The effects of tamoxifen on blood markers that are associated with cardiovascular risk, such as C-reactive protein, apolipoprotein A-1, and apolipoprotein B-100 were evaluated in women undergoing chemotherapy for breast cancer.
Keywords: Cardiovascular risk profile, Breast cancer, Estrogen receptor, Adjuvant chemotherapy
Abstract
Objectives.
The objective of this study was to evaluate the effect of tamoxifen on blood markers that are associated with cardiovascular risk, such as C-reactive protein (CRP), apolipoprotein A-1 (Apo-A), and apolipoprotein B-100 (Apo-B), in women undergoing chemotherapy for breast cancer.
Methods.
Over a period of 12 months, we followed 60 women with breast cancer. The women were divided into the following groups: a group that received only chemotherapy (n = 23), a group that received chemotherapy plus tamoxifen (n = 21), and a group that received only tamoxifen (n = 16). Plasma CRP levels were assessed at 0, 3, 6, and 12 months, and Apo-A and Apo B levels as well as the Apo-B/Apo-A ratio were assessed at 0 and 12 months.
Results.
We found increases in the plasma concentration of CRP in the chemotherapy alone and chemotherapy plus tamoxifen groups after 3 and 6 months of treatment (before the introduction of tamoxifen). However, after 12 months of treatment, women who used tamoxifen (the chemotherapy plus tamoxifen and tamoxifen alone groups) showed a significant reduction in CRP and Apo-B levels and a decrease in the Apo-B/Apo-A ratio. A significant increase in serum Apo-A levels was observed in the group receiving chemotherapy alone as a treatment for breast cancer.
Conclusion.
The use of tamoxifen after chemotherapy for the treatment of breast cancer significantly reduces the levels of cardiovascular disease risk markers (CRP, Apo-B, and the Apo-B/Apo-A ratio).
Introduction
The occurrence of different types of cancer and cardiovascular disease (CVD) has been closely associated with an increase in the levels of molecules related to chronic inflammatory processes [1]. C-reactive protein (CRP) is an acute-phase protein that is secreted by hepatocytes in response to cytokines, including interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α. These cytokines are considered to be important ischemic markers of inflammation [2]. Leukocytes and monocytes stimulate the production of IL-1 and IL-6 and provide a proangiogenic and protumor microenvironment for tumor growth [3]. The regular use of anti-inflammatory drugs reduces the occurrence of breast cancer by decreasing disease progression [4]. Elevated levels of CRP have been found in patients with breast cancer after radiation therapy [5] and chemotherapy [6]. With regard to the cardiovascular system, several studies have shown a strong correlation between high blood CRP level and CVD [7, 8]. In acute coronary syndrome, high CRP levels have been found in the coronary sinus, suggesting the presence of CRP in cardiac tissue [9]. Postmenopausal women have an elevated risk for coronary heart disease and atherosclerotic events resulting from reduced estrogen levels and loss of the cardioprotective action of estrogen [10, 11]. These cardiovascular complications are in part a result of the deprivation of estrogen and its subsequent inability to signal through its receptors (estrogen receptor [ER]-α and ER-β), which are distributed in different tissues and are involved in cellular regulation, proliferation, and differentiation. ER signaling also mediates physiological effects on the cardiovascular system by releasing nitric oxide, prostacyclins, and endothelium-derived hyperpolarizing factor [12]. Inversely, estrogen has the ability to enhance the effects of breast cancer by inducing the growth of breast tissue, thereby increasing the potential for genetic changes and tumor development [13, 14]. Therefore, in addition to the use of conventional chemotherapy and radiotherapy in the treatment of estrogen-dependent breast cancers, hormone therapy with tamoxifen may be included in the treatment regimen. Tamoxifen, a selective modulator of ER, acts as an estrogen antagonist in breast tissue [15] and an agonist in other tissues, such as in the cardiovascular system [16]. Tamoxifen is known to be cardioprotective by reducing blood levels of total cholesterol, low density lipoproteins (LDLs) [10, 17], and CRP in healthy women [18] and in women with estrogen-dependent breast cancers [19]. It has been observed that the use of tamoxifen also increases apolipoprotein A-1 (Apo-A) and reduces apolipoprotein B-100 (Apo-B) levels; these markers are considered to be more reliable markers of CVD risk than high density lipoprotein (HDL) and LDL cholesterol levels [20]. Although treatment with tamoxifen has shown beneficial effects on the cardiovascular system, studies have also shown that the use of chemotherapy drugs, especially those from the anthracycline family, have cardiotoxic effects [21] in the short and long term [21, 22] and can contribute to an elevated risk for CVD.
The objective of this study was to evaluate whether or not hormone therapy with tamoxifen could modify the plasma concentrations of important CVD markers (CRP, Apo-A, and Apo-B) in women undergoing chemotherapy.
