant main effects of cocaine dose (F2,4 = 9.21; p = 0.032) and Ro 60-0175 dose (F2,4 = 24.79; p = 0.006) but not a significant interaction (F4,8 = 1.14; p = 0.41). Tukey's post hoc tests revealed that, averaged across all doses of cocaine, pretreatment with 0.3 mg/kg Ro 60-0175 significantly reduced cocaine self-administration behavior (p < 0.05, compared with vehicle). More specifically, after pretreatment with 0.3 mg/kg Ro 60-0175 response rates maintained by the EDMax cocaine unit dose were reduced to ∼63% compared with baseline, whereas response rates maintained by the lower dose of cocaine were reduced to levels approximating the extinction criterion of 20%. In addition, it should be noted that increasing the unit dose of cocaine available one half-log unit above the EDMax unit dose did not overcome the effects of Ro 60-0175, indicative of a downward shift, rather than a parallel leftward shift, of the cocaine dose-response function. Pretreatment with Ro 60-0175 did not significantly alter the number of cocaine infusions earned throughout the duration of the session (F2,4 = 1.65; p = 0.30; data not shown). The effects of Ro 60-0175 pretreatments on responding emerged on the first day of administration, and there was no evidence of tolerance across three consecutive test sessions. In addition, on the cocaine self-administration day immediately after the last pretreatment of each week response rates consistently recovered to near the 100% baseline.
Fig. 5.
Effects of pretreatment with Ro 60-0175 (veh, 0.1–0.3 mg/kg) on second-order cocaine self-administration responding in squirrel monkeys (n = 3). Data (mean ± S.E.M.) are expressed as the percentage of responding maintained during EDMax cocaine self-administration sessions after vehicle Ro 60-0175 pretreatment. Abscissa, unit dose of cocaine available for self-administration, relative to the maximally effective unit dose of cocaine (EDMax) for each individual subject. Ordinate, normalized response rate.
Second-Order Stimulus Termination: Effects of mCPP and Ro 60-0175.
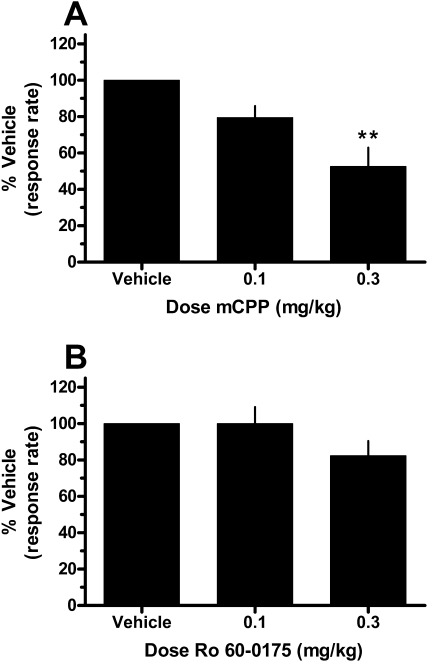
The effects of pretreatment with the preferential 5-HT2CR agonist mCPP on responding maintained by a second-order schedule of stimulus termination are shown in Fig. 6A. The mean response rate (± S.E.M.) after vehicle mCPP pretreatment was 0.91 ± 0.06 responses/s. mCPP significantly attenuated response rates in a dose- dependent manner (one-way repeated-measures ANOVA; F2,6 = 17.37; p = 0.003). Post hoc Tukey's tests indicated that 0.3 mg/kg mCPP significantly reduced response rates to ∼53% of baseline (p = 0.003). In addition, one-way repeated-measures ANOVA revealed that pretreatment with 0.3 mg/kg mCPP significantly reduced the number of reinforcers earned throughout the duration of the session compared with vehicle (F2,6 = 15.14; p = 0.005; Tukey's test, p = 0.004). Administration of 0.1 mg/kg mCPP trended toward producing a similar effect but failed to reach significance (p = 0.061).
Fig. 6.
Effects of pretreatment with mCPP (A) or Ro 60-0175 (B) on responding maintained by a second-order schedule of stimulus termination in squirrel monkeys (n = 4). Data (mean ± S.E.M.) are expressed as a percentage of responding after administration of the drug vehicle. **, significant difference (p < 0.01) for a given data point compared with vehicle pretreatment. Abscissae, dose of pretreatment. Ordinates, normalized response rate.
