The management of hormone receptor–positive, human epidermal growth factor receptor 2–positive, and triple-negative breast cancers is reviewed, emphasizing changes that occurred in recent years and focusing on potential mechanisms of drug resistance. Strategies to prevent or overcome resistance to specific therapeutic agents are also highlighted.
Abstract
The management of breast cancer has changed dramatically over the past 20 years. Based on gene expression profiles, or proteomics of three or four biomarkers, it is apparent that there are multiple subtypes with different clinical characteristics, clinical courses, and sensitivities to existing therapies. This manuscript reviews the management of hormone receptor–positive, human epidermal growth factor receptor 2–positive, and triple-negative breast cancers, emphasizing changes that have occurred in recent years and focusing on potential mechanisms of drug resistance. I also highlight strategies to prevent or overcome resistance to specific therapeutic agents. As a result of enhanced biological understanding of the molecular anomalies that drive the development, progression, and dissemination of breast cancer, a number of novel, molecularly targeted agents have been added to standard therapies. Chemotherapy, endocrine therapy, and targeted treatments have markedly reduced the risk for recurrence and mortality after primary treatment of breast cancer and have increased the 5- and 10-year survival rates. The challenges with novel therapeutics include the absence of accurate predictive biomarkers to identify those patient who will derive substantial benefit and those patients whose tumors are resistant to specific antitumor agents. As we move forward with increasing molecular segmentation of breast cancer and develop new, highly targeted agents, molecular diagnostics must accompany molecular therapeutics to implement the concept of personalized cancer therapy.
Introduction
There is no greater professional honor than the recognition provided by one's peers. I am humbled and deeply moved by receiving the Bob Pinedo Prize. Bob has been a friend and colleague for the past three decades and a guiding light in translational oncology. His contributions to clinical pharmacology and new drug development have been many and he always coupled his passion for good science with single-minded dedication to the welfare of his patients. When I learned of my selection as the 2011 awardee, I turned to Google to try to learn the secret of Bob's success [1]. As an amateur genealogist, I thought I could find his secret in his roots. The Pinedo name can be traced back in history to the Cartulario de Valpuesta, a document written in the year 804 [2]. Pinedo is a town located on the border of the Basque country and Castile, and is a quaint rural village in scenic hill country named after the “pino,” or pine trees common in that area [1]. There are many variations on the name, but a cursory review of source documents finds many illustrious Pinedos (Table 1) [3]. Melchor Pinedo was listed in the Voyagers to America in 1527; Diego de Pinedo, from Burgos (Spain), arrived in Mexico in 1533 and settled in Nueva Galicia. Alonso Alvarez de Pinedo was a Spanish explorer and cartographer of the 16th century and Gonzalo Diaz de Pinedo was a well-known Spanish conquistador. Thomas de Pinedo was a 17th century Portuguese scholar educated by the Jesuits in Spain; persecuted by the Inquisition, he fled to Holland. Other Pinedos appear as Jesuit missionaries, theater company managers, a Governor of Paraguay, politicians, diplomats, and historians. There were also painters, sculptors, actors, singers, and dancers. A line of Pinedos from Amsterdam settled in Curaçao in the early 1700s, and Abraham Pinedo was one of the most powerful businessmen in Curaçao during the 18th century. From Curaçao, some of the Pinedos migrated to Venezuela, Colombia, Panama, Mexico, and the U.S. As summarized above, Bob Pinedo had a lot of talent in his ancestry, and the convergence of all these skills coalesced in him, the gentleman, scholar, scientist, and compassionate physician who is an example to us all.
Table 1.
Pinedo: An internet search
Experienced physicians have long known about the variability of disease in general and cancer in particular (Fig. 1). Some patients with metastatic breast cancer develop rapidly progressive, fulminant metastatic disease that causes death in just a few months despite all available therapy, whereas others exhibit an indolent course, characterized by periods of slow progression alternating with extended periods of stability or response to therapy, resulting in extended survival, sometimes measured in decades [4]. Similarly, patients with small, early breast cancer can have very different clinical courses despite receiving identical treatments. For much of the past century, treatment for breast cancer was guided by the Halsted radical mastectomy [5]. Pioneering observations by several thought leaders in the second half of the 20th century indicated this variability in outcomes and led to systematic assessment of prognostic indicators, some based on the characteristics of the host, some on the histological features of her breast cancer. The seminal observation that some breast cancers retained the hormonal sensitivity of normal breast tissue whereas others did not initiated the era of personalized therapy [6]. The objectives of personalized or individualized therapy for breast cancer are to deliver the treatments with the highest probability of producing antitumor effects with the lowest risk for toxicity or side effects to individual patients, based on the molecular characteristics of the tumor and the genetic profile of the host.