Materials and Methods
Study Patients
This study was conducted by the Laboratory of Neurohumoral Regulation of Circulation of the postgraduate program in physiological sciences at the Federal University of Espirito Santo, Brazil. The study was conducted between February 2008 and December 2010 in women with a confirmed history of breast cancer and who were undergoing chemotherapy and/or hormone therapy with tamoxifen. For inclusion in the study, patients aged 40–60 years with early-stage breast cancer without metastasis were selected. Selected patients were clinically monitored by the same oncologist to ensure that there was no difference among the types of treatment. All women agreed to participate in the study by signing the Free and Informed Consent Form. Women who were smokers, who suffered from hypertension, who had diabetes or pre-established CVD, and who had a CRP level >2 mg/dL in the initial laboratory test were excluded from the study. This study was previously reviewed and approved by the Human Research Ethics Committee under registration number 23/2008.
Study Protocol
At the beginning and the end of the study, blood pressure (BP), heart rate (HR) (Omron, Tokyo, Japan, HEM, 742, automatic), and body weight and height (Welmy® scale, São Paulo, Brazil, with readings of 0.1 kg and 0.5 cm, respectively) were measured.
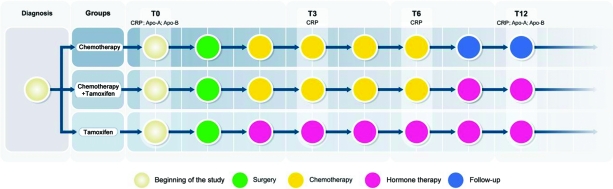
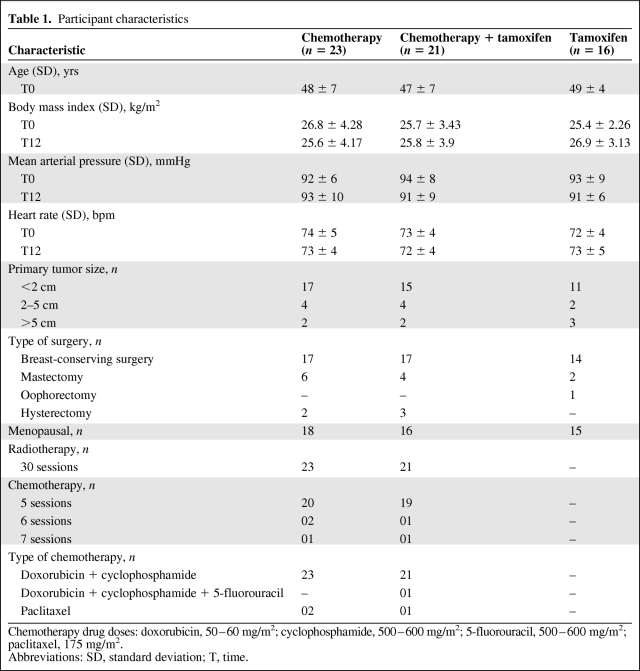
Blood samples were taken after 0 (T0), 3 (T3), 6 (T6), and 12 (T12) months of treatment for determination of serum CRP levels. Initiation of treatment (T0) was considered as the period between the diagnosis of breast cancer and the start of chemotherapy or hormone therapy. Assessments of Apo-A and Apo-B levels were made at T0 and T12. The groups were divided as follows (Fig. 1): (a) women who received chemotherapy treatment for 6 months (n = 23), (b) women who received chemotherapy for 6 months followed by an additional 6 months of tamoxifen (n = 21), and (c) women who received tamoxifen only for 12 months (n = 16). The daily doses of tamoxifen were 20 mg (tamoxifen hydrochloride, 1 mg/kg, Sanofi-Aventis, Paris, France). The types and amounts of chemotherapy drugs used and the amounts of radiation are shown in Table 1.
Figure 1.
Group division: women who received chemotherapy treatment for 6 months, women who received chemotherapy for 6 months followed by an additional 6 months of tamoxifen, and women who received tamoxifen for 12 months.
Abbreviations: Apo-A, apolipoprotein A-1; APO-B, apolipoprotein B-100 ; CRP, C-reactive protein; T, time.
Table 1.
Participant characteristics
Chemotherapy drug doses: doxorubicin, 50–60 mg/m2; cyclophosphamide, 500–600 mg/m2; 5-fluorouracil, 500–600 mg/m2; paclitaxel, 175 mg/m2.
Abbreviations: SD, standard deviation; T, time.
Biochemical Analysis
All samples included in the first biochemical analysis (T0) were from patients who had not received any treatment for breast cancer or other cancers. Blood collection was performed after 12 hours of fasting and consisted of the withdrawal of 20 ml of antecubital venous blood using Vacutainer™ tubes (Becton Dickinson, Franklin Lakes, NJ). Plasma separation was conducted by centrifugation for 10 minutes at 4,000 rpm at 4°C. Detection of plasma levels of CRP, Apo-A, and Apo-B was performed using hypersensitive immunonephelometry. CRP levels <2 mg/dL [23], Apo-B levels <90 mg/dL [24], and Apo-B/Apo-A ratios <0.7 mg/dL [25] were considered normal.
Mean Arterial Pressure and Body Mass Index
After measuring BP at T0 and T12, the mean arterial pressure (MAP) was calculated using the formula, MAP = (systolic blood pressure + 2 × diastolic blood pressure)/3. Body mass was also assessed at T0 and T12 using the body mass index (BMI), which was calculated as weight (kg) × height (m−2).
Statistical Analysis
To assess differences between chemotherapy and hormone therapy in patients with breast cancer, we used a one-way analysis of variance followed by a Bonferroni test (post hoc) to compare means. Statistical significance was considered at p < .05. Data are expressed as the mean ± standard deviation.
