Abstract
Impaired α7 nicotinic acetylcholine receptor (nAChR) function and GABAergic transmission in the hippocampus and elevated brain levels of kynurenic acid (KYNA), an astrocyte-derived metabolite of the kynurenine pathway, are key features of schizophrenia. KYNA acts as a noncompetitive antagonist with respect to agonists at both α7 nAChRs and N-methyl-d-aspartate receptors. Here, we tested the hypothesis that in hippocampal slices tonically active α7 nAChRs control GABAergic transmission to CA1 pyramidal neurons and are sensitive to inhibition by rising levels of KYNA. The α7 nAChR-selective antagonist α-bungarotoxin (α-BGT; 100 nM) and methyllycaconitine (MLA; 10 nM), an antagonist at α7 and other nAChRs, reduced by 51.3 ± 1.3 and 65.2 ± 1.5%, respectively, the frequency of GABAergic postsynaptic currents (PSCs) recorded from CA1 pyramidal neurons. MLA had no effect on miniature GABAergic PSCs. Thus, GABAergic synaptic activity in CA1 pyramidal neurons is maintained, in part, by tonically active α7 nAChRs located on the preterminal region of axons and/or the somatodendritic region of interneurons that synapse onto the neurons under study. l-Kynurenine (20 or 200 μM) or KYNA (20–200 μM) suppressed concentration-dependently the frequency of GABAergic PSCs; the inhibitory effect of 20 μM l-kynurenine had an onset time of approximately 35 min and could not be detected in the presence of 100 nM α-BGT. These results suggest that KYNA levels generated from 20 μM kynurenine inhibit tonically active α7 nAChR-dependent GABAergic transmission to the pyramidal neurons. Disruption of nAChR-dependent GABAergic transmission by mildly elevated levels of KYNA can be an important determinant of the cognitive deficits presented by patients with schizophrenia.
Introduction
Hippocampal dysfunction and a specific defect in hippocampal interneurons are consistent findings in the brains of patients that suffer from schizophrenia (Tamminga et al., 2010; Konradi et al., 2011), a disease that afflicts approximately 1% of the population worldwide. The contribution of hippocampal dysfunctions to the complex phenotype of the disease is underscored by numerous pharmacological studies. For instance, microinfusion of the GABAA receptor antagonist picrotoxin in the ventral hippocampus of rats impairs prepulse inhibition of the startle reflex response, a measure of sensorimotor gating that is deficient in patients with schizophrenia (Bast et al., 2001). Intraventricular administration of the α7 nicotinic acetylcholine receptor (nAChR) antagonist α-bungarotoxin (α-BGT) to rats also impairs sensory gating as measured by the disruption of the attenuation of the amplitude of the second auditory-evoked potential in a pair of stimuli in the CA3 region of the hippocampus (Luntz-Leybman et al., 1992). Finally, systemic treatment of rats with NMDA receptor antagonists recapitulates major clinical features of schizophrenia that are ameliorated by treatment with nicotine and α7 nAChR-selective agonists (see references in Timofeeva and Levin, 2011). Among these features are deficits of working memory and decreased levels of glutamic acid decarboxylase 67, one of the enzyme isoforms that catalyzes the synthesis of GABA, and parvalbumin, a Ca2+-binding protein expressed by interneurons, primarily in the stratum pyramidale.
Evidence also exists that a number of metabolic pathways are affected in the brains of patients with schizophrenia. The kynurenine pathway of the tryptophan metabolism is one of these pathways. Cerebral cortical levels of kynurenic acid (KYNA), an astrocyte-derived kynurenine metabolite, have been found to be higher in patients with schizophrenia than in age-matched control subjects (Schwarcz et al., 2001). KYNA is a neuroactive metabolite that interacts with a multitude of molecular targets in the brain. At concentrations ranging from tens to hundreds micromolar, KYNA acts as a competitive antagonist of glycine at NMDA receptors. The IC50 values for KYNA to inhibit NMDA receptors are approximately 15 μM in the absence of glycine and 230 μM in the presence of 10 μM glycine (Hilmas et al., 2001). With an IC50 of approximately 7 μM, KYNA blocks noncompetitively α7 nAChRs (Hilmas et al., 2001; Lopes et al., 2007). Finally, with EC50 values of 7 and 39 μM, KYNA activates the rat and the human orphan G-protein receptor 35, respectively (Wang et al., 2006).
Studies of mice with a null mutation in the gene that encodes kynurenine aminotransferase II (KAT II), an enzyme responsible for more than 70% of the astrocytic synthesis of KYNA from kynurenine, have revealed the role of the metabolite in modulating hippocampal α7 nAChR activity (Alkondon et al., 2004). Decreased hippocampal KYNA levels in the mKat-2(−/−) mice resulted in increased α7 nAChR activity in CA1 stratum radiatum interneurons and increased GABAergic transmission onto CA1 pyramidal neurons (Alkondon et al., 2004). Additional support for the ability of KYNA to regulate the activity of multiple neurotransmitter systems was obtained from recent studies in which KYNA was synthesized de novo in hippocampal slices, while synaptic transmission, action potentials, and nAChR activity were recorded from interneurons. Increasing de novo production of KYNA decreased agonist-induced activation of α7 nAChRs and inhibited tonically active NMDA receptors in stratum radiatum interneurons, in addition to reducing the rate of firing of these neurons (Alkondon et al., 2011a,b).
Tracing the consequences of increased production of KYNA on α7 nAChR activity and GABAergic synaptic transmission to CA1 pyramidal neurons, the major output of the hippocampus, is a significant step toward understanding the pathophysiology of schizophrenia. Therefore, the present study was designed to test the hypothesis that, under resting conditions, in a fully functional neurocircuitry, activation of α7 nAChRs by basal levels of choline and/or acetylcholine in hippocampal slices contributes to maintain GABAergic synaptic activity in CA1 pyramidal neurons and is reduced by increasing de novo synthesis of KYNA. To test this hypothesis, GABAergic postsynaptic currents (PSCs) were recorded from the somata of CA1 pyramidal neurons in slices that were subjected to incubation and/or superfusion with nAChR subtype-selective antagonists and different concentrations of kynurenine, the precursor of KYNA.
Materials and Methods
Hippocampal Slices.
Male Sprague-Dawley rats at ages ranging between 30 and 35 days were used in this study. Animal care and handling were done strictly in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee of the University of Maryland. Rats were euthanized by CO2 narcosis followed by decapitation. Their brains were removed in ice-cold artificial cerebrospinal fluid (ACSF), which was composed of 125 mM NaCl, 25 mM NaHCO3, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, 1 mM MgCl2, and 25 mM dextrose. The ACSF was bubbled with 95% O2 and 5% CO2. The hippocampi were dissected out and mounted on the stage of a Vibratome (Leica VT1000S; Leica Microsystems Inc., Bannockburn, IL), which was used to cut transversal hippocampal slices of 300- to 350-μm thickness. Slices were stored at room temperature for at least 45 min in an immersion chamber containing ACSF continuously bubbled with 95% O2 and 5% CO2 before recordings. Some of the slices were transferred to a chamber containing ACSF with test compounds that was continuously bubbled with 95% O2 and 5% CO2. Slices were incubated with the test compounds for 2 to 5 h at room temperature, except l-kynurenine-incubated slices were maintained at 30°C to maximize the activity of KAT II.
Electrophysiological Recordings.
PSCs were recorded from the soma of CA1 pyramidal neurons at a holding potential of 0 mV according to the standard whole-cell mode of the patch-clamp technique by using an LM-EPC7 amplifier (List Electronics, Darmstadt, Germany). In the recording chamber, hippocampal slices were superfused with ACSF at 2 ml/min. All recordings were obtained in the presence of the muscarinic receptor antagonist atropine (0.5 μM), a concentration that does not affect α7 nAChR currents and does not modify KYNA-induced α7 nAChR inhibition (Lopes et al., 2007; Alkondon et al., 2011b). Test compounds were applied to the slices via bath perfusion. Signals were filtered at 3 kHz, digitized at 10 kHz through Digidata 1322A (Molecular Devices, Sunnyvale, CA), and recorded by using the Clampex module of pCLAMP9 software (Molecular Devices). The frequency of PSCs in control condition remained nearly the same even at a sampling rate of 20 kHz, so we sampled at the rate of 10 kHz throughout for ease of handling the data.