The effects of pretreatment with the selective 5-HT2CR agonist Ro 60-0175 on response rates are shown in Fig. 6B. The mean response rate (± S.E.M.) after vehicle Ro 60-0175 pretreatment was 0.83 ± 0.05 responses/s. In contrast to the effects of mCPP, pretreatment with Ro 60-0175 did not significantly alter responding (one-way repeated-measures ANOVA, F2,6 = 2.40; p = 0.17). The highest dose of Ro 60-0175 tested (0.3 mg/kg) had a weak effect on responding as response rates were ∼82% of the baseline. Also in contrast to the effects of mCPP, neither dose of Ro 60-0175 significantly altered the number of reinforcers earned throughout the duration of the session compared with vehicle (0.1 mg/kg, p = 0.54; 0.3 mg/kg, p = 0.092; data not shown).
Neurochemical Effects of Cocaine
Caudate Nucleus.
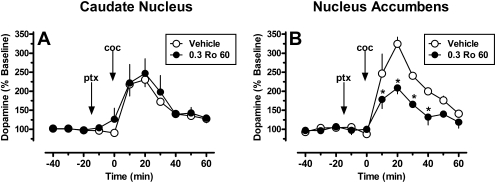
Mean (± S.E.M.) basal DA levels uncorrected for probe recovery were 4.90 ± 0.98 nM. As reported previously, 1.0 mg/kg cocaine after vehicle (saline) pretreatment increased extracellular DA in the caudate nucleus to ∼230% of basal DA levels within 20 min after cocaine administration that returned to near-baseline levels within 60 min after drug injection (Czoty et al., 2000, 2002). Pretreatment with the selective 5-HT2CR agonist Ro 60-0175 15 min before cocaine did not alter the subsequent time course or maximal increase in DA levels within the caudate nucleus (Fig. 7A). Two-way repeated-measures ANOVA revealed a significant main effect of time (F10,30 = 28.57; p < 0.001), but not for the main effect of Ro 60-0175 pretreatment (F1,3 = 0.40; p = 0.57) or their interaction (F10,29 = 0.14; p = 1.0).
Fig. 7.
Effects of cocaine (1.0 mg/kg) on extracellular levels of DA in the caudate nucleus (A; n = 4) or nucleus accumbens (B; n = 3) after pretreatment with 0.3 mg/kg Ro 60-0175 (●) or its vehicle (○) in squirrel monkeys. Data points (mean ± S.E.M.) are expressed as the percentage of DA levels before drug administration. Arrows designate time of administration of Ro 60-0175 or its vehicle (ptx, pretreatment) and cocaine (coc). *, significant difference (p < 0.05) for a given data point compared with vehicle pretreatment within the same time point. Abscissae, time relative to cocaine administration. Ordinates, normalized DA concentration.
Nucleus Accumbens.
Mean (± S.E.M.) basal DA levels uncorrected for probe recovery in the nucleus accumbens were 2.68 ± 1.35 nM. Administration of 1.0 mg/kg cocaine produced a peak increase of ∼324% of basal DA levels at 20 min after cocaine administration (Fig. 7B). The time course of cocaine-induced changes in DA levels were similar to those observed within the caudate nucleus as the effect peaked at 20 min after cocaine administration and returned to near-baseline levels within 60 min. In contrast to a lack of effect in the caudate nucleus, pretreatment with Ro 60-0175 attenuated the effects of cocaine on extracellular DA levels. Two-way repeated-measures ANOVA revealed significant main effects of time (F10,20 = 61.75; p < 0.001) and pretreatment with Ro 60-0175 (F1,2 = 17.96; p = 0.05) and a significant interaction (F10,20 = 2.52; p = 0.038). Subsequent post hoc analyses indicated that although Ro 60-0175 had no effect on DA levels in the interval preceding cocaine administration, the effects of cocaine were significantly attenuated by Ro 60-0175 pretreatment at each sampling interval within the 40-min time period immediately after cocaine injection (p < 0.05).
Discussion
Although previous work has demonstrated a modulatory role for 5-HT on the behavioral and neurochemical effects of cocaine in nonhuman primates, the impact of specific 5-HT receptor subtypes has remained unclear. In the present study, using compounds that demonstrate greater intersubtype selectivity than those used previously, we now report that selective 5-HT2CR activation attenuates the behavioral and neurochemical effects of cocaine in squirrel monkeys.