Results
Characteristics of Patients
The data for the study participants are described in Table 1. Among all the women evaluated within the 12-month period, two in the chemotherapy alone group died as a result of multiple organ failure caused by metastasis. The average age was 48 ± 6 years and the average BMI was 26 ± 3.7 kg/m2; the average age and BMI were equivalent among the groups. With regard to tumor size, 43 women (72%) had tumors <2 cm, 10 (17%) had tumors 2–5 cm, and only four (11%) had tumors >5 cm. The predominant chemotherapy regimen was five sessions (65%), and the chemotherapy drugs most frequently used were a combination of doxorubicin (50–60 mg/m2) and cyclophosphamide (500–600 mg/m2) (73%).
Cardiovascular Profile
Table 1 also shows the cardiovascular profile of the women at T0 and T12. When MAP was evaluated, we found no significant differences in measures taken at T0 and T12 in the chemotherapy (92 ± 6 mmHg and 93 ± 10 mmHg, respectively), chemotherapy plus tamoxifen (94 ± 8 mmHg and 91 ± 9 mmHg, respectively), and tamoxifen alone (93 ± 9 mmHg and 91 ± 6 mmHg, respectively) groups.
Likewise, there were no significant differences among the groups when HR was measured at T0 and T12 (chemotherapy, 74 ± 5 bpm and 73 ± 4 bpm, respectively; chemotherapy plus tamoxifen, 73 ± 4 bpm and 72 ± 4 bpm, respectively; tamoxifen, 72 ± 4 bpm and 73 ± 5 bpm, respectively).
CVD Risk Factors
CRP
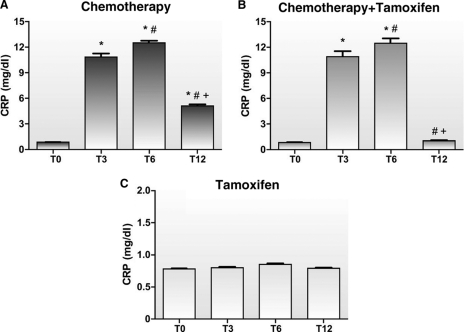
Figure 2 shows the plasma concentrations of CRP at different times after treatment. In the chemotherapy alone group, we observed higher CRP levels at T3 (10.85 ± 0.02 mg/dL), T6 (12.5 ± 1.95 mg/dL), and T12 (5.13 ± 0.75 mg/dL) than at T0 (0.84 ± 0.02 mg/dL) (p < .01) (Fig. 2A). Figure 2B shows that plasma levels of CRP were significantly greater at T3 (10.81 ± 2.97 mg/dL) and T6 (12.36 ± 2.64 mg/dL) than at T0 (0.82 ± 0.16 mg/dL) (p < .01) in the chemotherapy plus tamoxifen group. However, the use of tamoxifen caused a significant reduction in CRP levels in this group at T12 (1.04 ± 0.38 mg/dL), and patients demonstrated values similar to those observed at T0 (0.82 ± 0.16 mg/dL) (p < .01).
Figure 2.
Plasma concentrations of C-reactive protein (CRP) in the groups treated with chemotherapy alone (A), chemotherapy followed by tamoxifen (B), and tamoxifen alone (C).
*p < .01 compared with the beginning (T0).
#p < .01 compared with the third month (T3).
+p < .01 compared with the sixth month (T6).
No differences were found in the levels of CRP in the tamoxifen group when the levels at times T0, T3, T6, and T12 (0.78 ± 0.05 mg/dL, 0.80 ± 0.06 mg/dL, 0.85 ± 0.07 mg/dL, and 0.79 ± 0.04 mg/dL, respectively) were evaluated (Fig. 2C).
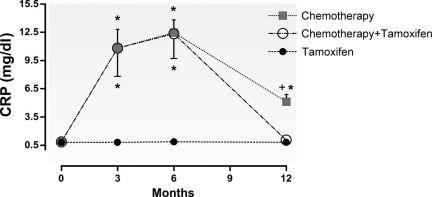
Figure 3 shows the CRP concentrations in the different breast cancer treatment groups. All participants initially had a CRP level <1 mg/dL with no difference among the chemotherapy, chemotherapy plus tamoxifen, and tamoxifen alone groups (0.84 ± 0.02 mg/dL, 0.82 ± 0.02 mg/dL, and 0.78 ± 0.05 mg/dL, respectively). We found that, at T3, the chemotherapy and chemotherapy plus tamoxifen groups exhibited significantly higher plasma CRP levels (10.85 ± 1.95 mg/dL and 10.8 ± 2.97 mg/dL, respectively) than the tamoxifen group (0.8 ± 0.06 mg/dL) (p < .01). Similarly, when CRP levels were evaluated at T6, the chemotherapy alone and chemotherapy plus tamoxifen groups continued to exhibit higher values (12.5 ± 1.31 mg/dL and 12.36 ± 2.64 mg/dL, respectively) than the tamoxifen group (0.85 ± 0.07 mg/dL) (p < .01). However, at T12, we found that, although the overall CRP levels were lower, the chemotherapy alone group demonstrated higher plasma levels than the chemotherapy plus tamoxifen and tamoxifen alone groups (5.13 ± 0.75 mg/dL, 1.04 ± 0.38 mg/dL, and 0.79 ± 0.04 mg/dL, respectively) (p < .01).