Patch pipettes were pulled from borosilicate glass capillaries (1.2 mm o.d.; World Precision Instruments, Inc., Sarasota, FL) with a P-97 Flaming-Brown puller (Sutter Instrument Company, Novato, CA). When filled with internal solution the patch pipettes had resistances between 4 and 6 MΩ. The leak current was generally between 50 and 150 pA, and when it exceeded 200 pA, the data were not included in the analysis. The access resistance was monitored during the course of the experiments and ranged between 15 and 20 MΩ. Data from any neuron were not considered for analysis when the access resistance increased more than 20% from the initial values. The internal pipette solution contained 0.5% biocytin in addition to 10 mM ethylene-glycol bis(β-amino-ethyl ether)-N-N′-tetraacetic acid, 10 mM HEPES, 130 mM Cs-methane sulfonate, 10 mM CsCl, 2 mM MgCl2, and 5 mM N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium bromide (QX-314), pH adjusted to 7.3 with CsOH. All recordings were done at room temperature (20–22°C). Only a single neuron was studied per slice. Therefore, the number of neurons represents the number of hippocampal slices analyzed. Biocytin staining was developed, and the morphology of biocytin-filled neurons identified them as pyramidal neurons.
Data Analysis and Statistics.
PSCs were analyzed in 5-min recordings by using the Clampfit module of pCLAMP 9.0 software (Molecular Devices). Frequency, peak amplitude, rise time (10–90%), and decay-time constant (τd) of synaptic events were measured. The threshold amplitude for detecting PSCs was set at 10 pA, and the PSCs detected by the software were visually inspected to minimize errors. Events that did not show a typical synaptic waveform were rejected manually. For kinetic analysis, only single events with a sharp rising phase and an exponential decay were chosen during visual inspection of the recordings. Double- and multiple-peak currents were excluded for determination of PSC properties but included for the calculation of frequency of PSCs. Rise times and τd were determined during the analysis of the averaged chosen single events aligned at half rise time. Data are expressed as mean ± S.E.M. of results obtained from various animals, and statistical significance was analyzed by using one-way ANOVA or t test in Sigmaplot 11.0 (Systat Software, Inc., San Jose, CA). Furthermore, the cumulative distributions of events in control versus treatment groups were compared by using the Kolmogorov-Smirnov (K-S) test. For this, events from different neurons in each group were pooled together and then subjected to the K-S test by using the Clampfit module of pCLAMP 9.0 software.
Drugs.
(−)Bicuculline methochloride, atropine sulfate, l-kynurenine sulfate (kynurenine), KYNA, QX-314, 2-amino-5-phosphonovaleric acid (APV), and tetrodotoxin (TTX) were purchased from Sigma (St. Louis, MO). 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX) was purchased from Sigma/RBI (Natick, MA). α-BGT was purchased from Biotoxins Inc. (St. Cloud, FL). Methyllycaconitine (MLA) was a gift from Professor M. H. Benn (University of Calgary, Calgary, Alberta, Canada). Kynurenine-containing ACSF was always prepared on the day of the experiments. A stock solution of 500 mM KYNA was made in 1 M NaOH and subsequently serially diluted in regular ACSF as needed. The pH of KYNA-containing and all other ACSF solutions, measured within 2 to 3 min after bubbling with 95% O2/ 5% CO2, was approximately 7.4. Stock solutions of all other compounds were made in distilled water, kept frozen, and subsequently diluted in ACSF.
Results
Spontaneous GABAergic Synaptic Activity in CA1 Pyramidal Neurons.
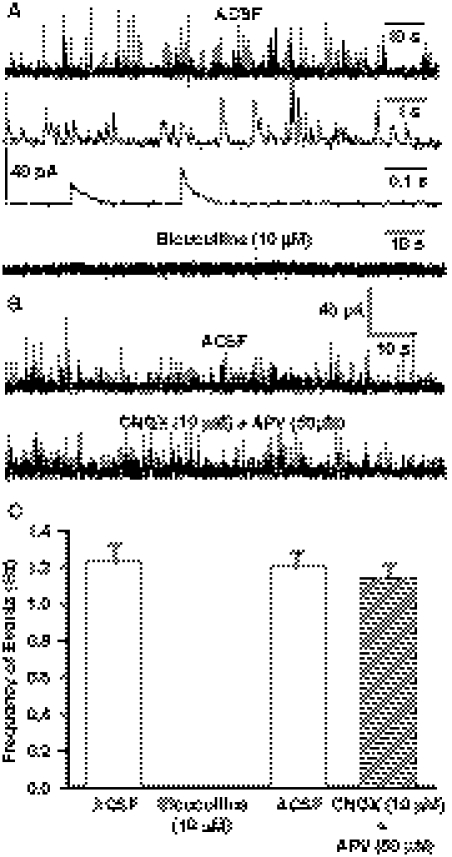
Spontaneous synaptic currents recorded from CA1 pyramidal neurons at 0 mV appeared as outward events (see traces in Fig. 1, A and B). Under control conditions, the frequency of these events ranged from 1.05 to 1.27 Hz (mean ± S.E.M. = 1.18 ± 0.08; n = 94 neurons from 94 slices from 63 rats). These spontaneously occurring synaptic currents were completely blocked after 15-min superfusion of the hippocampal slices with ACSF containing 10 μM bicuculline (Fig. 1, A and C), indicating that they were mediated via GABAA receptors. The slow decay-time constant of the outward currents (approximately 35 ms; see Table 1) was consistent with the notion that they were GABAergic in nature. To examine the extent to which glutamatergic excitation of GABAergic interneurons that synapse onto the CA1 pyramidal neurons regulate the spontaneous GABAergic synaptic activity recorded from the latter, hippocampal slices were superfused with ACSF containing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and NMDA receptor antagonists CNQX (10 μM) and APV (50 μM). The frequency of GABAergic PSCs recorded from the CA1 pyramidal neurons in the presence of both CNQX and APV was comparable with that recorded under control conditions (Fig. 1, B and C). These results suggest that the spontaneous GABAergic synaptic activity recorded from the pyramidal neurons is not regulated by the basal glutamatergic activity in the slices.
Fig. 1.
Spontaneous GABAergic PSCs from CA1 pyramidal neurons. A, sample recordings of spontaneous GABAergic PSCs at 0 mV obtained from CA1 pyramidal neurons of 30-day-old rats under the control condition (top trace). The second and third traces show spontaneous PSCs at an expanded time scale under the control condition. Bottom trace shows recordings at 0 mV 15 min after superfusion of the slice with ACSF containing the GABAA receptor antagonist bicuculline (10 μM). B, representative recordings of PSCs from another neuron at 0 mV before (top trace) and during superfusion of slices with ACSF containing glutamate receptor antagonists APV (50 μM) and CNQX (10 μM) (bottom trace). Neurons had been superfused with the glutamate receptor antagonists for 15 min before beginning analysis. C, quantification of the effects of bicuculline and APV + CNQX on the spontaneous PSCs recorded from CA1 pyramidal neurons at 0 mV. Graph and error bars represent mean and S.E.M., respectively, of data obtained from five neurons from four rats in bicuculline and six neurons from three rats in CNQX + APV.
TABLE 1.
Characteristics of GABAergic PSCs recorded from CA1 pyramidal neurons in hippocampal slices in the continuous presence of 10 nM MLA
Recordings were obtained either during 15-min superfusion of the slices with 10 nM MLA or during superfusion that followed 2- to 5-h incubation with MLA. Data are presented as mean ± S.E.M. of results obtained from 10 neurons from five rats in ACSF, eight neurons from five rats in the MLA incubation, and six neurons from six rats in the MLA bath application.
| Treatment | Amplitude | Rise Time 10 to 90% | τd |
|---|---|---|---|
| pA | ms | ||
| ACSF | 25.2 ± 0.73 | 2.34 ± 0.19 | 36.1 ± 1.72 |
| 10 nM MLA bath exposure | 20.4 ± 0.44 | 1.67 ± 0.19 | 42.1 ± 3.51 |
| 10 nM MLA incubation | 19.9 ± 0.92 | 1.78 ± 0.18 | 40.7 ± 2.63 |
Suppression of Spontaneous GABAergic Synaptic Activity in CA1 Pyramidal Neurons Exposed to α7 nAChR Antagonists.