Figure 1.

Comment from Sir William Osler on individual variability.
Assessment of the Risk for Recurrence or Death
An initial step in the assessment of each patient with newly diagnosed breast cancer is to assess the potential risk for developing distant metastases or local treatment failure. Initially, such an assessment was based entirely on the histopathological characteristics of the primary tumor, such as tumor size, grade, histological type, lymphovascular invasion, and the presence and quantity of regional lymph node involvement [7]. Over the past 40 years, these factors were combined in several multifactorial prognostic indices, both for metastatic and for primary breast cancers [8, 9]. In the latter case, the most commonly used indices were the Nottingham Prognostic Index followed, some 15 years later, by Adjuvant! Online [10, 11]. These indices predicted outcome for groups of patients with great success and were independently validated [12]. However, their ability to predict individual prognosis was less impressive. Technological progress led to high-throughput techniques, including gene and protein expression profiling. Some of these, MammaPrint® (Agendia, Inc., Amsterdam, The Netherlands) and Oncotype DX® (Genomic Health, Inc., Santa Clara, CA), were successful prototypes and were able to refine individual prediction of prognosis and, as early reports suggest, responsiveness to specific systemic treatments [13, 14].
Summary of Results From Adjuvant Systemic Therapy
While these changes in prognostic and predictive factors were taking place, much work was being done in the development of combined modality therapies. Thus, combinations of surgical excision and radiotherapy were shown to be superior to surgery alone, resulting in better local and regional control. The addition of (adjuvant) chemotherapy reduced the annual odds for recurrence by ∼50%–60%, with somewhat more modest reductions in the odds for death [15, 16]. Optimal endocrine therapy reduced the annual odds for recurrence by ∼60%–70%, again with a somewhat more modest reduction in the odds for mortality. As a result of multiple randomized trials and the collaborative work of hundreds of clinical investigators, the standard of care for treating primary breast cancer changed dramatically over the past 40 years, with marked reductions in the extent and aggressiveness of primary surgical treatment, including management of the axilla, and the rational integration of postoperative radiotherapy, chemotherapy, and endocrine therapy into the equation [17]. Survival outcomes of patients with breast cancer improved dramatically over this time and the population-based mortality figures dropped by more than one third [18–20]. These improvements made us question whether or not all patients with breast cancer benefited equally from current treatments.
Novel Prognostic Indices
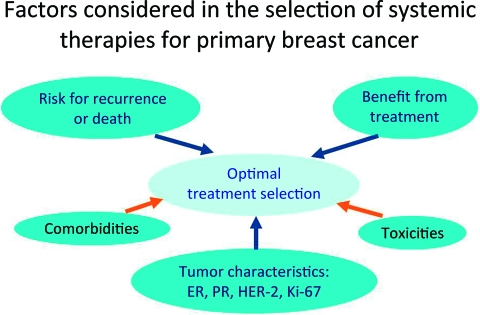
The effort dedicated to the development of prognostic factors and the technological progress emerging from the completion of the Human Genome Project resulted in rapid conceptual change and the redefinition of breast cancer classification systems from one based on anatomy and histology to more recent models based on gene and protein expression and a greater understanding of the underlying molecular biology. The seminal observations of Perou et al. [21] indicated that breast cancer was a conglomerate of multiple molecularly defined subtypes that differed not only in their molecular characteristics but also in their clinical course and responsiveness to available systemic therapies. Several novel classifications have been proposed, and at this time it is uncertain how many subtypes of breast cancer exist, or even how many are clinically relevant. What is unquestioned, however, is that the earlier paradigm in which the risk for recurrence or metastasis dictated the selection of systemic treatment has been replaced by one in which risk determines the potential need for additional therapy, whereas responsiveness to specific agents or modalities helps select the optimal systemic therapy (Fig. 2). Selection of the optimal treatment is a complex undertaking. Figure 3 summarizes the factors taken into consideration when selecting the optimal systemic therapy for an individual patient with breast cancer. Although gene and protein expression profiles are not widely available in the community, tumor classifications based on a few basic breast cancer biomarkers can identify a few, clinically relevant tumor subtypes. Estrogen receptor (ER)and progesterone receptor (PR) expression, human epidermal growth factor receptor (HER)-2 expression and HER-2 amplification, and the proliferative characteristics of the tumor (Ki-67) are the most important biomarkers in breast cancer today, and their systematic assessment and accurate quantification are essential in deciding how to treat every individual patient with breast cancer. Molecular subtypes defined by gene-expression profiling do not correspond exactly to the clinical subtypes defined by ER, PR, HER-2, and Ki-67 testing. However, subtyping based on clinically available assays (immunohistochemistry, fluorescence in situ hybridization) is the most practical approach to selecting therapy today. Based on these biomarkers, a simple clinical classification has evolved and serves as the basis for selecting the optimal therapy in the clinic (Fig. 4). Let's review briefly how such subtypes are managed today.