Figure 3.
Plasma concentrations of C-reactive protein (CRP) among the groups over time in the groups treated with chemotherapy alone (A), chemotherapy followed by tamoxifen (B), and tamoxifen alone (C).
*p < .01 compared with the tamoxifen alone group.
+p < .01 compared with the chemotherapy plus tamoxifen and tamoxifen alone groups.
Apo-A and Apo-B
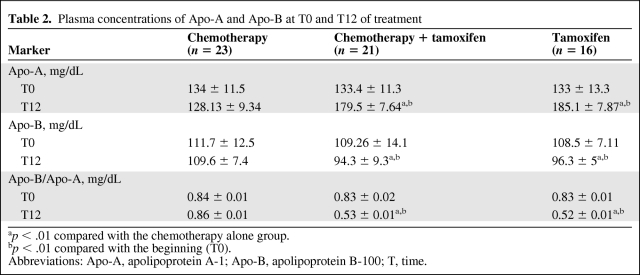
As shown in Table 2, the levels of Apo-A in the chemotherapy alone group showed no differences at T0 and T12 (134 ± 11.5 mg/dL and 128.13 ± 9.34 mg/dL, respectively), nor were the levels of Apo-B significantly different at T0 and T12 (111.7 ± 12.5 mg/dL and 109.6 ± 7.4 mg/dL, respectively). However, we found significantly greater Apo-A values when T0 and T12 were compared between the chemotherapy plus tamoxifen (133.4 ± 11.5 mg/dL and 179.5 ± 7.64 mg/dL, respectively) and tamoxifen alone groups (133 ± 13.3 mg/dL and 185.1 ± 7.87 mg/dL, respectively) (p < .01).
Table 2.
Plasma concentrations of Apo-A and Apo-B at T0 and T12 of treatment
ap < .01 compared with the chemotherapy alone group.
bp < .01 compared with the beginning (T0).
Abbreviations: Apo-A, apolipoprotein A-1; Apo-B, apolipoprotein B-100; T, time.
As for the levels of Apo-B at T0 and T12, a decrease was observed in both the chemotherapy plus tamoxifen (109.26 ± 14.13 mg/dL and 94.3 ± 9.3 mg/dL, respectively) and tamoxifen alone (108.5 ± 7.11 mg/dL and 96.26 ± 5.8 mg/dL, respectively) groups.
When the different forms of treatment were compared, we found that, at T0, the plasma levels of Apo-A in the chemotherapy, chemotherapy plus tamoxifen, and tamoxifen alone groups were similar (134 ± 11.5 mg/dL, 133.4 ± 13.3 mg/dL, and 133 ± 13.3 mg/dL, respectively) (Fig. 4A), as were the plasma levels of Apo-B (111.7 ± 12.5 mg/dL, 109.26 ± 14.1 mg/dL, and 108.5 ± 7.11 mg/dL, respectively) (Fig. 4B).
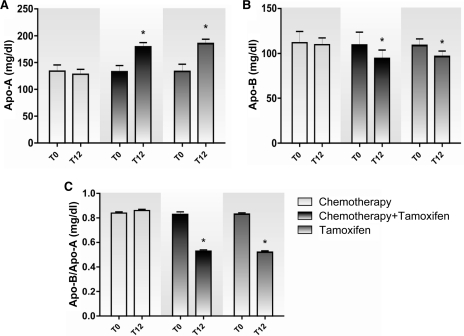
Figure 4.
Plasma concentrations of Apo-A (A), Apo-B (B), and the Apo-B/Apo-A ratio (C) in the groups treated with chemotherapy alone, chemotherapy followed by tamoxifen, and tamoxifen alone.
*p < .01 compared with the beginning (T0).
Abbreviations: Apo-A, apolipoprotein A-1; Apo-B, apolipoprotein B-100; T, time.
At T12, the values of Apo-A for the chemotherapy plus tamoxifen and tamoxifen alone groups were higher (179.5 ± 7.64 mg/dL and 185.1 ± 7.87 mg/dL, respectively) than those of the chemotherapy alone group (128.13 ± 9.34 mg/dL) (p < .01) (Fig. 4A). The values of Apo-B at T12 in the chemotherapy plus tamoxifen and tamoxifen alone groups were significant lower (94.3 ± 9.3 mg/dL and 96.26 ± 5.8 mg/dL, respectively) than those of the chemotherapy alone group (109.6 ± 7.4 mg/dL) (p < .01), and the chemotherapy group maintained levels similar to those observed at T0 (Fig. 4B).
Apo-B/Apo-A Ratio
The Apo-B/Apo-A ratio represents an important factor in assessing CVD risk [26]. We found that this ratio remained the same at T0 and T12 in the chemotherapy group (0.84 ± 0.01 mg/dL and 0.86 ± 0.01 mg/dL, respectively) but was reduced in the chemotherapy plus tamoxifen (0.83 ± 0.02 mg/dL and 0.52 ± 0.01 mg/dL, respectively) and tamoxifen alone (0.83 ± 0.01 mg/dL and 0.52 ± 0.01 mg/dL, respectively) groups (p < .01) (Table 2). When types of treatment were compared, the groups showed no differences at T0, but at T12, the Apo-B/Apo-A ratio in the chemotherapy plus tamoxifen and tamoxifen groups (0.53 ± 0.01 mg/dL and 0.52 ± 0.01 mg/dL, respectively) was lower than in the chemotherapy group (0.86 ± 0.01 mg/dL) (p < .01) (Fig. 4C).