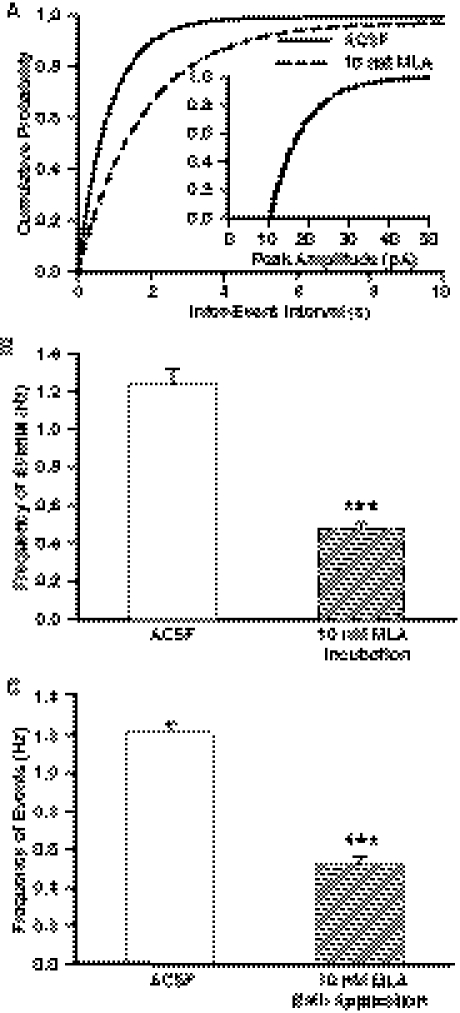
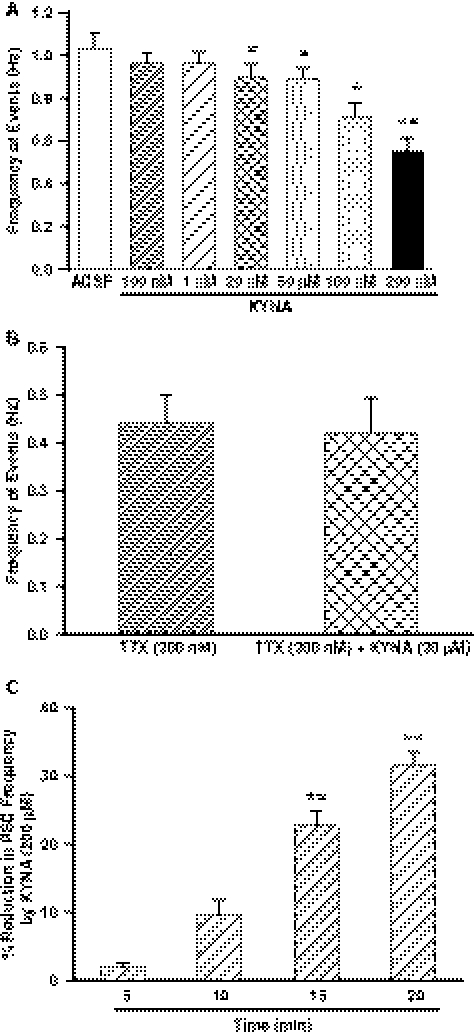
To determine whether GABAergic transmission to CA1 pyramidal neurons is regulated by tonically active α7 nAChRs in interneurons, hippocampal slices were incubated for 2 h in ACSF containing the α7 nAChR antagonist MLA (10 nM) and subsequently continuously superfused in MLA-containing ACSF. The concentration of MLA used in this study was sufficient to produce complete block of α7 nAChR currents (Alkondon et al., 2009). The cumulative plot of interevent intervals recorded in the presence of MLA was displaced toward longer intervals compared with control (Fig. 2A). In addition, the mean frequency of events recorded in the presence of MLA was significantly lower than that recorded under control conditions (Fig. 2B). In contrast, MLA had no significant effect on the τd, rise time, mean peak amplitude, or cumulative distribution of the peak amplitude of GABAergic PSCs (Table 1).
Fig. 2.
Effect of MLA on frequency of GABAergic PSCs. A, cumulative probability plots of interevent intervals and peak amplitude (inset) of PSCs recorded from control and MLA-incubated slices. Plots represent data from five neurons from four rats for control and eight neurons from four rats for MLA incubation. MLA caused a significant rightward displacement of the cumulative distribution of interevent intervals (p < 0.01 according to K-S test). B, mean frequency of GABAergic PSCs recorded 1) under control conditions and 2) in the continuous presence of MLA after 2-h incubation with MLA. Graph and error bars represent mean and S.E.M., respectively, of data obtained from 10 neurons from five rats in control and eight neurons from five rats in MLA. ***, p < 0.001 compared with control according to unpaired t test. C, mean frequency of GABAergic PSCs recorded under control condition followed by 15-min superfusion of the slices with MLA. Graph and error bars represent mean and S.E.M., respectively, of data obtained from seven neurons from seven rats. ***, p < 0.001 compared with control according to paired t test.
In another set of experiments, spontaneous GABAergic PSCs were recorded from pyramidal neurons before and during their superfusion with MLA (10 nM)-containing ACSF. Under this experimental condition, MLA significantly reduced the mean frequency of PSCs (Fig. 2C). The magnitude of the effect was nearly the same regardless of whether the slices were incubated for 2 h or superfused for 15 min with MLA-containing ACSF (Fig. 2, B and C).
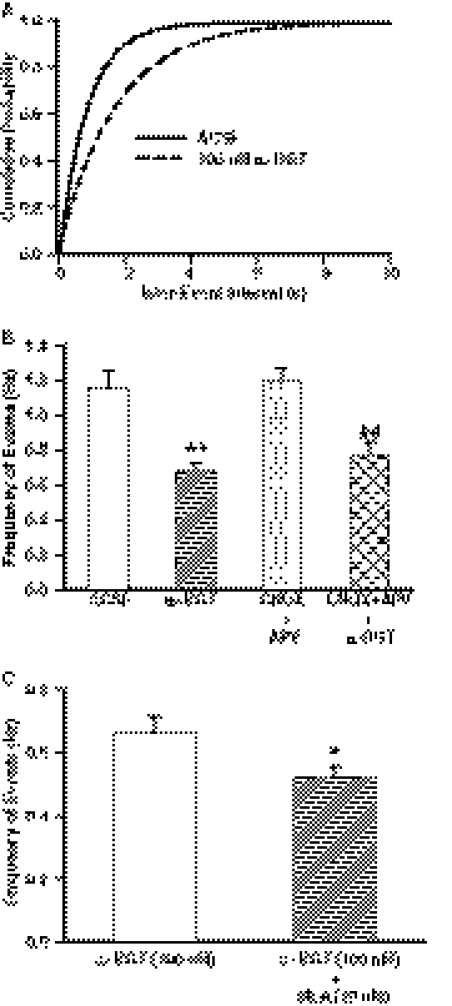
The frequency of GABAergic PSCs was also significantly lower in slices that had been incubated for 1 h in ACSF containing a saturating concentration of the α7 nAChR antagonist α-BGT (100 nM) than in control slices. α-BGT caused a displacement of the cumulative distribution of interevent intervals toward longer intervals (Fig. 3A) and reduced the mean frequency of PSCs (Fig. 3B). α-BGT also decreased the frequency of PSCs in the presence of the glutamate receptor blockers CNQX (10 μM) and APV (50 μM) (Fig. 3B). The magnitude of the effect of combined bath application of MLA (10 nM) and α-BGT (100 nM) in slices that had been preincubated with α-BGT was significantly larger than that of α-BGT alone (Fig. 3C).
Fig. 3.