Figure 2.

Conceptual evolution in selecting adjuvant systemic therapy.
Figure 3.
Factors considered in the selection of systemic therapies for primary breast cancer.
Abbreviations: ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Figure 4.
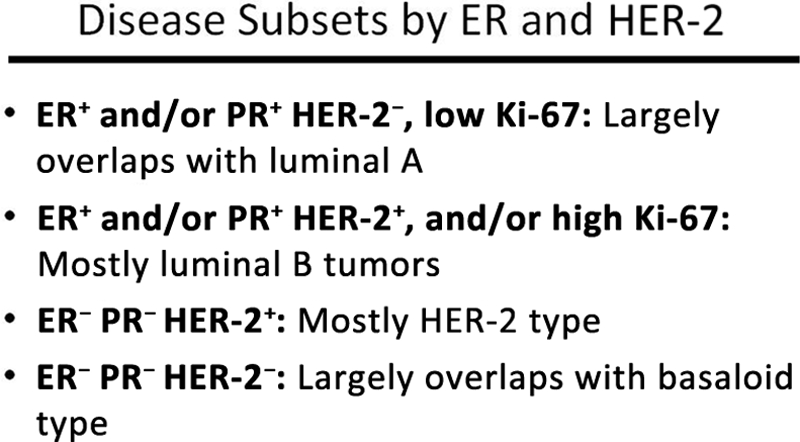
Breast cancer subtypes based on hormone receptor and HER-2 expression levels and Ki-67 staining.
Abbreviations: ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Management of Hormone Receptor–Positive HER-2− Primary Breast Cancer
Patients with ER+ and/or PR+ HER-2− breast cancer with low Ki-67 expression levels have, in general, exquisite sensitivity to endocrine therapy and benefit from antiestrogen treatment more than from chemotherapy. If premenopausal, the treatment of choice is either tamoxifen or ovarian suppression/ablation [22]. If postmenopausal, an aromatase inhibitor is the initial treatment of choice, with several other therapeutic options to be used in sequence in the metastatic setting. Although there are many outstanding questions about the optimal endocrine therapy, many of them will be answered by ongoing clinical trials. In the metastatic setting, patients with this subtype of breast cancer benefit from multiple sequentially applied single endocrine interventions and derive many months, sometimes years, of high-quality survival time based on these treatments [15].