Discussion
The present study investigated women with ER+ breast cancers who were undergoing chemotherapy, and we demonstrated that the use of tamoxifen decreased the plasma concentrations of some risk factors associated with CVD. Women who had tamoxifen introduced into their treatment regimen showed a reduction in plasma levels of CRP, Apo-B, and the Apo-B/Apo-A ratio and an increase in plasma levels of Apo-A. Despite the different menopausal status (postmenopausal, premenopausal, perimenopausal), all groups studied demonstrated initially similar levels of these markers of CVD risk. Chemotherapy promoted elevated levels of CRP in the first 6 months of breast cancer treatment, and even when treatment was stopped after 6 months, CRP levels remained high.
The Framingham study indicated that CRP is an important biomarker of inflammation, and it is used as a predictor of acute myocardial infarction, stroke [27], coronary artery disease (CAD) [28, 29], and arterial vascular events [30, 31]. Tissue injury, inflammation, and infection can stimulate CRP secretion by hepatocytes in response to inflammatory cytokines, such as IL-1, IL-6, and TNF-α [32, 33]. These cytokines induce the expression of cell adhesion molecules, including intercellular adhesion molecule-1, vascular cell adhesion molecule-1, E-selectin, and P-selectin, that facilitate leukocyte adhesion to endothelial cells and migration into the subintimal space of the arteries during vascular inflammation [34]. Furthermore, CRP increases the secretion of monocyte chemoattractant protein-1 [35], which promotes the recruitment of macrophages to the site of arterial injury [36] and contributes to the initiation of atherosclerotic processes [37, 38]. CRP has also been shown to increase the levels of oxygen free radicals [39] by inhibiting the production of nitric oxide synthase in vitro [40].
Estrogen appears to modulate IL-6 and TNF-α levels, because elevated levels of these biomarkers have been found in the plasma of postmenopausal women [41]. Thus, by simulating the effects of estrogen, tamoxifen may be acting on hepatic receptors [42, 43] to promote proestrogen activity, including reducing the production of IL-6 and TNF-α, which in turn reduces the serum CRP concentration [18, 44]. Tamoxifen also exerts anti-inflammatory effects through the upregulation of cytokines, such as transforming growth factor β, that protect the vessel wall from proatherogenic changes [45]. A reduced concentration of CRP resulting from the decreased production of cytokines upon tamoxifen treatment is likely the mechanism by which tamoxifen use after chemotherapy benefits women, particularly with regard to CVD.
In patients with cancer, a blood CRP level >0.5 mg/dL increases the risk for developing venous thromboembolism [37]. Thus, a high risk for the occurrence of these vascular complications is present in patients receiving chemotherapy. Monocytes that are stimulated by CRP also induce tissue CRP production [46] in smooth muscle cells and endothelial cells in vivo and in vitro [47]. It has been shown that CRP can also interact with the endothelium through lipids and can undergo conformational changes to become modified CRP, which is a natural isoform that is expressed predominantly in the endothelium [48] and can upregulate the P-selectin level, which stabilizes platelets and leads to thrombus formation [49].
Studies have shown that reducing CRP levels may occur indirectly through the enhancement of endothelial function or the loss of adipose tissue [50]. In our study, we found that the BMIs of the patients were similar in all groups and were therefore not a justification for the changes in CRP concentrations observed during chemotherapy.
Two important markers for CVD, Apo-A and Apo-B, are considered to be better predictors of CVD risk than serum HDL and LDL levels [20, 26, 51]. Similarly, the Apo-B/Apo-A ratio is considered to be a better predictor of CVD risk than the total cholesterol/HDL ratio [23, 26].
In our study, we demonstrated reduced plasma concentrations of Apo-B and increased plasma concentrations of Apo-A in the treatment groups that included tamoxifen in their regimen. Furthermore, groups in which tamoxifen was used showed a significant reduction in the Apo-B/Apo-A ratio at the end of the treatment period. In contrast, the exclusive use of chemotherapy as a treatment for breast cancer promoted stable levels of Apo-B and Apo-A during the treatment period. The chemotherapy alone group also showed a high Apo-B/Apo-A ratio (>0.7 mg/dL), a result that is associated with an elevated CVD risk [25].
It is known that tamoxifen can act on the liver by increasing the biosynthesis of Apo-A through effects mediated by ERs [42]. Apo-A functions as an antioxidant and anti-inflammatory agent that assists in the production of nitric oxide [52]. It also acts as a carrier for cholesterol excretion in conjunction with molecules such as ATP-binding cassette transporter A1 (ABCA1), ABCG1, scavenger receptor class B member 1, caveolin, and cytochrome P-450 27A1, which have been associated with cholesterol efflux [53–55]. In a recent study, higher levels of CRP were shown to downregulate ABCA1 and ABCG1 in macrophages [56], thus contributing to the progression of atherosclerosis in humans.