Effect of α-BGT on frequency of GABAergic PSCs. A, cumulative probability of interevent intervals of PSCs recorded under control conditions and after 1-h incubation in ACSF containing 100 nM α-BGT. Plots represent data from four neurons from four rats in control and eight neurons from four rats in α-BGT. The cumulative distribution of interevent intervals obtained in the presence of α-BGT was significantly displaced to the right in comparison with control (p < 0.01 according to K-S test). B, quantification of the effects of α-BGT, CNQX + APV, and CNQX + APV + α-BGT. Results obtained from control slices were compared with those obtained from slices after 1-h incubation with CNQX + APV or 1-h incubation with CNQX + APV + α-BGT. Graph and error bars represent mean and S.E.M., respectively, of data obtained from five neurons from five rats in the control condition, eight neurons from five rats in α-BGT incubation, five neurons from three rats in CNQX + APV incubation, and six neurons from three rats in CNQX + APV + α-BGT. **, p < 0.01 compared with control according to unpaired t test. ##, p < 0.01 compared with CNQX + APV by unpaired t test. C, frequency of PSCs recorded in the continuous presence of α-BGT (100 nM) was compared with that recorded in the continuous presence of α-BGT (100 nM) + MLA (10 nM). In these experiments, all slices were incubated for 1 h in α-BGT (100 nM)-containing ACSF and subsequently superfused with ACSF containing only α-BGT (100 nM) or both α-BGT (100 nM) and MLA (10 nM). Graph and error bars represent mean and S.E.M., respectively, of data obtained from seven neurons from four rats in α-BGT and seven neurons from four rats in α-BGT + MLA. *, p < 0.01 according to paired t test.
Suppression of GABAergic Synaptic Activity in CA1 Pyramidal Neurons Exposed to the Na+-Channel Blocker Tetrodotoxin.
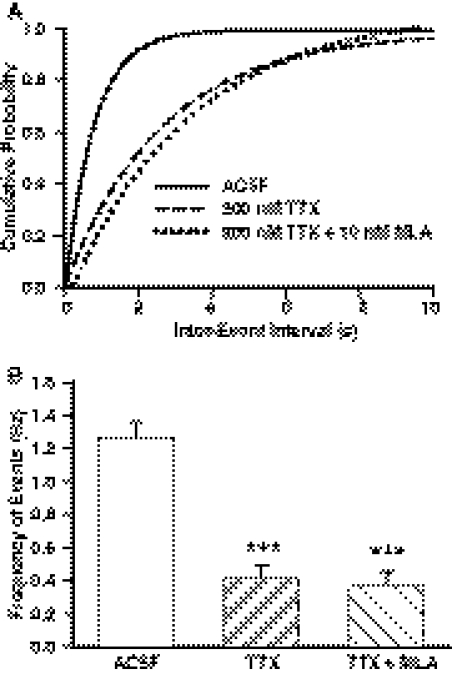
To isolate action potential-independent from action potential-dependent GABAergic PSCs, hippocampal slices were first incubated for 1 h in TTX (200 nM)-containing ACSF and subsequently superfused with the same solution. The cumulative distribution of interevent intervals recorded in the presence of TTX was displaced toward longer intervals in comparison with control (Fig. 4A). TTX also reduced the mean frequency and the mean amplitude of GABAergic events by 68.3 ± 2.0 and 49.4 ± 1.3%, respectively (Fig. 4B; Table 2). Neither the rise time nor τd of the GABAergic PSCs were affected by TTX (Table 2). These results are consistent with TTX-induced block of the action potential-dependent GABAergic transmission; only miniature PSCs (mPSCs) remained in the presence of TTX.
Fig. 4.
Effect of TTX on the frequency of GABAergic PSCs. A, cumulative distribution of interevent intervals of PSCs recorded under control conditions, in the continuous presence of 200 nM TTX, or in the continuous presence of 200 nM TTX + 10 nM MLA. In comparison with control, the cumulative distribution of interevent intervals obtained in the presence of TTX or TTX + MLA was displaced to the right (p < 0.001 according to K-S test). B, mean frequency of PSCs recorded under the same experimental conditions as in A. Graph and error bars represent mean and S.E.M., respectively, of data obtained from five neurons from five rats in control, 10 neurons from six rats in TTX, and six neurons from four rats in TTX + MLA. ***, p < 0.001 compared with control according to one-way ANOVA followed by Dunnett post hoc test.
TABLE 2.
Characteristics of GABAergic PSCs recorded from CA1 pyramidal neurons in hippocampal slices incubated with TTX (200 nM) alone or together with MLA (10 nM)
Data are presented as mean ± S.E.M. of results obtained from five neurons from five rats in control, 10 neurons from six rats in TTX, and six neurons from four rats in TTX plus MLA.
| Treatment | Amplitude | Rise Time 10 to 90% | τd |
|---|---|---|---|
| pA | ms | ||
| ACSF | 26.5 ± 0.69 | 2.19 ± 0.18 | 34.8 ± 1.59 |
| 200 nM TTX | 13.4 ± 0.52** | 1.87 ± 0.15 | 38.6 ± 2.98 |
| 200 nM TTX plus 10 nM MLA | 14.1 ± 0.84** | 1.81 ± 0.24 | 39.9 ± 3.04 |
P < 0.01 compared with control according to one-way ANOVA followed by Dunnett post hoc test.
In the continuous presence of TTX, MLA affected neither the cumulative distribution of interevent intervals (Fig. 4A) nor the mean frequency of mPSCs (Fig. 4B) recorded from CA1 pyramidal neurons. MLA was also devoid of any effect on the mean amplitude, rise time, and τd of mPSCs (Table 2).
GABAergic Synaptic Activity Recorded from CA1 Pyramidal Neurons Decreased in the Presence of Increasing Concentrations of l-Kynurenine.
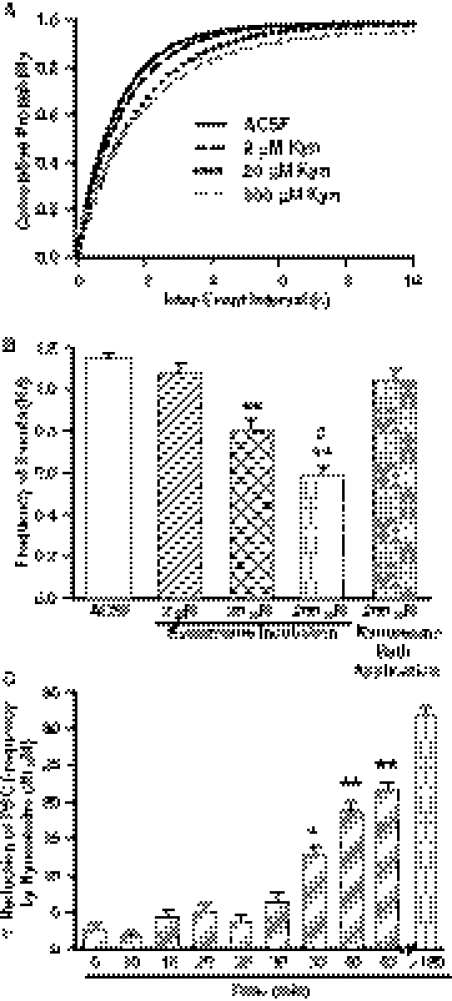
Studies from our laboratory and others have shown that incubation of rat hippocampal slices with kynurenine increases the production of KYNA in situ (Scharfman et al., 1999; Alkondon et al., 2011b). To examine the effects of newly synthesized KYNA on GABAergic transmission in CA1 pyramidal neurons, hippocampal slices were first incubated for 2 to 5 h in ACSF containing 2, 20, or 200 μM kynurenine. At the end of the incubation time, slices were transferred to the recording chamber, where they were continuously superfused with kynurenine-containing ACSF while GABAergic PSCs were recorded from CA1 pyramidal neurons. Control slices were incubated and maintained in kynreunine-free ACSF.
Kynurenine caused a concentration-dependent reduction of the mean frequency of GABAergic PSCs (Fig. 5, A and B). At 20 and 200 μM kynurenine also caused a significant displacement of the cumulative distribution of interevent intervals toward longer intervals (Fig. 5A). The mean peak amplitude, rise time, and τd of PSCs were not affected by the test concentrations of kynurenine (Table 3).
Fig. 5.