Patients with hormone receptor–positive breast cancer with a high proliferative index and/or HER-2 amplification are somewhat less likely to benefit from endocrine treatment and their disease has a more aggressive course [21]. Randomized trials have shown that patients with ER+ tumors benefit from the addition of chemotherapy to endocrine therapy, especially when the two treatment options are given sequentially, not concurrently [16]. What is less well defined is how to determine which of these patients will benefit the most and which will benefit the least or not at all from the addition of chemotherapy [23]. Several retrospective analyses have suggested that patients with the most hormone-responsive tumors are the least likely to benefit from chemotherapy, and vice versa [24, 25]. These observations were strengthened by recent molecular assays. As mentioned earlier, the MammaPrint® assay identifies low- and high-risk tumors based on the expression of 70 genes [13]. This assay adds to the information provided by clinical and pathological factors, and the combination of all seems to be superior to each component [26]. Similarly, the Oncotype DX® assay, based on reverse transcription-polymerase chain reaction of 21 genes, provides reproducible prognostic information, the recurrence score (RS), and also seems to predict benefit from systemic therapies [24, 25]. Based on multiple retrospective analyses of patients treated in clinical trials, patients with low and intermediate risk Oncotype DX® RSs benefit from tamoxifen, whereas those with high risk RSs do not [25]. Conversely, those with high risk RSs benefit significantly from adjuvant chemotherapy, whereas those with low and intermediate risk RSs seem not to [24]. The results of these retrospective analyses are currently being validated prospectively in three large multicenter, multinational trials: Trial Assigning IndividuaLized Options for Treatment (Rx), Microarray In Node negative Disease may Avoid ChemoTherapy, and Rx for Positive Node, Endocrine Responsive Breast Cancer [27–29]. The outcomes of those trials will define the accuracy of the molecular predictors and will inform future decisions regarding the indication for chemotherapy in patients with ER+ early breast cancer.
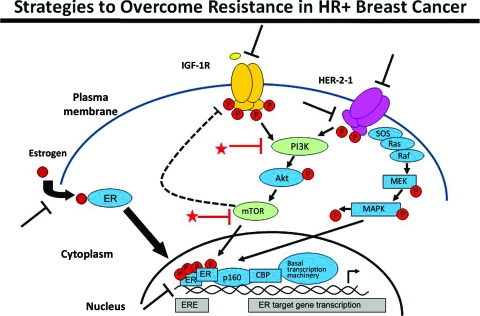
Although endocrine therapies are effective, only ∼30% of patients with metastatic ER+ breast cancer have an objective response, and at most 50% gain clinical benefit from endocrine treatment [15]. Large clinical trials have established that only ∼50% of patients with ER+ primary breast cancer benefit from adjuvant endocrine therapy. The rest have inherent endocrine resistance, and many who initially benefit from endocrine therapy later develop secondary resistance. Thus, there is much interest in identifying and understanding all potential mechanisms of endocrine resistance [30]. Figure 5 describes some of the commonly considered mechanisms of endocrine resistance [31]. Activation of cell surface growth factor receptors—epidermal growth factor receptor (EGFR), HER-2, and insulin-like growth factor 1 receptor (IGF-1R) are a few examples—has been shown to activate the ER signaling pathway in the absence of its natural ligand. Furthermore, downstream elements of the receptor tyrosine kinases—phosphatidylinositol 3-kinase (PI3K), Akt, mammalian target of rapamycin (mTOR)—can be activated by a number of additional intracellular and extracellular mechanisms. Thus, dysregulation of any of these pathways can contribute to ligand-independent activation of the hormone receptor pathways, rendering these resistant to common therapeutic interventions, such as antiestrogens or other methods of estrogen deprivation. These challenges can also be considered therapeutic opportunities: with greater understanding of these mechanisms of resistance, we can now start to introduce selective inhibitors of these alternate signaling pathways in association with endocrine therapy. In this manner, combinations of endocrine treatments with EGFR inhibitors and HER-2 inhibitors were shown to lead to a longer progression-free survival interval in four separate, although somewhat underpowered, trials (Table 2) [32–35].
Figure 5.
Strategies to overcome resistance in hormone receptor–positive breast cancer.
Abbreviations: CBP, CREB-binding protein; ER, estrogen receptor; ERE, estrogen response element; HER-2, human epidermal growth factor receptor 2; IGF-1R, insulin-like growth factor 1 receptor; MAPK, mitogen-activated protein kinase; MEK, MAPK/extracellular signal–related kinase kinase; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase; PR, progesterone receptor; SOS, son of sevenless.
Nat Rev Clin Oncol 2010;7:139–147, with permission from Nature Publishing Group.
Table 2.
Clinical evidence for cotargeting growth factor receptors in ER+ MBC
aSlightly longer in exploratory analysis of centrally confirmed ER status, 3.8 months versus 5.6 months.
bStratum 1 of trial defined as never receiving tamoxifen or completed adjuvant tamoxifen >1 year prior.