It is known that chemotherapeutic agents, such as anthracyclines and cyclophosphamides, can cause cardiac toxicity through mechanisms related to oxidative stress and lipid peroxidation in cardiomyocites [57]. High plasma CRP levels (10–20 mg/dL), which were observed in the chemotherapy group in our study, increase the production of superoxide anion as a result of mitochondrial dysfunction and promote the upregulation of NADPH oxidase subunits, which induce the activation of extracellular signal–related kinase (ERK)-1 and ERK-2. The ERK-1/ERK-2 signaling pathway is activated by CRP and has been found to inhibit the efflux of cholesterol from macrophage-derived foam cells [56].
The Prospective Epidemiological Study of Myocardial Infarction [58] demonstrated that a reduction in serum Apo-A levels is associated with CAD and is independent of HDL cholesterol levels.
Similarly, Apo-B levels more accurately predict the concentrations of proatherogenic particles, including very low density lipoprotein and LDL [59]. CRP can induce reactive oxygen species and promote proinflammatory cytokine release, thereby increasing the absorption of oxidized LDL by macrophages [60]. This process is important for the formation of atherosclerotic plaques [61, 62].
Conclusion
This study showed that chronic use of tamoxifen significantly reduced the plasma concentrations of CVD biomarkers (CRP, Apo B, and the Apo-B/Apo-A ratio) in women with breast cancer who underwent chemotherapy. The results indicate the need for a careful cardiovascular evaluation and the medical monitoring of women with breast cancer to reduce the morbidity and mortality associated with cancer treatment complications.
Acknowledgment
This research was supported by grants and fellowship from FAPES (Vitória, Brazil).
Author Contributions
Conception/Design: Sonia A. Gouvêa, Walckiria G. Romero, Gláucia R. Abreu
Provision of study material or patients: Sonia A. Gouvêa, Walckiria G. Romero
Collection and/or assembly of data: Sonia A. Gouvêa, Walckiria G. Romero, Fabrício B. Silva, Mariana V. Borgo
Data analysis and interpretation: Sonia A. Gouvêa, Walckiria G. Romero, Nazaré S. Bissoli, Gláucia R. Abreu
Manuscript writing: Sonia A. Gouvêa, Walckiria G. Romero
Final approval of manuscript: Sonia A. Gouvêa, Walckiria G. Romero, Gláucia R. Abreu
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koukourakis MI, Kambouromiti G, Pitsiava D, et al. Serum C-reactive protein (CPR) levels in cancer patients are linked with tumor burden and are reduced by anti-hypertensive medication. Inflammation. 2009;32:169–175. doi: 10.1007/s10753-009-9116-4. [DOI] [PubMed] [Google Scholar]
- 3.DeNardo DG, Coussens LM. Inflammation and breast cancer: Balancing immune response—crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212–222. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: Promise, perils, and pharmacogenetics. Nat Rev Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 5.Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 6.Pierce BL, Neuhouser ML, Wener MH, et al. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Treat. 2009;114:155–167. doi: 10.1007/s10549-008-9985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 8.Smeeth L, Thomas SL, Hall AJ, et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa T, Imamura T, Hatakeyama K, et al. Possible contribution of C-reactive protein within coronary plaque to increasing its own plasma levels across coronary circulation. Am J Cardiol. 2004;93:611–614. doi: 10.1016/j.amjcard.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Ali S, Buluwela L, Coombes RC. Antiestrogens and their therapeutic applications in breast cancer and other diseases. Annu Rev Med. 2011;62:217–232. doi: 10.1146/annurev-med-052209-100305. [DOI] [PubMed] [Google Scholar]
- 11.Brincat MP, Galea R, Baron M. Selective estrogen receptor modulations. Curr Obstet Gynaecol. 1999;9:229–234. [Google Scholar]
- 12.Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signaling through ERα and ERβ. J Steroid Biochem Mol Biol. 2010;122:74–81. doi: 10.1016/j.jsbmb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Hulka BS, Moorman PG. Breast cancer: Hormones and other risk factors. Maturitas. 2008;61:203–213. doi: 10.1016/j.maturitas.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 14.McDonald Wade S, 3rd, Hackney MH, Khatcheressian J, et al. Ovarian suppression in the management of premenopausal breast cancer: Methods and efficacy in adjuvant and metastatic settings. Oncology. 2008;75:192–202. doi: 10.1159/000163059. [DOI] [PubMed] [Google Scholar]
- 15.Mobasseri S, Liebson PR, Klein LW. Hormone therapy and selective estrogen receptor modulators for prevention of coronary heart disease in postmenopausal women: Estrogen replacement from the cardiologist's perspective. Cardiol Rev. 2004;12:287–298. doi: 10.1097/01.crd.0000131189.50041.d1. [DOI] [PubMed] [Google Scholar]