Concentration-dependent effect of kynurenine (Kyn) on the frequency of spontaneous PSCs. A, cumulative probability plot of interevent intervals of PSCs recorded under control conditions or in the continuous presence of kynurenine (2–200 μM) after 2- to 5-h incubation in ACSF containing the corresponding concentration of kynurenine. The plots were obtained from data in 11 neurons from 11 rats in control, seven neurons from four rats in 2 μM kynurenine, 10 neurons from six rats in 20 μM kynurenine, and six neurons from four rats in 200 μM kynurenine. Cumulative distributions of interevent intervals recorded from neurons in the presence of 20 and 200 μM kynurenine were significantly displaced to the right in comparison with control (p < 0.05 and 0.01, respectively, according to K-S test) compared with control. B, mean frequency of PSCs recorded 1) under control conditions, 2) in the continuous presence of 2, 20, or 200 μM kynurenine after 2- to 5-h incubation with the corresponding concentration of kynurenine, or 3) during 15-min perfusion with 200 μM kynurenine. Compared with control, kynurenine reduced significantly the mean frequency of PSCs: *, p < 0.05; **, p < 0.01 according to one-way ANOVA followed by Dunnett post hoc test. The magnitude of the effect of 200 μM kynurenine was larger than that of 20 μM kynurenine (#, p < 0.05 according to one-way ANOVA followed by Tukey post hoc test). Graph and error bars represent mean and S.E.M., respectively, of data obtained from same number of neurons and rats as in A. Data from five neurons from three rats were used in the 200 μM kynurenine bath application. C, graph shows time-dependent percentage reduction in PSC frequency by continuous superfusion of ACSF containing kynurenine (20 μM). The maximum inhibition obtained during an incubation protocol (data from B) is provided in the last column for comparison. The onset time for the effect kynurenine was ∼35 min. *, p < 0.05; **, p < 0.01 according to one-way ANOVA followed by Dunnett post hoc test.
TABLE 3.
Characteristics of GABAergic PSCs recorded from CA1 pyramidal neurons in hippocampal slices incubated with kynurenine (200 μM) alone or together with 10 nM MLA
Data are presented as mean ± S.E.M. of results obtained from eight neurons from eight rats in ACSF, six neurons from four rats in 200 μM l-kynurenine, and eight neurons from four rats in 200 μM l-kynurenine plus MLA.
| Treatment | Amplitude | Rise Time 10 to 90% | τd |
|---|---|---|---|
| pA | ms | ||
| ACSF | 24.9 ± 0.83 | 2.25 ± 0.21 | 39.3 ± 2.14 |
| 200 μM Kynurenine | 19.9 ± 0.96 | 2.07 ± 0.12 | 43.5 ± 2.25 |
| 200 μM Kynurenine plus 10 nM MLA | 15.3 ± 0.44* | 1.73 ± 0.18 | 52.4 ± 2.98 |
| 200 μM KYNA | 20.2 ± 0.89 | 2.02 ± 0.24 | 40.8 ± 2.61 |
P < 0.05 compared with control according to one-way ANOVA followed by Dunnett post hoc test.
In the absence of preincubation, 15-min superfusion of hippocampal slices with ACSF containing kynurenine (200 μM) had no significant effect on the frequency of GABAergic PSCs (Fig. 5B). The slow onset of the action of kynurenine on GABAergic transmission is in agreement with the concept that this action is the result of the metabolic conversion of kynurenine into a neuroactive metabolite. The onset of inhibition was further assessed in experiments in which recordings were obtained from neurons before and during their superfusion with ACSF containing 20 μM kynurenine. As illustrated in Fig. 5C, a significant reduction in the frequency of GABAergic PSCs was detected after 35 min of bath application of kynurenine. The percentage of inhibition increased from approximately 13% at 35 min to nearly 22% at 45 min, the maximum recording time within which there was no noticeable change in access resistance. The effect of 20 μM kynurenine had not fully developed by 45 min, because the frequency of GABAergic PSCs recorded from CA1 pyramidal neurons in slices incubated for 2 to 5 h with 20 μM kynurenine was approximately 32% lower than that recorded under control conditions (Fig. 5, B and C). Furthermore, in the kynurenine (20 μM)-incubated slices, the frequency of GABAergic PSCs was 0.76 ± 0.02 Hz (n = 5 neurons) at 2 to 3 h and 0.74 ± 0.02 Hz at 3 to 5 h (n = 5 neurons), suggesting that maximum inhibition was achieved by 2- to 3-h incubation with the drug.
Exogenously Applied KYNA Reduced GABAergic Synaptic Activity in CA1 Pyramidal Neurons.
To analyze the effects of exogenously applied KYNA on GABAergic transmission to CA1 pyramidal neurons, hippocampal slices were first incubated for 2 to 5 h in ACSF containing a test concentration of KYNA (100 nM-200 μM) and subsequently superfused with KYNA-containing ACSF. The mean frequency of GABA PSCs recorded in the continuous presence of ≥20 μM KYNA was lower than that recorded under control conditions (Fig. 6A). The magnitude of the effect increased with increasing concentrations of KYNA (Fig. 6A) and was comparable with that of equimolar concentrations of kynurenine, particularly at the highest concentrations. In the presence of 200 nM TTX, incubation of the slices with KYNA (20 μM) had no significant effect on the frequency of mIPSCs (Fig. 6B).
Fig. 6.
Concentration-dependent effect of exogenously applied KYNA on the frequency of spontaneous PSCs. A, mean frequency of PSCs recorded under control condition or in the continuous presence of KYNA (0.1–200 μM) after 2- to 5-h incubation in ACSF containing the corresponding concentration of KYNA. Graph and error bars represent mean and S.E.M., respectively, of data obtained from 17 neurons from 17 rats in control, four neurons from three rats in 100 nM KYNA, six neurons from three rats in 1 μM KYNA, eight neurons from five rats in 20 μM KYNA, six neurons from four rats in 50 μM KYNA, five neurons from three rats in 100 μM KYNA, and six neurons from four rats in 200 μM KYNA. *, p < 0.05; **, p < 0.01 compared with control according to one-way ANOVA followed by Dunnett post hoc test. B, mean frequency of PSCs recorded in the continuous presence of TTX (200 nM) or in the continuous presence of TTX (200 nM) plus KYNA (20 μM). Graph and error bars represent mean and S.E.M., respectively, of data obtained from 12 neurons from eight rats in TTX and five neurons from two rats in TTX + KYNA. C, graph showing time-dependent percentage reduction in PSC frequency by continuous superfusion of ACSF containing KYNA (200 μM). In this set of experiments, ACSF contained 10 μM CNQX + 50 μM APV during and 10 min before application of KYNA. The onset time for the effect KYNA was approximately 15 min. **, p < 0.01 according to one-way ANOVA followed by Dunnett post hoc test.
To determine the onset time for KYNA to suppress GABAergic PSCs, the frequency of events recorded from neurons before and during superfusion with ACSF containing 200 μM KYNA was analyzed. In this set of experiments, slices were first exposed to ACSF containing CNQX (10 μM) and APV (50 μM) followed by the addition of KYNA (200 μM). As shown in Fig. 6C, the frequency of GABAergic PSCs was significantly reduced at 15 min of bath application of KYNA, and the magnitude of the effect increased further with time.
Effects of the Admixture of Kynurenine and the α7 nAChR Antagonist α-BGT on GABAergic Synaptic Activity in CA1 Pyramidal Neurons.
To determine whether α7 nAChRs contribute to the effects of kynurenine on GABAergic transmission, hippocampal slices were first incubated for 1 h in ACSF containing a saturating concentration of the α7 nAChR antagonist α-BGT (100 nM) and for an additional 2 to 5 h in ACSF containing both α-BGT (100 nM) and kynurenine (20 or 200 μM).
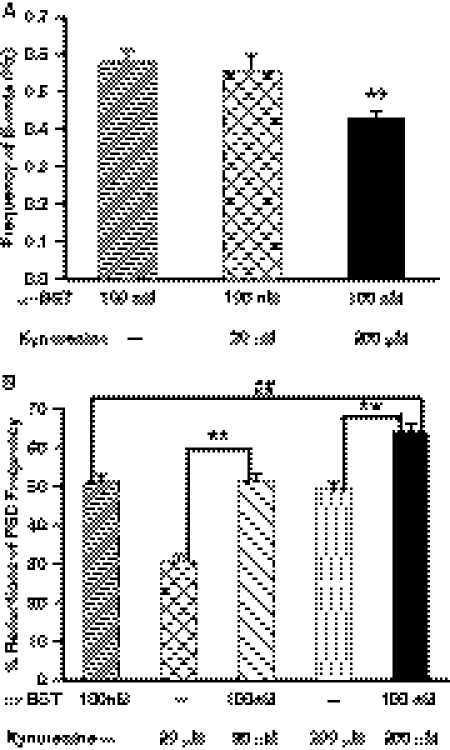
The mean frequency of GABAergic PSCs recorded in the presence of α-BGT alone was not statistically different from that recorded in the presence of α-BGT plus 20 μM kynurenine (Fig. 7A). α-BGT (100 nM) alone decreased the frequency of PSCs by 51.3 ± 1.3% (Fig. 7B). After incubation of the slices with kynurenine (20 μM) alone, the mean frequency of events was 30.7 ± 1.5% lower than that recorded under control conditions (Fig. 7B). The magnitude of the effect of the admixture of α-BGT (100 nM) and kynurenine (20 μM) on the frequency of GABAergic PSCs was comparable with that of α-BGT alone (Fig. 7, A and B), indicating that there is no additive inhibitory effect for the combined treatment.
