The results of several phase II and phase III clinical trials, as well as large observational studies, to address the use of bevacizumab in the treatment of patients with metastatic colorectal cancer in the first-line setting.
Keywords: Bevacizumab, Colorectal cancer, Drug toxicity, Combination drug therapy, Vascular endothelial growth factor
Abstract
Since its approval for the first-line treatment of metastatic colorectal cancer (mCRC), bevacizumab has become a standard treatment option in combination with chemotherapy for patients with mCRC. Bevacizumab has demonstrated efficacy in combination with a number of different backbone chemotherapy regimens, and its widespread use has introduced several important questions regarding the selection and optimization of bevacizumab-based treatment regimens, its use in various patient populations, and the identification of associated adverse events. This review discusses the results of several phase II and phase III clinical trials, as well as large observational studies, to address the use of bevacizumab in the treatment of patients with mCRC in the first-line setting.
Introduction
The U.S. Food and Drug Administration (FDA) approved bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA) for the first-line treatment of patients with metastatic colorectal cancer (mCRC) in 2004. Subsequently, bevacizumab became a standard first-line treatment option in combination with chemotherapy. Despite nearly a decade of experience with bevacizumab, important questions still remain regarding its optimal use, the ideal patient population, potential adverse events, and predictive biomarkers of response. This review discusses the selection and optimization of bevacizumab-based chemotherapy regimens in the first-line treatment of patients with mCRC.
Bevacizumab: An Overview
Tumor-related blood vessels depend on vascular endothelial growth factor (VEGF or VEGF-A) for growth and survival. Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that neutralizes VEGF-A and prevents its binding to VEGF receptor 2 (VEGFR-2), its primary receptor. This blockade inhibits endothelial cell responses related to permeability, proliferation, migration, and survival [1]. Bevacizumab inhibits tumor angiogenesis, growth, and metastasis in numerous tumor models [2–8] while reducing intratumoral interstitial pressure, thereby potentially promoting the delivery of cytotoxic chemotherapy [9]. Bevacizumab has several other proposed mechanisms of action, many of which have been extensively reviewed [10–13]. Several other anti-VEGF agents have been tested for the treatment of mCRC, although none are currently approved by the FDA [14–19].