Abbreviations: AI, aromatase inhibitor; CI, confidence interval; ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; HR, hazard ratio; ITT, intent to treat; PFS, progression-free survival; Rx, treatment; TAnDEM, TrAstuzumab in Dual HER2 ER-Positive Metastatic breast cancer.
Our investigations have determined that the PI3K pathway is one of the most frequently altered pathways in breast cancer [36]. About 20% of primary breast cancers carry PI3K mutations, and a significant additional fraction exhibits other alterations in this pathway. Mutations in the gene encoding phosphatase and tensin homologue deleted on chromosome ten (PTEN) are much less frequent, but PTEN function loss by other mechanisms is more prevalent. These molecular abnormalities lead to constitutive activation of signaling or to an imbalance between activating and suppressive pathways. The observations suggest that inhibiting PI3K pathway signaling should partially reverse endocrine resistance. The most compelling evidence in favor of this hypothesis originates from the everolimus in combination with exemestane in the treatment of postmenopausal women with estrogen receptor positive locally advance or metastatic Breast cancer who are RefractOrv to LEtrozole or anastROzole (BOLERO)-2 trial [37]. In that study, postmenopausal patients whose metastatic breast cancer was progressing on a nonsteroidal aromatase inhibitor were offered therapy with exemestane and were randomly assigned to either oral everolimus (an mTOR inhibitor) or placebo. A two-to-one randomization, after appropriate stratification, was applied to 705 patients. At the first interim analysis, there was a highly statistically significant longer progression-free survival time in the combination group than in the placebo group: the hazard ratio was 0.43 (p < .0001).
Management of Hormone Receptor–Negative HER-2+ Breast Cancer
Patients with this subtype of breast cancer had the worst outcomes prior to the development of HER-2–targeted agents [38]. Since the introduction of trastuzumab, their prognosis has improved to the extent that this group of patients has one of the more favorable prognoses of all breast cancer patients [39]. The identification of HER-2 amplification and HER-2 overexpression as an adverse prognostic factor almost 25 years ago was a watershed event in breast oncology and corresponds to the real initiation of molecularly targeted therapies [38]. HER-2 amplification is found in 15%–20% of breast cancers [40]. The presence of this molecular abnormality is associated with greater proliferation, angiogenesis, invasion, and metastasis [38]. The monoclonal antibody trastuzumab binds to the extracellular domain of the HER-2 oncoprotein and has multiple molecular effects, resulting in a favorable therapeutic outcome. In the adjuvant setting, and in combination with standard adjuvant chemotherapy, trastuzumab results in a 50% lower odds for recurrence and death and a markedly higher survival rate [41]. The accepted standard is to administer trastuzumab for 1 year, although provocative preliminary data suggest that durations as short as 9 weeks might be similarly effective [42]. There are five ongoing randomized trials exploring different durations of trastuzumab therapy in the adjuvant setting.
Although trastuzumab works well in up to 50% of patients with HER-2+ breast cancer, it seems ineffective in the rest. Various potential mechanisms of resistance to trastuzumab have been proposed: mucin overgrowth of the extracellular domain that “hides” the relevant epitope from the antibody, activation of complementary signaling pathways (EGFR, HER-3, IGF-1R, etc.), constitutive activation of the PI3K–Akt–mTOR pathway or loss of PTEN expression or function, reduced p27kip1, etc. [43]. Understanding these mechanisms of resistance is paramount to designing appropriate and effective treatment regimens for patients that host resistant tumors. Recent clinical trial results have provided exciting information about dual inhibition of the HER-2 signaling pathway. Thus, the combination of trastuzumab and the small molecule tyrosine kinase inhibitor (TKI) lapatinib (a dual inhibitor of EGFR and HER-2) is clearly more effective than either lapatinib or trastuzumab alone [44]. Similarly, the combination of two anti–HER-2 antibodies (trastuzumab and pertuzumab) is clearly more effective than either antibody alone [45]. Finally, the combination of trastuzumab and an mTOR-inhibitor (everolimus) seems to be significantly effective in patients progressing on trastuzumab-containing regimens [46]. Other novel agents under development in this field include the TKI neratinib, the antibody–drug conjugate trastuzumab emtansine, also referred to as T-DM1, and downstream signaling inhibitors, such as PI3K or mTOR inhibitors and heat shock protein-90 inhibitors, among others. It is uncertain at this point which combination or sequence of these various agents will provide the greatest therapeutic benefit, although it is clear that optimal outcomes will require the use of two or more HER-2–directed agents.