Fig. 7.
Effect of the admixture of kynurenine and α-BGT on the frequency of PSCs. A, mean frequency of PSCs recorded 1) after 1-h incubation with α-BGT (100 nM), 2) after 1-h incubation with ACSF containing α-BGT (100 nM) followed by 2- to 5-h incubation with α-BGT (100 nM) plus kynurenine (20 μM), or 3) after 1-h incubation with ACSF containing α-BGT (100 nM) followed by 2- to 5-h incubation with α-BGT (100 nM) + kynurenine (200 μM). In each experimental group, the ACSF used to superfuse the slices was the same as that used during the incubation time. Graph and error bars represent mean and S.E.M., respectively, of data obtained from 10 neurons from six rats in α-BGT, six neurons from four rats in α-BGT + 20 μM kynurenine, and six neurons from four rats in α-BGT + 200 μM kynurenine. The effect of admixture of α-BGT (100 nM) + kynurenine (200 μM) is significantly higher than that of α-BGT alone (**, p < 0.01 according to one-way ANOVA followed by Tukey post hoc test). B, graph shows the percentage reduction of PSC frequency in the continuous presence of α-BGT (100 nM), kynurenine (20 μM), α-BGT (100 nM) + kynurenine (20 μM), kynurenine (200 μM), or α-BGT (100 nM) + kynurenine (200 μM). The magnitude of effect of admixture of α-BGT (100 nM) + kynurenine (20 μM) was significantly larger than kynurenine (20 μM) alone and not different from that of α-BGT (100 nM). The effect of α-BGT (100 nM) + kynurenine (200 μM) was significantly larger than that of kynurenine (200 μM) or α-BGT (100 nM) alone. ** and ##, p < 0.01 according to one-way ANOVA followed by Tukey post hoc test.
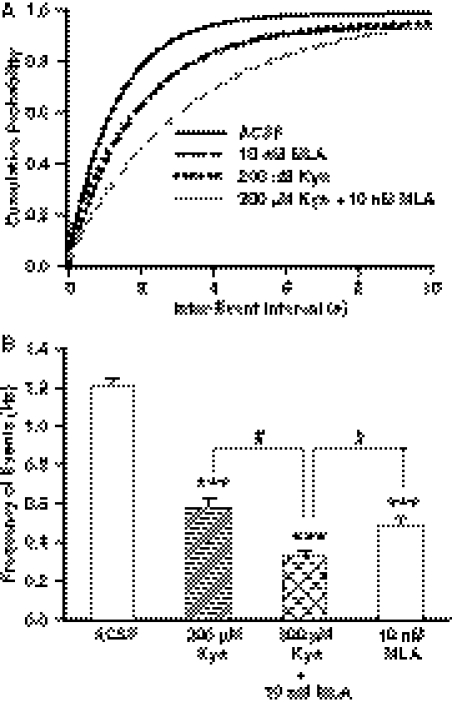
After 2- to 5-h incubation in ACSF containing 200 μM kynurenine, the frequency of GABAergic PSCs was 49.2 ± 1.9% lower than that recorded under control conditions (Fig. 7B). The effect of the admixture of 100 nM α-BGT and 200 μM kynurenine on the mean PSC frequency was larger than that of either chemical alone (Fig. 7). Likewise, the magnitude of the effect of the admixture of 10 nM MLA plus 200 μM kynurenine on the frequency of GABAergic PSCs was larger than that seen with either 10 nM MLA or 200 μM kynurenine (Fig. 8). Both rightward displacement of the cumulative plot of interevent intervals and reduction of the mean frequency of PSCs were more pronounced in the presence of both 10 nM MLA and 200 μM kynurenine than in the presence of either chemical alone (Fig. 8). In addition, the admixture of 10 nM MLA and 200 μM kynurenine caused a significant reduction of the amplitude of PSCs (Table 3).
Fig. 8.
Combined effect of kynurenine and MLA on the frequency of PSCs. A, cumulative probability plots of interevent intervals of PSCs recorded under the control condition and after 2- to 5-h incubation in ACSF containing different agents. In each experimental group, the ACSF used to superfuse the slices was the same as that used during the incubation time. Compared with control, cumulative distributions of interevent intervals were displaced to the right by 10 nM MLA (p < 0.001, according to K-S test), 200 μM kynurenine (p < 0.01, according to K-S test), and 200 μM kynurenine + 10 nM MLA (p < 0.001, according to K-S test). Cumulative probability plots of data were obtained from 19 neurons from 19 rats in control, eight neurons from four rats in MLA, six neurons from four rats in 200 μM kynurenine, and eight neurons from four rats in 200 μM kynurenine + 10 nM MLA. B, mean frequency of PSCs recorded under the same experimental conditions as in A. Graph and error bars represent mean and S.E.M., respectively, of data obtained from the same number of neurons as in A. In the presence of 200 μM kynurenine, 200 μM kynurenine + 10 nM MLA and 10 nM MLA, the mean frequency of PSCs was significantly lower than that of control (***, p < 0.001 according to one-way ANOVA followed by Dunnet post hoc test). Mean frequency of PSCs recorded in the presence of 200 μM kynurenine + 10 nM MLA was significantly lower than that recorded in the presence of kynurenine (200 μM) or MLA (10 nM) alone (#, p < 0.05 according to one-way ANOVA followed by Tukey post hoc test).
Discussion
The results presented in the present study demonstrate that 1) under resting conditions, α7 nAChRs located on interneurons are active in hippocampal slices and contribute to maintaining GABAergic synaptic input to CA1 pyramidal neurons, and 2) incubation of hippocampal slices with low micromolar concentrations of kynurenine, the precursor of KYNA, leads to the de novo synthesis of levels of KYNA that are sufficient to inhibit basal activation of α7 nAChRs, and, thereby, suppress α7 nAChR-dependent GABAergic synaptic transmission to the pyramidal neurons. The physiological and clinical relevance of these findings is discussed herein.
The Use of nAChR Antagonists Reveals that There Is a Basal Level of α7 nAChR Activity in Hippocampal Slices that Controls GABAergic Synaptic Inputs to CA1 Pyramidal Neurons.
Glutamatergic activity provides major excitatory input to many neuron types. Yet, in the present study, blockade of both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and NMDA receptors in the slices had no significant effect on the frequency of GABAergic PSCs recorded from the pyramidal neurons (see Fig. 1). These results strongly suggest that GABAergic synaptic activity recorded from CA1 pyramidal neurons is controlled primarily by factors other than the prototypical glutamate inputs.
In numerous studies, the use of nicotinic agonists and antagonists demonstrated that hippocampal interneurons express a number of pharmacologically distinct nAChR subtypes, the most prevalent being the α-BGT-sensitive α7 nAChRs (Alkondon et al., 1997; Jones and Yakel, 1997; Frazier et al., 1998; McQuiston and Madison, 1999; Ji and Dani, 2000). Electrical stimulation of specific hippocampal pathways have led to the identification of α7 nAChR-mediated synaptic transmission in small subsets of CA1 stratum radiatum and stratum pyramidale interneurons and in CA1 and CA3 pyramidal neurons (Alkondon et al., 1998; Frazier et al., 1998; Hefft et al., 1999; Stone, 2007; Albuquerque et al., 2009; Grybko et al., 2011). The low probability of finding synaptic transmission mediated by α7 nAChRs in the hippocampus has been accounted for by the predominant nonsynaptic localization of these receptors (Umbriaco et al., 1995; Aznavour et al., 2005). Here, the use of nAChR subtype-selective antagonists led to the identification of hippocampal neurocircuitries in which basal levels of cholinergic transmitter activate α7 nAChRs to control GABAergic synaptic activity in the pyramidal neurons.