Initial phase I studies with bevacizumab demonstrated favorable tolerability [20, 21]. The plasma half-life is ∼20 days, which allows dosing every 2 or 3 weeks. Bevacizumab has demonstrated clinical benefit for patients with multiple cancers, leading to regulatory approvals for its use in mCRC, non-small cell lung cancer, renal cell cancer, and glioblastoma. Its approval for breast cancer was recently rescinded by the FDA, but the National Comprehensive Cancer Network Breast Cancer Guidelines Panel has so far affirmed its support for bevacizumab with paclitaxel as a first-line treatment option for metastatic breast cancer [22].
Current Questions on the Optimal Use of Bevacizumab in First-Line Treatment
Chemotherapy Backbone
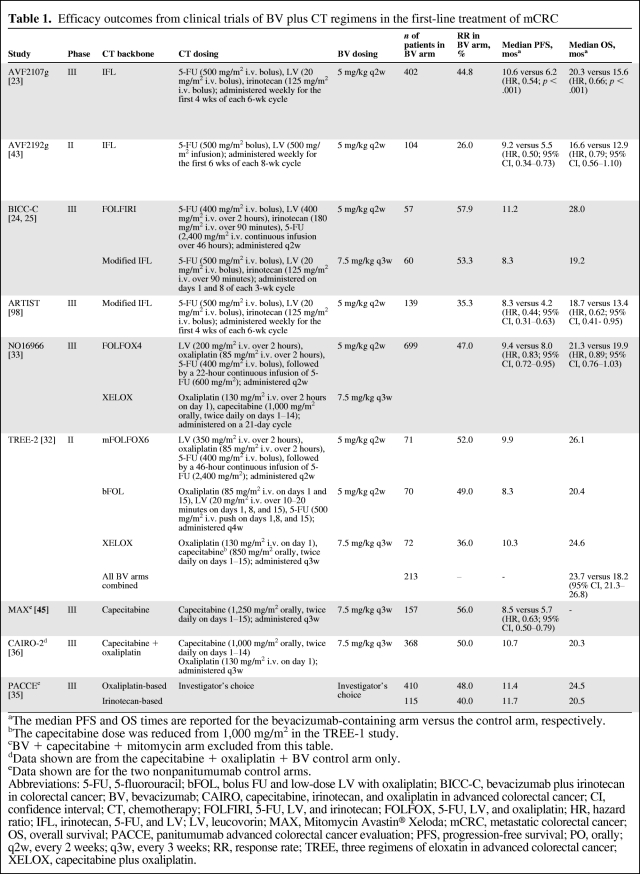
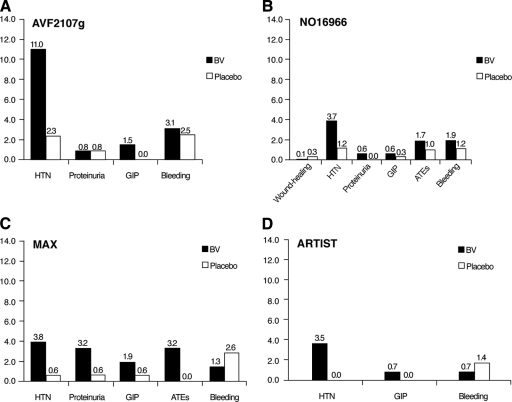
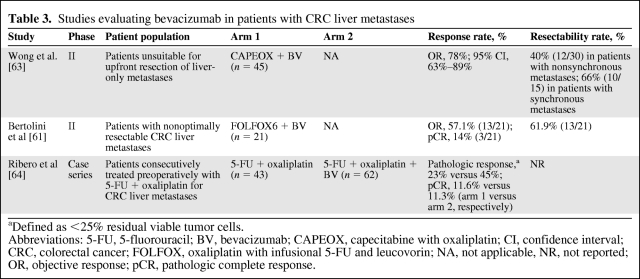
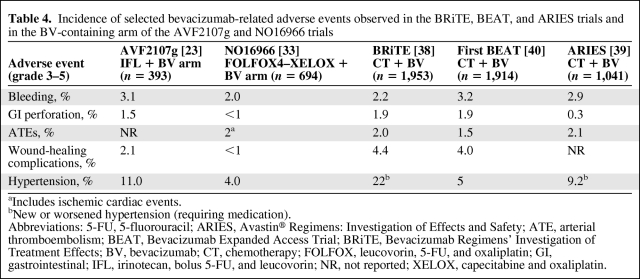
For mCRC, bevacizumab has demonstrated clinical benefit in combination with fluoropyrimidines alone and with fluoropyrimidines combined with either oxaliplatin or irinotecan. In the U.S., bevacizumab is FDA approved with infusional 5-fluorouracil (5-FU), whereas in Europe and many other countries, bevacizumab is approved with oral or infusional 5-FU. A partial listing of key clinical trials of bevacizumab in the first-line setting is shown in Table 1, and the incidence rates of key bevacizumab-related adverse events are listed in Figure 1.
Table 1.
Efficacy outcomes from clinical trials of BV plus CT regimens in the first-line treatment of mCRC
aThe median PFS and OS times are reported for the bevacizumab-containing arm versus the control arm, respectively.
bThe capecitabine dose was reduced from 1,000 mg/m2 in the TREE-1 study.
cBV + capecitabine + mitomycin arm excluded from this table.
dData shown are from the capecitabine + oxaliplatin + BV control arm only.
eData shown are for the two nonpanitumumab control arms.
Abbreviations: 5-FU, 5-fluorouracil; bFOL, bolus FU and low-dose LV with oxaliplatin; BICC-C, bevacizumab plus irinotecan in colorectal cancer; BV, bevacizumab; CAIRO, capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer; CI, confidence interval; CT, chemotherapy; FOLFIRI, 5-FU, LV, and irinotecan; FOLFOX, 5-FU, LV, and oxaliplatin; HR, hazard ratio; IFL, irinotecan, 5-FU, and LV; LV, leucovorin; MAX, Mitomycin Avastin® Xeloda; mCRC, metastatic colorectal cancer; OS, overall survival; PACCE, panitumumab advanced colorectal cancer evaluation; PFS, progression-free survival; PO, orally; q2w, every 2 weeks; q3w, every 3 weeks; RR, response rate; TREE, three regimens of eloxatin in advanced colorectal cancer; XELOX, capecitabine plus oxaliplatin.
Figure 1.
Incidence of key bevacizumab-related adverse events in selected phase III randomized clinical trials.
Abbreviations: ATE, arterial thromboembolism; BV, bevacizumab; GIP, gastrointestinal perforation; HTN, hypertension; MAX, Mitomycin Avastin® Xeloda.