Studies in rodents have indicated that selective 5-HT2CR agonists attenuate the locomotor, reinforcing, and reinstatement effects of cocaine (for review, see Bubar and Cunningham, 2008). However, to the best of our knowledge, selective 5-HT2CR agonists or antagonists have not been previously administered to nonhuman primates. We therefore were initially concerned about the safety and tolerability of the administration of such compounds. To confirm the 5-HT2CR as a pharmacotherapeutic target, we first assessed the impact of mCPP pretreatment on the behavioral effects of cocaine. mCPP is commonly described as a “preferential” 5-HT2CR agonist because 1) it exhibits modest selectivity for the 5-HT2CR compared with the 5-HT2A receptor (pEC50 = 7.09 versus 6.65, respectively), 2) it is a more efficacious agonist at the 5-HT2CR compared with the 5-HT2A receptor, and 3) its behavioral effects in rodents are blocked by 5-HT2CR antagonists, but not antagonists of other receptor subtypes (Porter et al., 1999; Bubar and Cunningham, 2008). It is noteworthy that we have previously administered mCPP at doses up to 2.5 mg/kg i.v. in rhesus monkeys without deleterious or adverse consequence (Murnane et al., 2010). In the present study, pretreatment with mCPP attenuated the behavioral stimulant and reinstatement effects of cocaine in squirrel monkeys, and these effects were prevented by administration of the highly selective 5-HT2CR antagonist SB 242084, suggesting that the effects of mCPP were indeed mediated through the 5-HT2CR.
It must be noted that mCPP also caused reductions in basal rates of responding when administered alone. In addition to direct agonist activity at 5-HT receptors, mCPP functions as a 5-HT releaser, increasing extracellular concentrations of 5-HT (Baumann et al., 1993; Eriksson et al., 1999; Murnane et al., 2010). This is an important consideration because pharmacological enhancement of 5-HT levels via administration of indirect agonists decreases response rates in squirrel monkeys maintained on a fixed-interval schedule of stimulus termination (Spealman et al., 1989; Howell and Byrd, 1995). We therefore replicated the mCPP experiments with Ro 60-0175, a drug that, compared with mCPP, exhibits greater selectivity and agonist efficacy for the 5-HT2CR (Porter et al., 1999). The results demonstrate that the modulation of cocaine-induced behavioral effects after pretreatment with Ro 60-0175 shared a pattern that was similar to mCPP, although the maximal reductions in cocaine-induced reinstatement and behavioral stimulant effects resulting from Ro 60-0175 were of noticeably lower magnitude compared with those produced by mCPP. It is noteworthy that, in contrast to mCPP, doses of Ro 60-0175 that were found to significantly attenuate the behavioral effects of cocaine did not produce significant reductions in basal responding regardless of the operant schedule used. The effects of both agonists can more succinctly be summarized as follows: 1) mCPP attenuated the behavioral effects of cocaine with greater magnitude compared with Ro 60-0175, but only at doses that also reduced nondrug-maintained responding, and 2) the modulation of the behavioral effects of cocaine after Ro 60-0175 pretreatment were slightly weaker than those of mCPP, but Ro 60-0175 demonstrated a better degree of behavioral selectivity.
One may speculate that increasing the pretreatment dose of Ro 60-0175 would have produced an even stronger attenuation of the behavioral effects of cocaine. However, in a pilot study, increasing the dose of Ro 60-0175 to 1.0 mg/kg in two squirrel monkeys reduced baseline responding to 45% in the fixed-interval stimulus termination procedure in a SB 242084-reversible manner (data not shown) and was therefore not tested in interaction studies with cocaine. These results suggest that the nonspecific rate-decreasing effects of both mCPP and Ro 60-0175 are at least partially mediated through the 5-HT2CR, and as such, it cannot be argued that greater pharmacological selectivity for the 5-HT2CR would fully eliminate the possibility of nonspecific disruptions in operant performance. However, our data do indicate that the selective 5-HT2CR agonist Ro 60-0175 reduced the behavioral stimulant, reinforcing, and reinstatement effects of cocaine at a dose (0.3 mg/kg) that did not demonstrate nonspecific rate-decreasing effects in either the fixed-interval or second-order schedules of stimulus termination, whereas mCPP exerted similar effects only at doses that also engendered nonspecific disruptions in operant responding. Therefore, we suggest that greater selectivity and/or efficacy for the 5-HT2CR engenders superior behavioral specificity of drug effects on responding maintained by drug versus nondrug reinforcers. The development and availability of pharmacological tools with even greater selectivity and/or efficacy at the 5-HT2CR would allow us to test this hypothesis to better effect.