- 16.Canton M, Neverova I, Menabo R, et al. Evidence of myofibrillar protein oxidation induced by postischemic reperfusion in isolated rat hearts. Am J Physiol Heart Circ Physiol. 2004;286:H870–H877. doi: 10.1152/ajpheart.00714.2003. [DOI] [PubMed] [Google Scholar]
- 17.Nordenskjold B, Rossell J, Rutqvist LE, et al. Coronary heart disease mortality after 5 years of adjuvant tamoxifen therapy: Results from a randomized trial. J Natl Cancer Inst. 2005;97:1609–1610. doi: 10.1093/jnci/dji342. [DOI] [PubMed] [Google Scholar]
- 18.Bonanni B, Johansson H, Gandini S, et al. Effect of tamoxifen at low doses on ultrasensitive C-reactive protein in healthy women. J Thromb Haemost. 2003;1(10):2149–2152. doi: 10.1046/j.1538-7836.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- 19.Decensi A, Robertson C, Viale G, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–790. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 20.van der Steeg WA, Boekholdt SM, Stein EA, et al. Role of the apolipoprotein B-apolipoprotein A-I ratio in cardiovascular risk assessment: A case-control analysis in EPIC-Norfolk. Ann Intern Med. 2007;146:640–648. doi: 10.7326/0003-4819-146-9-200705010-00007. [DOI] [PubMed] [Google Scholar]
- 21.Jones LW, Haykowsky M, Pituskin EN, et al. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor–positive operable breast cancer. The Oncologist. 2007;12:1156–1164. doi: 10.1634/theoncologist.12-10-1156. [DOI] [PubMed] [Google Scholar]
- 22.Floyd JD, Nguyen DT, Lobins RL, et al. Cardiotoxicity of cancer therapy. J Clin Oncol. 2005;23:7685–7696. doi: 10.1200/JCO.2005.08.789. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM. National Cholesterol Education Program (NCEP)–The National Cholesterol Guidelines in 2001, Adult Treatment Panel (ATP) III. Approach to lipoprotein management in 2001 National Cholesterol Guidelines. Am J Cardiol. 2002;90:11i–21i. doi: 10.1016/s0002-9149(02)02631-0. [DOI] [PubMed] [Google Scholar]
- 25.Davidson MH. Emerging therapeutic strategies for the management of dyslipidemia in patients with the metabolic syndrome. Am J Cardiol. 2004;93:3C–11C. doi: 10.1016/j.amjcard.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 26.McQueen MJ, Hawken S, Wang X, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): A case-control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 27.Laaksonen DE, Niskanen L, Nyyssönen K, et al. C-reactive protein in the prediction of cardiovascular and overall mortality in middle-aged men: A population-based cohort study. Eur Heart J. 2005;26:1783–1789. doi: 10.1093/eurheartj/ehi237. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM. C-reactive protein: Eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clin Chem. 2009;55:209–215. doi: 10.1373/clinchem.2008.119214. [DOI] [PubMed] [Google Scholar]
- 29.Scirica BM, Cannon CP, Sabatine MS, et al. Concentrations of C-reactive protein and B-type natriuretic peptide 30 days after acute coronary syndromes independently predict hospitalization for heart failure and cardiovascular death. Clin Chem. 2009;55:265–273. doi: 10.1373/clinchem.2008.117192. [DOI] [PubMed] [Google Scholar]
- 30.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emerging Risk Factors Collaboration. Danesh J, Erqou S, Walker M, et al. The Emerging Risk Factors Collaboration: Analysis of individual data on lipid, inflammatory and other markers in over 1.1 million participants in 104 prospective studies of cardiovascular disease. Eur J Epidemiolol. 2007;22:839–869. doi: 10.1007/s10654-007-9165-7. [DOI] [PubMed] [Google Scholar]
- 32.Sun H, Koike T, Ichikawa T, et al. C-reactive protein in atherosclerotic lesions: Its origin and pathophysiological significance. Am J Pathol. 2005;167:1139–1148. doi: 10.1016/S0002-9440(10)61202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119:166.e17–166.e28. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 34.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 35.Pasceri V, Cheng JS, Willerson JT, et al. Modulation of C-reactive protein-mediated monocyte chemo-attractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103:2531–2534. doi: 10.1161/01.cir.103.21.2531. [DOI] [PubMed] [Google Scholar]
- 36.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 37.Kröger K, Weiland D, Ose C, et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol. 2006;17:297–303. doi: 10.1093/annonc/mdj068. [DOI] [PubMed] [Google Scholar]
- 38.Jialal I, Deveraj S, Venugopal SK. C-reactive protein: Risk marker or mediator in atherothrombosis? Hypertension. 2004;44:6–11. doi: 10.1161/01.HYP.0000130484.20501.df. [DOI] [PubMed] [Google Scholar]
- 39.Prasad K. C-reactive protein increases oxygen radical generation by neutrophils. J Cardiovasc Pharmacol Ther. 2004;9:203–209. doi: 10.1177/107424840400900308. [DOI] [PubMed] [Google Scholar]
- 40.Jialal I, Verma S, Devaraj S. Inhibition of endothelial nitric oxide synthase by C-reactive protein: Clinical relevance. Clin Chem. 2009;55:206–208. doi: 10.1373/clinchem.2008.119206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cioffi M, Esposito K, Vietri MT, et al. Cytokine pattern in postmenopause. Maturitas. 2002;41:187–192. doi: 10.1016/s0378-5122(01)00286-9. [DOI] [PubMed] [Google Scholar]