For patients with hormone receptor–positive HER-2+ breast cancer, the need to add an antiestrogen to the HER-2–directed therapy increases the complexity of developing more effective therapies.
Another important molecular subset of breast cancers is loosely denominated triple -negative breast cancer (TNBC). This group is defined by the absence of ER, PR, and normal HER-2 expression. It largely, but not completely, overlaps with the “basal-like” breast cancer subtype identified by gene-expression profiling. TNBC is heterogeneous in its clinical course, and certainly in its molecular characteristics. TNBCs are highly proliferative and have p53 mutations in ∼50% of cases, and most BRCA1-mutated tumors fall into this category. As a group, patients with TNBC have a shorter time to progression and overall survival time in the metastatic setting and shorter disease-free survival interval after primary breast cancer [47]. From neoadjuvant chemotherapy trials, we know that some patients with TNBC are totally resistant to standard regimens whereas others seem to have exquisitely sensitive tumors. Pathological complete responses (pCRs) are quite common in this group, and for patients who achieve a pCR the long-term prognosis is excellent [48, 49]. However, for those who do not achieve a pCR, the long-term survival outcome is poor. Because patients with TNBC only have chemotherapy as a standard therapy available to them, there is much interest and emphasis in identifying novel therapeutic targets. Expanding knowledge about the molecular biology of TNBC has pointed to EGFR, mitogen-activated protein kinase, Notch, Met, poly(ADP-ribose) polymerase (PARP), and DNA repair pathways as logical targets to explore, validate, and develop inhibitors. These potential targets are unlikely to be present on all TNBCs and are more likely “driver” molecular anomalies in discrete subsets. It is, therefore, critically important to also develop relevant and reproducible biomarkers to identify the subpopulation most likely to benefit from each targeted strategy. Patients with asymptomatic metastatic TNBC can be appropriately managed with sequential single-agent chemotherapy. Frequently, these patients present with rapidly progressive, symptomatic disease, sometimes visceral crisis. In such cases, combination chemotherapy and chemotherapy with bevacizumab are appropriate initial interventions to maximize the probability of an early response and control symptoms or organ dysfunction. Patients with primary TNBC benefit from taxane–anthracycline–alkylator combinations. Although there is much interest in platinum compounds, clinical evidence of their superiority, or indeed even equivalence, to standard agents is lacking. Because there is a clear unmet need, there is much emphasis in the identification of novel drugs for TNBC treatment. Of all the agents under evaluation, the PARP inhibitors seem to have more solid evidence of activity, although, based on recent controlled trials, such activity has only been clearly shown in BRCA mutation carriers [50]. Ongoing research has shown the enormous molecular complexity of breast cancer. Results of gene sequencing demonstrate the large number of seemingly random mutations in individual tumors, although some patterns indicating dysfunction of specific signaling pathways, if not uniformity in a specific molecular anomaly, are starting to appear. It is apparent that monotherapy is unlikely to be of substantial clinical benefit for the great majority of patients; the challenge is to determine the driving mutation(s) of each tumor and to develop rational combinations that will produce the greatest therapeutic benefit with limited toxicity. The observation that specific dominant molecular lesions might be present in 2%–5% of breast cancers (excluding HER-2 and hormonal receptors) requires a paradigm change in drug discovery and emphasizes the critical importance of molecular diagnostics, heretofore an underemphasized area of investigation. We need to screen for multiple molecular abnormalities (or at least for all those for which we have validated therapeutic agents) in order to offer maximum therapeutic benefits and minimize the costs and delays involved in identifying relevant targets for individual patients.