Several studies have indicated that the mesolimbic DA system is modulated by signaling at the 5-HT2CR. Both the 5-HT2CR mRNA and protein have been described to localize predominantly on GABAergic interneurons within the rodent VTA (Eberle-Wang et al., 1997; Bubar and Cunningham, 2007), and local administration of mCPP increased the firing rate of these cells (Di Giovanni et al., 2001). Accordingly, Navailles et al. (2008) demonstrated that intra-VTA administration of the 5-HT2CR agonist Ro 60-0175 attenuated the DA-increasing effects of systemically administered cocaine in anesthetized rats. It therefore seems likely that 5-HT2CR activation within the VTA functionally inhibits DA release within mesolimbic terminal regions by stimulating local GABA release onto DA-releasing neurons.
In agreement with this hypothesis, systemic administration of the 5-HT2CR-selective agonist Ro 60-0175 significantly attenuated cocaine-induced increases in DA levels within the NAc, but not the caudate nucleus, of squirrel monkeys. These results suggest that, in contrast to the mesolimbic DA system, the nigrostriatal pathway seems to be unaffected by signaling through the 5-HT2CR in nonhuman primates, a result that is consistent with a previous receptor localization study in which the 5-HT2CR mRNA was found within the VTA and NAc, but not the substantia nigra pars compacta or dorsolateral aspects of the striatum, of nonhuman primates (López-Giménez et al., 2001). Although these data raise the intriguing possibility that the modulation of the mesolimbic DA-increasing effects of cocaine after Ro 60-0175 administration may underlie the observed attenuation of cocaine-induced behavioral effects, this hypothesis remains speculative at present. Furthermore, because Ro 60-0175 was administered systemically in the present study, the primary sites of its inhibitory influence on cocaine-induced behavioral effects cannot be definitively identified and may include regions other than the NAc.
It is noteworthy that Ro 60-0175 pretreatment did not affect basal DA levels in the 15-min period preceding cocaine administration, although basal DA levels were reduced by systemic administration of 5-HT2CR agonists in rodents (Di Matteo et al., 1999; Di Giovanni et al., 2000). The reason for this discrepancy is unclear, but several possible factors may be responsible. First, the time required for the onset of Ro 60-0175 effects has not been determined in nonhuman primates. We chose the 15-min pretreatment time in the microdialysis study because it was found to be effective in our behavioral studies involving interactions with cocaine. However, reductions in basal DA levels might have occurred after the 15-min pretreatment time had elapsed. Alternatively, Ro 60-0175 at the dose used presently may have selectively attenuated cocaine-induced elevations in DA without affecting basal levels in nonhuman primates, as has been shown previously with administration of other compounds (Czoty et al., 2002; Bauzo et al., 2009). Future studies examining changes in DA levels within the NAc of squirrel monkeys after administration of multiple doses of Ro 60-0175 alone will clarify this issue.
In summary, our present results demonstrate for the first time that pretreatment with the selective 5-HT2CR agonist Ro 60-0175 insurmountably reduced the abuse-related effects of cocaine in a dose-dependent and antagonist-reversible manner in nonhuman primates. Furthermore, by comparing the effects of a preferential agonist (mCPP) with those of a more selective agonist (Ro 60-0175), our results suggest that increased pharmacological selectivity and efficacy for the 5-HT2CR confer a greater index of behavioral specificity, because doses of Ro 60-0175 that attenuated cocaine-induced behavioral effects failed to significantly alter nondrug-maintained responding. Finally, neurochemical evidence suggests that the behavioral effects of Ro 60-0175 may be mediated by selective reductions of cocaine-induced DA increases within the mesolimbic DA system. Taken together, these results suggest that 5-HT2CR agonists may be useful pharmacotherapeutics for the treatment of cocaine abuse as these compounds functionally antagonize the behavioral and neurochemical effects of cocaine in nonhuman primates.
Acknowledgments
We thank Mi Zhou, Juliet Brown, and Lisa Neidert for expert technical assistance; and the Yerkes National Primate Research Center animal care, veterinary care, and facilities staff for exceptional services.
These studies were funded by the National Institutes of Health National Institute on Drug Abuse [Grants DA12514, DA00517, F31-DA026262]; the National Institutes of Health National Center for Research Resources [Grant RR00165]; and the American Recovery and Reinvestment Act of 2009 [Grant F31DA026262].