Two α7 nAChR antagonists were used in this study: α-BGT and MLA. The finding that α-BGT reduced the frequency without altering the amplitude of GABAergic PSCs recorded from CA1 pyramidal neurons demonstrated that in the hippocampal slices synaptic release of GABA onto the pyramidal neurons is maintained, in part, by α7 nAChRs that are activated by basal levels of acetylcholine and/or choline. MLA also reduced the frequency of PSCs recorded from the pyramidal neurons, although to a greater extent than did α-BGT. The larger effect of MLA compared with that of α-BGT (see Figs. 2 and 3) may be caused by the ability of MLA to block some heteromeric α7 or non-α7 nAChRs. The α7 nAChR currents in hippocampal neurons that are sensitive to both α-BGT and MLA (Albuquerque et al., 2009) arise from single channels with conductance in the range of 73 to 91 pS (Castro and Albuquerque, 1993; Mike et al., 2000). In chick sympathetic neurons, however, Yu and Role (1998) described the existence of both homomeric and heteromeric α7 nAChRs with single-channel conductance of 18 pS being sensitive to α-BGT and 35 pS channels being sensitive to MLA. Because hippocampal interneurons coexpress β2 and α5 subunits in abundance with α7 subunits (Sudweeks and Yakel, 2000; Son and Winzer-Serhan, 2008), it is conceivable that MLA-sensitive/α-BGT-insensitive α7 nAChRs that contain β2 and/or α5 subunits contribute to regulation of the activity of GABAergic synaptic inputs to CA1 pyramidal neurons seen here. Alternatively, some of the MLA effects may arise from blockade of non-α7 heteromers such as α4α6α5(β2)2 or α3α4α5(β2)2 nAChRs, as proposed by Klink et al. (2001) for some GABA neurons in the substantia nigra.
The frequency of GABAergic mPSCs recorded from the CA1 pyramidal neurons in the presence of TTX was not affected by MLA. Therefore, the α7 and non-α7 nAChRs that are sensitive to MLA and modulate GABAergic transmission to CA1 pyramidal neurons are not located on the presynaptic terminals of interneurons. Instead, these receptors are located on the somatodendritic and/or preterminal axon region of interneurons that synapse onto the pyramidal neurons. These receptors may also be located on the somatodendritic, preterminal, or axon regions of glutamatergic neurons that synapse onto interneurons that in turn synapse onto the pyramidal neurons from which recordings are obtained. The contribution of nAChRs present on glutamate neurons is limited because glutamate receptor blockers CNQX and APV had no significant effect on the frequency of spontaneous GABAergic PSCs (see Fig. 1) and did not prevent the inhibitory effect of α-BGT (Fig. 3B). However, α7 nAChRs present on glutamate neurons play an important role in regulating glutamate input activity to CA1 pyramidal neurons, and such activity is suppressed by concentrations of KYNA as low as 1 μM (Banerjee et al., 2012). In situ hybridization studies have demonstrated that the majority of the hippocampal interneurons express mRNA encoding the α7 nAChR, whereas subsets of stratum oriens interneurons express mRNA encoding the α2 nAChR subunit, and subsets of stratum radiatum and stratum lacunosum moleculare interneurons express mRNAs that encode the α5 nAChR subunit (Winzer-Serhan and Leslie, 2005; Son and Winzer-Serhan, 2008). Low levels of mRNA encoding the α3 nAChR subunit have also been detected in the hippocampus, but not in interneurons (Son and Winzer-Serhan, 2008). Functional nAChRs that have the pharmacological properties of α3-containing nAChRs have been found in glutamatergic neurons/axons that synapse onto stratum radiatum interneurons (Alkondon et al., 2004).
Typically, CA1 pyramidal neurons receive GABAergic inputs from multiple interneuron types located in various layers of the CA1 region (Miles et al., 1996). Interneurons located in the stratum radiatum and lacunosum moleculare innervate distal regions of the apical dendrites. As a result, GABAergic inputs from these interneurons are of very low amplitude and slow rise times and are rarely detected in voltage-clamp recordings obtained from the soma of pyramidal neurons (Ouardouz and Lacaille, 1997; Buhler and Dunwiddie, 2002). Two more lines of evidence rule out the contribution of GABAergic PSCs from stratum radiatum interneurons. First, APV, although able to suppress the excitability of stratum radiatum interneurons by 70% (Alkondon et al., 2011a), had no significant effect on the frequency of GABAergic PSCs (see Fig. 1). Second, MLA, which is least effective in suppressing the spontaneous action potential frequency in the stratum radiatum interneurons (Alkondon et al., 2011a), was found to be highly effective in suppressing the frequency of GABAergic PSCs recorded from CA1 pyramidal neurons (see Fig. 2). It is therefore conceivable that most of the GABAergic PSCs recorded from the pyramidal neurons in the present study arose from interneurons in the stratum pyramidale and the stratum oriens. Interneurons in the stratum pyramidale, classified in other studies as basket cells, provide multiple innervation sites around the pyramidal cell soma and axon initial segment (Cope et al., 2002). Because nicotinic synaptic potentials sensitive to MLA and α-BGT have been detected in basket cells (Stone, 2007), it is likely that the activation of α7 nAChRs by basal levels of acetylcholine and/or choline in the slices causes excitation of these basket cells, and, thereby, contributes to most of the nicotinic regulation of GABAergic synaptic activity in the CA1 pyramidal neurons.
KYNA Generated by Low Micromolar Concentrations of Kynurenine Disrupts GABAergic Synaptic Transmission in CA1 Pyramidal Neurons via Inhibition of α7 nAChR Activity: Clinical Relevance.
Astrocyte processes intermingle with neuronal structures, thereby providing an ideal environment for multiple types of neuron–glia interaction (Black and Waxman, 1988; Butt et al., 1994; Theodosis and Poulain, 1999). The KAT II enzyme present primarily in astrocytes is actively involved in the conversion of the substrate kynurenine into KYNA (Guidetti et al., 2007). Previous studies have demonstrated that in vitro kynurenine can be converted into KYNA in hippocampal slices (Scharfman et al., 1999; Alkondon et al., 2011b). In the present study, incubation of hippocampal slices with kynurenine (20 or 200 μM) resulted in a concentration-dependent suppression of the frequency of GABAergic PSCs in the CA1 pyramidal neurons. The slow onset of the effect of kynurenine (see Fig. 5C) suggested that the effect was mediated by kynurenine-derived KYNA.
After inhibition of the slices with α-BGT, 20 μM kynurenine had no inhibitory effect on GABAergic synaptic transmission. This finding supported the contention that suppression of GABAergic transmission by levels of KYNA generated in slices incubated with low micromolar concentrations of kynurenine was the result of KYNA-induced inhibition of α7 nAChRs. On the other hand, the admixture of 200 μM kynurenine with 100 nM α-BGT or 10 nM MLA produced more suppression of GABAergic transmission than each chemical alone. Therefore, levels of KYNA generated by exposure of the slices to high concentrations of kynurenine affect additional targets that contribute to the regulation of GABAergic synaptic activity in the pyramidal neurons. Indeed, it has been shown that concentrations of KYNA generated by exposure of hippocampal slices to 200 μM kynurenine are sufficient to inhibit extrasynaptic NMDA receptors in CA1 interneurons (Alkondon et al., 2011b). Inhibition of these receptors can decrease the excitability of the interneurons, and thereby, suppress GABAergic transmission to the pyramidal neurons. In addition, the potential contribution of metabolites other than KYNA to the effects of high concentrations of kynurenine on GABAergic transmission cannot be ruled out.
The present demonstration that GABAergic transmission to CA1 pyramidal neurons is suppressed as a result of inhibition of α7 nAChRs by mild increases of de novo synthesis of KYNA has significant clinical relevance. Brain tissue levels of KYNA have been found to be higher in patients with schizophrenia as opposed to those detected in age-matched controls (Schwarcz et al., 2001). Disruption of hippocampal GABAergic inhibition, particularly originating from parvalbumin-positive interneurons, i.e., interneurons in the stratum pyramidale of the hippocampus, is a hallmark of the disease and seems to be a major determinant of the cognitive deficits that these patients present (Freedman et al., 2000; Freedman and Goldowitz, 2010; Konradi et al., 2011; Timofeeva and Levin, 2011). Thus, it is tempting to speculate that suppression of the activity of GABAergic synaptic inputs to CA1 pyramidal neurons caused by KYNA-induced inhibition of α7 nAChRs, particularly in parvalbumin-positive interneurons, contributes to the pathophysiology of this catastrophic disorder.