- 42.Draper MW. The role of selective estrogen receptor modulators (SERMs) in postmenopausal health. Ann N Y Acad Sci. 2003;997:373–377. doi: 10.1196/annals.1290.040. [DOI] [PubMed] [Google Scholar]
- 43.Lamon-Fava S, Micherone D. Regulation of apo-A-I gene expression: Mechanism of action of estrogen and genistein. J Lipid Res. 2004;45:106–112. doi: 10.1194/jlr.M300179-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Cushman M, Costantino JP, Tracy RP, et al. Tamoxifen and cardiac risk factors in healthy women: Suggestion of an anti-inflammatory effect. Arterioscler Thromb Vasc Biol. 2001;21:255–261. doi: 10.1161/01.atv.21.2.255. [DOI] [PubMed] [Google Scholar]
- 45.Grainger DJ. Transforming growth factor beta and atherosclerosis: So far, so good for the protective cytokine hypothesis. Arterioscler Thromb Vasc Biol. 2004;24:399–404. doi: 10.1161/01.ATV.0000114567.76772.33. [DOI] [PubMed] [Google Scholar]
- 46.Devaraj S, Dasu MR, Singh U, et al. C-reactive protein stimulates superoxide anion release and tissue factor activity in vivo. Atherosclerosis. 2009;203:67–74. doi: 10.1016/j.atherosclerosis.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cirillo P, Golino P, Calabrò P, et al. C-reactive protein induces tissue factor expression and promotes smooth muscle and endothelial cell proliferation. Cardiovasc Res. 2005;68:47–55. doi: 10.1016/j.cardiores.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Ji SR, Ma L, Bai CJ, et al. Monomeric C-reactive protein activates endothelial cells via interaction with lipid raft microdomains. FASEB J. 2009;23:1806–1816. doi: 10.1096/fj.08-116962. [DOI] [PubMed] [Google Scholar]
- 49.Molins B, Pena E, Vilahur G, et al. C-reactive protein isoforms differ in their effects on thrombus growth. Arterioscler Thromb Vasc Biol. 2008;28:2239–2246. doi: 10.1161/ATVBAHA.108.174359. [DOI] [PubMed] [Google Scholar]
- 50.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J Am Coll Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 51.Talmud PJ, Hawe E, Miller GJ, et al. Nonfasting apolipoprotein B and triglyceride levels as a useful predictor of coronary heart disease risk in middle-age UK men. Arterioscler Thromb Vasc Biol. 2002;22:1918–1923. doi: 10.1161/01.atv.0000035521.22199.c7. [DOI] [PubMed] [Google Scholar]
- 52.Walldius G, Junger I. The apoB/apoA-1 ratio: A strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy—a review of the evidence. J Intern Med. 2006;259:493–519. doi: 10.1111/j.1365-2796.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 53.Fichtlscherer S, Breuer S, Schächinger V, et al. C-reactive protein levels determine systemic nitric oxide bioavailability in patients with coronary artery disease. Eur Heart J. 2004;25:1412–1418. doi: 10.1016/j.ehj.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 54.Qamirani E, Ren Y, Kuo L, et al. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2005;25:995–1001. doi: 10.1161/01.ATV.0000159890.10526.1e. [DOI] [PubMed] [Google Scholar]
- 55.Ohashi R, Mu H, Wang X, et al. Reverse cholesterol transport and cholesterol efllux in atherosclerosis. QJM. 2005;98:845–856. doi: 10.1093/qjmed/hci136. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Liao D, Bharadwaj U, et al. C-reactive protein inhibits cholesterol efflux from human macrophage-derived foam cells. Arterioscler Thromb Vasc Biol. 2008;28:519–526. doi: 10.1161/ATVBAHA.107.159467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castelli WP, Anderson K, Wilson PW, et al. Lipids and risk of coronary heart disease. The Framingham Study. Ann Epidemiol. 1992;2:23–28. doi: 10.1016/1047-2797(92)90033-m. [DOI] [PubMed] [Google Scholar]
- 58.Kalil Filho R, Hajjar LA, Bacal F, et al. [I Brazilian guideline for cardio-oncology from Sociedade Brasileira de Cardiologia] Arq Bras Cardiol. 2011;96(suppl 1):1–52. [PubMed] [Google Scholar]
- 59.Walldius G, Jungner I. Apolipoprotein B and apolipoprotein A-I: Risk indicators of coronary heart disease and targets for lipid-modifying therapy. J Intern Med. 2004;255:188–205. doi: 10.1046/j.1365-2796.2003.01276.x. [DOI] [PubMed] [Google Scholar]
- 60.Han KH, Hong KH, Park JH, et al. C-reactive protein promotes monocyte chemoattractant protein-1–mediated chemotaxis through upregulating CC chemokine receptor 2 expression in human monocytes. Circulation. 2004;109:2566–2571. doi: 10.1161/01.CIR.0000131160.94926.6E. [DOI] [PubMed] [Google Scholar]
- 61.Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: Implications for atherosclerosis. Circulation. 2001;103:1194–1197. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 62.Chang MK, Binder CJ, Torzewski M, et al. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proc Natl Acad Sci U S A. 2002;99:13043–13048. doi: 10.1073/pnas.192399699. [DOI] [PMC free article] [PubMed] [Google Scholar]