These studies represent partial fulfillment of D.F.M.'s Ph.D. dissertation research at Emory University.
Preliminary findings from these experiments were presented previously: Manvich DF and Howell LL (2011) Cocaine-induced reinstatement is differentially modulated by agonism and antagonism of the serotonin 5-HT2C receptor in nonhuman primates, at the American Society for Pharmacology and Experimental Therapeutics meeting; 2011 April 9–13; Washington, DC. American Society for Pharmacology and Experimental Therapeutics, Bethesda, MD. Manvich DF and Howell LL (2010) m-Chlorophenylpiperazine (mCPP) attenuates the behavioral-stimulant effects of cocaine via activation of the 5-HT2C receptor in nonhuman primates, at the American Society for Pharmacology and Experimental Therapeutics meeting; 2010 April 24–28; Anaheim, CA. American Society for Pharmacology and Experimental Therapeutics, Bethesda, MD.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- DA
- dopamine
- 5-HT
- 5-hydroxytryptamine
- 5-HT2CR
- 5-HT2C receptor
- ANOVA
- analysis of variance
- mCPP
- m-chlorophenylpiperazine
- NAc
- nucleus accumbens
- VTA
- ventral tegmental area
- veh
- vehicle
- FR20
- fixed-ratio 20
- aCSF
- artificial cerebrospinal fluid
- Ro 60-0175
- (α-S)-6-chloro-5-fluoro-α-methyl-1H-indole-1-ethanamine fumarate
- SB 242084
- 6-chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride.
Authorship Contributions
Participated in research design: Manvich, Kimmel, and Howell.
Conducted experiments: Manvich.
Performed data analysis: Manvich and Kimmel.
Wrote or contributed to the writing of the manuscript: Manvich, Kimmel, and Howell.
References
- Azmitia EC, Gannon PJ. (1986) The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Adv Neurol 43:407–468 [PubMed] [Google Scholar]
- Banks ML, Manvich DF, Bauzo RM, Howell LL. (2009) Effects of histamine H3 receptor activation on the behavioral-stimulant effects of methamphetamine and cocaine in mice and squirrel monkeys. Pharmacology 83:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Rutter JJ, Auerbach SB. (1993) Intravenous administration of the serotonin agonist m-chlorophenylpiperazine (mCPP) increases extracellular serotonin in the diencephalon of awake rats. Neuropharmacology 32:1381–1386 [DOI] [PubMed] [Google Scholar]
- Bauzo RM, Kimmel HL, Howell LL. (2009) Interactions between the mGluR2/3 agonist, LY379268, and cocaine on in vivo neurochemistry and behavior in squirrel monkeys. Pharmacol Biochem Behav 94:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. (1989) Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther 251:150–155 [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. (2007) Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience 146:286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. (2008) Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res 172:319–346 [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. (2008) Stimulation of serotonin2C receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berl) 196:15–27 [DOI] [PubMed] [Google Scholar]
- Callahan PM, Cunningham KA. (1995) Modulation of the discriminative stimulus properties of cocaine by 5-HT1B and 5-HT2C receptors. J Pharmacol Exp Ther 274:1414–1424 [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. (2002) Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 300:831–837 [DOI] [PubMed] [Google Scholar]
- Czoty PW, Justice JB, Jr, Howell LL. (2000) Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology (Berl) 148:299–306 [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Di Mascio M, Esposito E. (2000) Preferential modulation of mesolimbic vs. nigrostriatal dopaminergic function by serotonin(2C/2B) receptor agonists: a combined in vivo electrophysiological and microdialysis study. Synapse 35:53–61 [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, La Grutta V, Esposito E. (2001) m-Chlorophenylpiperazine excites non-dopaminergic neurons in the rat substantia nigra and ventral tegmental area by activating serotonin-2C receptors. Neuroscience 103:111–116 [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. (1999) SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology 38:1195–1205 [DOI] [PubMed] [Google Scholar]
- Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet MF. (1997) Pattern of expression of the serotonin2C receptor messenger RNA in the basal ganglia of adult rats. J Comp Neurol 384:233–247 [PubMed] [Google Scholar]
- Eriksson E, Engberg G, Bing O, Nissbrandt H. (1999) Effects of mCPP on the extracellular concentrations of serotonin and dopamine in rat brain. Neuropsychopharmacology 20:287–296 [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Bauzo RM, Manvich DM, Morales JC, Votaw JR, Goodman MM, Howell LL. (2009) Role of dopamine transporters in the behavioral effects of 3,4-methylenedioxymethamphetamine (MDMA) in nonhuman primates. Psychopharmacology (Berl) 205:337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA. (2004) Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: acute and chronic pharmacological analyses. J Pharmacol Exp Ther 310:1246–1254 [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. (2008) The 5-HT2C receptor agonist Ro60–0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology 33:1402–1412 [DOI] [PubMed] [Google Scholar]