Acknowledgments
We are indebted to Dr. Yasco Aracava for reading the manuscript and providing helpful comments.
We thank Mabel Zelle and Bhagavathy Alkondon for technical assistance.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS25296] (to E.X.A.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- nAChR
- nicotinic acetylcholine receptor
- α-BGT
- α- bungarotoxin
- ACSF
- artificial cerebrospinal fluid
- ANOVA
- analysis of variance
- APV
- 2-amino-5-phosphonovaleric acid
- CNQX
- 6-cyano-7-nitroquinoxaline-2,3-dione
- KAT II
- kynurenine aminotransferase II
- K-S
- Kolmogorov-Smirnov
- KYNA
- kynurenic acid
- MLA
- methyllycaconitine
- NMDA
- N-methyl-d-aspartate
- PSC
- postsynaptic current
- mPSC
- miniature PSC
- TTX
- tetrodotoxin
- QX-314
- N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium bromide
- τd
- decay-time constant.
Authorship Contributions
Participated in research design: Banerjee, Alkondon, Pereira, and Albuquerque.
Conducted experiments: Banerjee.
Performed data analysis: Banerjee and Pereira.
Wrote or contributed to the writing of the manuscript: Banerjee, Alkondon, Pereira, and Albuquerque.
References
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89:73–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Aracava Y, Pereira EF, Albuquerque EX. (2009) A single in vivo application of cholinesterase inhibitors has neuron type-specific effects on nicotinic receptor activity in guinea pig hippocampus. J Pharmacol Exp Ther 328:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. (1998) α-Bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res 810:257–263 [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. (2011a) Endogenous activation of nAChRs and NMDA receptors contributes to the excitability of CA1 stratum radiatum interneurons in rat hippocampal slices: effects of kynurenic acid. Biochem Pharmacol 82:842–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. (1997) Neuronal nicotinic acetylcholine receptor activation modulates γ-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther 283:1396–1411 [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Kajii Y, Schwarcz R, Albuquerque EX. (2011b) Age dependency of inhibition of α7 nicotinic receptors and tonically active N-methyl-d-aspartate receptors by endogenously produced kynurenic acid in the brain. J Pharmacol Exp Ther 337:572–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Yu P, Arruda EZ, Almeida LE, Guidetti P, Fawcett WP, Sapko MT, Randall WR, Schwarcz R, et al. (2004) Targeted deletion of the kynurenine aminotransferase II gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via α7 nicotinic receptors in the hippocampus. J Neurosci 24:4635–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznavour N, Watkins KC, Descarries L. (2005) Postnatal development of the cholinergic innervation in the dorsal hippocampus of rat: quantitative light and electron microscopic immunocytochemical study. J Comp Neurol 486:61–75 [DOI] [PubMed] [Google Scholar]
- Banerjee J, Alkondon M, Albuquerque EX, Pereira EF. (2012) Kynurenic acid blocks tonically active α7 nicotinic receptors and reduces glutamate transmission to CA1 pyramidal neurons. 43rd Annual Meeting of the American Society of Neurochemistry; 2012 Mar 3–7; Baltimore, MD American Society for Neurochemistry, Windermere, FL [Google Scholar]
- Bast T, Zhang WN, Feldon J. (2001) Hyperactivity, decreased startle reactivity, and disrupted prepulse inhibition following disinhibition of the rat ventral hippocampus by the GABAA receptor antagonist picrotoxin. Psychopharmacology 156:225–233 [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG. (1988) The perinodal astrocyte. Glia 1:169–183 [DOI] [PubMed] [Google Scholar]
- Buhler AV, Dunwiddie TV. (2002) α7 Nicotinic acetylcholine receptors on GABAergic interneurons evoke dendritic and somatic inhibition of hippocampal neurons. J Neurophysiol 87:548–557 [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Berry M. (1994) Astrocyte associations with nodes of Ranvier: ultrastructural analysis of HRP-filled astrocytes in the mouse optic nerve. J Neurocytol 23:486–499 [DOI] [PubMed] [Google Scholar]
- Castro NG, Albuquerque EX. (1993) Brief-lifetime, fast-inactivating ion channels account for the α-bungarotoxin-sensitive nicotinic response in hippocampal neurons. Neurosci Lett 164:137–140 [DOI] [PubMed] [Google Scholar]
- Cope DW, Maccaferri G, Márton LF, Roberts JD, Cobden PM, Somogyi P. (2002) Cholecystokinin-immunopositive basket and Schaffer collateral-associated interneurones target different domains of pyramidal cells in the CA1 area of the rat hippocampus. Neuroscience 109:63–80 [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. (1998) Synaptic potentials mediated via α-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci 18:8228–8235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Adams CE, Leonard S. (2000) The α7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanat 20:299–306 [DOI] [PubMed] [Google Scholar]
- Freedman R, Goldowitz D. (2010) Studies on the hippocampal formation: from basic development to clinical applications: studies on schizophrenia. Prog Neurobiol 90:263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grybko MJ, Hahm ET, Perrine W, Parnes JA, Chick WS, Sharma G, Finger TE, Vijayaraghavan S. (2011) A transgenic mouse model reveals fast nicotinic transmission in hippocampal pyramidal neurons. Eur J Neurosci 33:1786–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. (2007) Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia 55:78–92 [DOI] [PubMed] [Google Scholar]
- Hefft S, Hulo S, Bertrand D, Muller D. (1999) Synaptic transmission at nicotinic acetylcholine receptors in rat hippocampal organotypic cultures and slices. J Physiol 515:769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. (2001) The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. J Neurosci 21:7463–7473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Dani JA. (2000) Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol 83:2682–2690 [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel JL. (1997) Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol 504:603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21:1452–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. (2011) Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res 131:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes C, Pereira EF, Wu HQ, Purushottamachar P, Njar V, Schwarcz R, Albuquerque EX. (2007) Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at α7* nicotinic receptors. J Pharmacol Exp Ther 322:48–58 [DOI] [PubMed] [Google Scholar]
- Luntz-Leybman V, Bickford PC, Freedman R. (1992) Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res 587:130–136 [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. (1999) Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci 19:2887–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mike A, Castro NG, Albuquerque EX. (2000) Choline and acetylcholine have similar kinetic properties of activation and desensitization on the α7 nicotinic receptors in rat hippocampal neurons. Brain Res 882:155–168 [DOI] [PubMed] [Google Scholar]
- Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF. (1996) Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16:815–823 [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Lacaille JC. (1997) Properties of unitary IPSCs in hippocampal pyramidal cells originating from different types of interneurons in young rats. J Neurophysiol 77:1939–1949 [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hodgkins PS, Lee SC, Schwarcz R. (1999) Quantitative differences in the effects of de novo produced and exogenous kynurenic acid in rat brain slices. Neurosci Lett 274:111–114 [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. (2001) Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 50:521–530 [DOI] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. (2008) Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs in rat hippocampal GABAergic interneurons. J Comp Neurol 511:286–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW. (2007) Kynurenic acid blocks nicotinic synaptic transmission to hippocampal interneurons in young rats. Eur J Neurosci 25:2656–2665 [DOI] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. (2000) Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal interneurons. J Physiol 527:515–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. (2010) The hippocampal formation in schizophrenia. Am J Psychiatry 167:1178–1193 [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA. (1999) Contribution of astrocytes to activity-dependent structural plasticity in the adult brain. Adv Exp Med Biol 468:175–182 [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Levin ED. (2011) Glutamate and nicotinic receptor interactions in working memory: importance for the cognitive impairment of schizophrenia. Neuroscience 195:21–36 [DOI] [PubMed] [Google Scholar]
- Umbriaco D, Garcia S, Beaulieu C, Descarries L. (1995) Relational features of acetylcholine, noradrenaline, serotonin and GABA axon terminals in the stratum radiatum of adult rat hippocampus (CA1). Hippocampus 5:605–620 [DOI] [PubMed] [Google Scholar]
- Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L. (2006) Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem 281:22021–22028 [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. (2005) Expression of α5 nicotinic acetylcholine receptor subunit mRNA during hippocampal and cortical development. J Comp Neurol 481:19–30 [DOI] [PubMed] [Google Scholar]
- Yu CR, Role LW. (1998) Functional contribution of the α7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurones. J Physiol 509:651–665 [DOI] [PMC free article] [PubMed] [Google Scholar]