Conclusion
In summary, breast cancer is a conglomerate of multiple, molecularly defined syndromes with different natural histories and sensitivities to therapeutics. ER, PR, HER-2, and Ki-67 represent critical molecular markers that identify the largest molecular subsets, and therapeutic decisions without such information are unwise and probably suboptimal. It is clear that molecular diagnostics is an indispensable companion to molecular therapeutics and that we will need novel, perhaps unconventional, clinical trial designs in order to rapidly advance the field toward the goal of personalized cancer therapies.
Acknowledgment
Presented on September 7, 2011 as the Bob Pinedo Cancer Care Prize Keynote Lecture during the 2nd Annual Meeting of the Society of Translational Oncology, Belfast, Northern Ireland.
For a link to the lecture: individualizedbreastcancertherapy.theoncologist.com.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
References
- 1.HouseofNames.com. Pinedo Family Crest and Name History. [accessed September 28, 2011]. Available at http://www.houseofnames.com/pinedo-family-crest.
- 2.Wikipedia. Cartularios de Valpuesta. [accessed September 28, 2011]. Available at http://en.wikipedia.org/wiki/Cartularios_de_Valpuesta.
- 3. [accessed September 28, 2011]. Available at http://www.google.com/search?q=famous+Pinedos&rls=com.microsoft:*&ie=UTF-8&oe=UTF-8&startIndex=&startPage=1.
- 4.Swenerton KD, Legha SS, Smith T, et al. Prognostic factors in metastatic breast cancer treated with combination chemotherapy. Cancer Res. 1979;39:1552–1562. [PubMed] [Google Scholar]
- 5.Halsted WS. I. The Results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg. 1894;20:497–555. doi: 10.1097/00000658-189407000-00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment, with illustrative cases. Lancet. 1896;ii:162–165. [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher ER, Fisher B. Relationship of pathologic and some clinical discriminants to the spread of breast cancer. Int J Radiat Oncol Phys. 1977;2:747–750. doi: 10.1016/0360-3016(77)90058-x. [DOI] [PubMed] [Google Scholar]
- 8.Hortobagyi GN, Smith TL, Legha SS, et al. Multivariate analysis of prognostic factors in metastatic breast cancer. J Clin Oncol. 1983;1:776–786. doi: 10.1200/JCO.1983.1.12.776. [DOI] [PubMed] [Google Scholar]
- 9.Fisher ER, Anderson S, Redmond C, et al. Pathologic findings from the National Surgical Adjuvant Breast Project protocol B-06. 10-year pathologic and clinical prognostic discriminants. Cancer. 1993;71:2507–2514. doi: 10.1002/1097-0142(19930415)71:8<2507::aid-cncr2820710813>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Haybittle JL, Blamey RW, Elston CW, et al. A prognostic index in primary breast cancer. Br J Cancer. 1982;45:361–366. doi: 10.1038/bjc.1982.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravdin PM, Clark GM, Hilsenbeck SG, et al. A demonstration that breast cancer recurrence can be predicted by neural network analysis. Breast Cancer Res Treat. 1992;21:47–53. doi: 10.1007/BF01811963. [DOI] [PubMed] [Google Scholar]
- 12.Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 13.van't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 14.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 15.Hortobagyi GN. Treatment of breast cancer. N Engl J Med. 1998;339:974–984. doi: 10.1056/NEJM199810013391407. [DOI] [PubMed] [Google Scholar]
- 16.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 17.Clarke M, Collins R, Darby S, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 18.Ross MB, Buzdar AU, Smith TL, et al. Improved survival of patients with metastatic breast cancer receiving combination chemotherapy. Cancer. 1985;55:341–346. doi: 10.1002/1097-0142(19850115)55:2<341::aid-cncr2820550206>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Giordano SH, Buzdar AU, Smith TL, et al. Is breast cancer survival improving? Cancer. 2004;100:44–52. doi: 10.1002/cncr.11859. [DOI] [PubMed] [Google Scholar]
- 20.Peto R, Boreham J, Clarke M, et al. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- 21.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 22.Cigler T, Goss PE. Breast cancer adjuvant endocrine therapy. Cancer J. 2007;13:148–155. doi: 10.1097/PPO.0b013e318074d363. [DOI] [PubMed] [Google Scholar]