- Frankel PS, Cunningham KA. (2004) m-Chlorophenylpiperazine (mCPP) modulates the discriminative stimulus effects of cocaine through actions at the 5-HT2C receptor. Behav Neurosci 118:157–162 [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Kimmel HL, Carroll FI, Goodman MM, Howell LL. (2005) Interaction of cocaine and dopamine transporter inhibitors on behavior and neurochemistry in monkeys. Pharmacol Biochem Behav 80:481–491 [DOI] [PubMed] [Google Scholar]
- Glowa JR, Rice KC, Matecka D, Rothman RB. (1997) Phentermine/fenfluramine decreases cocaine self-administration in rhesus monkeys. Neuroreport 8:1347–1351 [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA. (2000) Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther 295:1183–1191 [PubMed] [Google Scholar]
- Howell LL, Byrd LD. (1995) Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther 275:1551–1559 [PubMed] [Google Scholar]
- Kimmel HL, Ginsburg BC, Howell LL. (2005) Changes in extracellular dopamine during cocaine self-administration in squirrel monkeys. Synapse 56:129–134 [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Manvich DF, Blough BE, Negus SS, Howell LL. (2009) Behavioral and neurochemical effects of amphetamine analogs that release monoamines in the squirrel monkey. Pharmacol Biochem Behav 94:278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, O'Connor JA, Carroll FI, Howell LL. (2007) Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav 86:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. (1993) Effects of three monoamine uptake inhibitors on behavior maintained by cocaine or food presentation in rhesus monkeys. Drug Alcohol Depend 31:149–158 [DOI] [PubMed] [Google Scholar]
- López-Giménez JF, Mengod G, Palacios JM, Vilaró MT. (2001) Regional distribution and cellular localization of 5-HT2C receptor mRNA in monkey brain: comparison with [3H]mesulergine binding sites and choline acetyltransferase mRNA. Synapse 42:12–26 [DOI] [PubMed] [Google Scholar]
- Murnane KS, Fantegrossi WE, Godfrey JR, Banks ML, Howell LL. (2010) Endocrine and neurochemical effects of 3,4-methylenedioxymethamphetamine and its stereoisomers in rhesus monkeys. J Pharmacol Exp Ther 334:642–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, Moison D, Cunningham KA, Spampinato U. (2008) Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology 33:237–246 [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. (2007) Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther 320:627–636 [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Acosta JI. (2007) Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol 18:791–800 [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. (1999) Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol 128:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Cone EJ, Kuhar MJ. (1990) Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci 46:635–645 [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237:1219–1223 [DOI] [PubMed] [Google Scholar]
- Rüedi-Bettschen D, Rowlett JK, Spealman RD, Platt DM. (2010) Attenuation of cocaine-induced reinstatement of drug seeking in squirrel monkeys: κ opioid and serotonergic mechanisms. Psychopharmacology (Berl) 210:169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Goldberg SR. (2002) Second-order schedules of drug self-administration in animals. Psychopharmacology (Berl) 163:327–344 [DOI] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. (1999) Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav 64:327–336 [DOI] [PubMed] [Google Scholar]
- Spealman RD, Madras BK, Bergman J. (1989) Effects of cocaine and related drugs in nonhuman primates. II. Stimulant effects on schedule-controlled behavior. J Pharmacol Exp Ther 251:142–149 [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, et al. (1997) Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386:827–830 [DOI] [PubMed] [Google Scholar]
- Wallman MJ, Gagnon D, Parent M. (2011) Serotonin innervation of human basal ganglia. Eur J Neurosci 33:1519–1532 [DOI] [PubMed] [Google Scholar]
- Walsh SL, Cunningham KA. (1997) Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology (Berl) 130:41–58 [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Sullivan JT, Fromme R, Bigelow GE. (1994) Fluoxetine alters the effects of intravenous cocaine in humans. J Clin Psychopharmacol 14:396–407 [PubMed] [Google Scholar]
- Wise RA. (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494 [DOI] [PubMed] [Google Scholar]