The phase III trial AVF2107g, which led to the initial approval of bevacizumab by the FDA, compared first-line irinotecan, bolus 5-FU, and leucovorin (LV) (the IFL regimen) with and without bevacizumab in patients with mCRC. In that study, the overall survival (OS) and median progression-free survival (PFS) times were longer in the IFL–bevacizumab arm [23]. The phase III Bevacizumab plus Irinotecan in Colorectal Cancer (BICC-C) clinical trial further clarified the optimal irinotecan-based regimen in combination with bevacizumab for first-line mCRC by comparing infusional 5-FU, LV, and irinotecan (FOLFIRI) plus bevacizumab with IFL plus bevacizumab [24, 25]. Patients in the FOLFIRI–bevacizumab had a longer median OS time than those in the IFL–bevacizumab arm. On the basis of this and other studies, FOLFIRI has largely replaced IFL as the preferred irinotecan-based backbone for bevacizumab. In the U.S., the combination of capecitabine and irinotecan (XELIRI) with bevacizumab has not been well studied at doses and schedules that are tolerable in patients. The XELIRI regimen has been studied more extensively in Europe [26–31].
The clinical benefit of bevacizumab combined with a fluoropyrimidine and oxaliplatin has been evaluated in several randomized trials [32, 33]. In the phase III NO16966 study, 1,401 patients were randomized in a two-by-two design to capecitabine and oxaliplatin (XELOX) compared with 5-FU, LV, and oxaliplatin (FOLFOX4) [33]. Both arms were further randomized to bevacizumab or placebo. Pooled outcomes from the chemotherapy–bevacizumab arm were compared with those from the chemotherapy–placebo arm. The addition of bevacizumab to chemotherapy resulted in a longer median OS time, 21.3 months versus 19.9 months, but this difference was not statistically significant (hazard ratio [HR], 0.89; p = .077). The response rates (RRs) were also similar in the two groups. There was a modestly longer median PFS interval for patients receiving bevacizumab than for those given placebo; this result was statistically significant (HR, 0.83; p = .002). Given the OS benefits observed with first-line IFL–bevacizumab [23] and with FOLFOX–bevacizumab in the second-line setting [34], the results of the NO16966 trial were surprising. Clinical outcomes in the NO16966 trial may have been influenced by high rates of treatment discontinuation prior to disease progression in both the bevacizumab and control groups (71% and 53%, respectively). The reasons for treatment discontinuation are not well understood but are likely related to the difficulties of remaining on prolonged therapy, including the complications of cumulative toxicities and the need to remain on protocol-defined treatment schedules. A preplanned analysis that adjusted for patient dropout for reasons other than death or disease progression demonstrated an HR for progression of 0.63 with chemotherapy plus bevacizumab compared with chemotherapy plus placebo. This difference has several implications. First, it emphasizes issues that complicate the interpretation of PFS outcomes. If a large fraction of patients stop protocol-defined therapy before progression, treatment benefits may be diluted. Second, it emphasizes the need for protocol-defined treatments to be more flexible and sustainable, particularly when patients may require prolonged treatment.
In clinical practice, the decision of which chemotherapy to combine with bevacizumab is often guided by practical considerations of convenience, cost, and patient preference. The XELOX–bevacizumab combination offers the convenience of infusions every 3 weeks, albeit with slightly higher rates of hand–foot symptoms and gastrointestinal toxicity. For patients remaining on treatment for a prolonged period, the convenience of less frequent infusion visits may be particularly attractive. Alternatively, the FOLFOX–bevacizumab combination may be better tolerated in some patients but requires an ambulatory infusional device and more frequent chemotherapy administration. The FOLFIRI–bevacizumab combination also requires infusion visits every 2 weeks, limiting the long-term convenience of this regimen.
Regardless of the chemotherapy backbone, patients treated with first-line chemotherapy plus bevacizumab consistently experience a median PFS interval in the range of 9–12 months and a median OS time of ∼2 years. These results have been replicated in the control arms of several trials, including the Capecitabine, Irinotecan, and Oxaliplatin in Advanced Colorectal Cancer (CAIRO)2, Panitumumab Advanced Colorectal Cancer Evaluation (PACCE), and HORIZON III trials [35–37]. Additionally, community-based observational registry studies have demonstrated PFS and OS results comparable with results obtained in randomized trials. In those studies, the doses and schedules of treatments are at the discretion of the physician, assessments of disease response and progression are based on clinician judgment rather than formal criteria, and broader conclusions about safety and efficacy are limited by the absence of a control arm. Observational registry studies are a useful way of benchmarking experiences reported in formal randomized trials and of exploring questions related to the impact of practice variance, which is minimized in formal trials. The Bevacizumab Regimens' Investigation of Treatment Effects (BRiTE) study prospectively evaluated the clinical outcomes of patients receiving bevacizumab combined with chemotherapy for the first-line treatment of mCRC [38]. Investigators enrolled 1,953 patients from 248 primarily community-based sites in the U.S. A total of 96% of patients received bevacizumab every 2 weeks, with the majority receiving FOLFOX plus bevacizumab. The median OS duration for patients receiving first-line FOLFOX–bevacizumab treatment was 24.4 months (95% confidence interval [CI], 22.6–26.0 months), and the median OS time with first-line FOLFIRI plus bevacizumab was 22.9 months (95% CI, 19.6–27.4 months).