- 23.Tao Y, Klause A, Vickers A, et al. Clinical and biomarker endpoint analysis in neoadjuvant endocrine therapy trials. J Steroid Biochem Mol Biol. 2005;95:91–95. doi: 10.1016/j.jsbmb.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Tang G, Cuzick J, Costantino JP, et al. Risk of recurrence and chemotherapy benefit for patients with node-negative, estrogen receptor-positive breast cancer: Recurrence score alone and integrated with pathologic and clinical factors. J Clin Oncol. 2011;29:4365–4372. doi: 10.1200/JCO.2011.35.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang G, Shak S, Paik S, et al. Comparison of the prognostic and predictive utilities of the 21-gene recurrence score assay and Adjuvant! for women with node-negative, ER-positive breast cancer: Results from NSABP B-14 and NSABP B-20. Breast Cancer Res Treat. 2011;127:133–142. doi: 10.1007/s10549-010-1331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buyse M, Loi S, van't Veer L, et al. TRANSBIG Consortium. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98:1183–1192. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]
- 27.Sparano JA. TAILORx: Trial assigning individualized options for treatment (Rx) Clin Breast Cancer. 2006;7:347–350. doi: 10.3816/CBC.2006.n.051. [DOI] [PubMed] [Google Scholar]
- 28.Rutgers E, Piccart-Gebhart MJ, Bogaerts J, et al. The EORTC 10041/BIG 03–04 MINDACT trial is feasible: Results of the pilot phase. Eur J Cancer. 2011;47:2742–2749. doi: 10.1016/j.ejca.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Angulo AM, Barlow WE, Gralow JR, et al. A randomized phase III clinical trial of standard adjuvant endocrine therapy ± chemotherapy in patients (pts) with 1–3 positive nodes, hormone receptor (HR)-positive and HER2-negative breast cancer with recurrence score (RS) of 25 or less: SWOG S1007 [abstract OT1–03-01] Cancer Res. 2011;71(24 suppl):607s. [Google Scholar]
- 30.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Cosimo S, Baselga J. Management of breast cancer with targeted agents: Importance of heterogeneity. Nat Rev Clin Oncol. 2010;7:139–147. doi: 10.1038/nrclinonc.2009.234. [DOI] [PubMed] [Google Scholar]
- 32.Osborne CK, Neven P, Dirix LY, et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: A randomized phase II study. Clin Cancer Res. 2011;17:1147–1159. doi: 10.1158/1078-0432.CCR-10-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 34.Cristofanilli M, Valero V, Mangalik A, et al. Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 2010;16:1904–1914. doi: 10.1158/1078-0432.CCR-09-2282. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: Results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther. 2011;10:1093–1101. doi: 10.1158/1535-7163.MCT-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2011;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 39.Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. The Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 41.Yin W, Jiang Y, Shen Z, et al. Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: A meta-analysis of published randomized controlled trials. PLoS One. 2011;6:e21030. doi: 10.1371/journal.pone.0021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: Final results of the FinHer Trial. J Clin Oncol. 2009;27:5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 43.Bailey TA, Luan H, Clubb RJ, et al. Mechanisms of trastuzumab resistance in ErbB2-driven breast cancer and newer opportunities to overcome therapy resistance. J Carcinog. 2011;10:28. doi: 10.4103/1477-3163.90442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 45.Baselga J, Cortés J, Kim SB, et al. CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carey L, Winer E, Viale G, et al. Triple-negative breast cancer: Disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–692. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 48.Pusztai L, Ayers M, Symmans FW, et al. Emerging science: Prospective validation of gene expression profiling-based prediction of complete pathologic response to neoadjuvant paclitaxel/FAC chemotherapy in breast cancer. J Clin Oncol. 2003;21(23 suppl):237. [Google Scholar]
- 49.Iwamoto T, Lee JS, Bianchini G, et al. First generation prognostic gene signatures for breast cancer predict both survival and chemotherapy sensitivity and identify overlapping patient populations. Breast Cancer Res Treat. 2011;130:155–164. doi: 10.1007/s10549-011-1706-9. [DOI] [PubMed] [Google Scholar]
- 50.Javle M, Curtin NJ. The potential for poly(ADP-ribose) polymerase inhibitors in cancer therapy. Ther Adv Med Oncol. 2011;3:257–267. doi: 10.1177/1758834011417039. [DOI] [PMC free article] [PubMed] [Google Scholar]