Similar OS and PFS results were observed in other large observational studies, including the U.S.-based Avastin® Regimens: Investigation of Effects and Safety (ARIES) trial, a study of first- or second-line bevacizumab for mCRC, and the international Bevacizumab Expanded Access Trial (BEAT), a nonrandomized study of the safety and efficacy of bevacizumab with first-line chemotherapy [39, 40]. In the ARIES study, the 715 patients receiving first-line FOLFOX plus bevacizumab had a median time to progression (TTP) and OS time of 9.7 and 23.5 months, respectively [41]. The 182 patients receiving FOLFIRI plus bevacizumab had a median TTP of 9.3 months and a median OS time of 26.3 months. Because there is no conclusive evidence that bevacizumab has superior activity when combined with a specific chemotherapy and rates of serious adverse events are similar for patients receiving FOLFOX, FOLFIRI, or XELOX, all these chemotherapy backbones may be considered appropriate for combination with bevacizumab in the first-line treatment setting.
For patients who are poor candidates for oxaliplatin or irinotecan, clinical trial evidence supports the use of bevacizumab with 5-FU and LV alone. A randomized phase II trial comparing 5-FU, LV, and bevacizumab with 5-FU, LV and placebo demonstrated a longer median OS time for the FU–LV–bevacizumab group of 16.6 months, versus 12.9 months for the 5-FU–LV–placebo group, but this difference did not reach statistical significance (p = .16). The PFS times were 5.5 months in the placebo-containing arm and 9.2 months in the bevacizumab-containing arm, which was statistically significant (p < .01) [42, 43]. A follow-up pooled analysis of three randomized studies comparing 5-FU with and without bevacizumab demonstrated a longer median OS time for patients receiving 5-FU with bevacizumab than for those receiving 5-FU alone (p = .008) and a statistically significant higher overall RR (p = .019) [44].
Similar benefits were seen in the Mitomycin Avastin® Xeloda (MAX) trial, a phase III study of capecitabine alone, capecitabine plus bevacizumab, and capecitabine, mitomycin, and bevacizumab [45]. Although the longer OS time for patients receiving capecitabine plus bevacizumab was not statistically significantly different from that seen with capecitabine alone, the median PFS intervals were 5.7 months for the capecitabine monotherapy arm and 8.5 months for the capecitabine–bevacizumab arm (p < .01). Taken together, the data from these studies support the use of single-agent 5-FU or capecitabine with bevacizumab in patients who are not candidates for combination chemotherapy but who are otherwise suitable for treatment.
Maintenance Treatment
Decisions about the duration of first-line chemotherapy, treatment breaks, and the use of maintenance chemotherapy remain controversial. The goal of maintenance therapy is to ensure sufficient tumor suppression while maintaining quality of life. Most combination chemotherapy regimens cannot be continued indefinitely without significant modification or interruption. Several clinical trials, such as the optimized leucovorin [LV]-fluorouracil [FU]-oxaliplatin (OPTIMOX)-1 and OPTIMOX-2 studies, offered modest support for the continuation of chemotherapy as tolerated, but bevacizumab was not included in those studies [46–48]. Most clinical trials studying bevacizumab have continued treatment as tolerated until disease progression [33, 34, 49]. To specifically address the issue of maintenance therapy with bevacizumab, the Maintenance in Colorectal Cancer (MACRO) study evaluated the clinical benefit of XELOX plus bevacizumab until progression compared with XELOX plus bevacizumab for six cycles followed by maintenance bevacizumab alone [50]. Many of the patients in the XELOX–bevacizumab arm stopped oxaliplatin because of cumulative toxicity, resulting in capecitabine and bevacizumab being given as unofficial maintenance therapy in that arm. Interim results, reported at a median follow-up of 16 months, demonstrated a modest PFS advantage for the XELOX–bevacizumab group (HR, 1.1; 95% CI, 0.89–1.37; p = .59). As expected, there were also higher rates of hand–foot syndrome and neuropathy in the group receiving continuous XELOX plus bevacizumab. Outside a clinical trial, the standard approach of continuing otherwise effective therapy is to simply modify or reduce the agents causing toxicity, with the goal of preempting severe or otherwise unacceptable toxicities. Several ongoing clinical trials are further addressing the issue of maintenance therapy with bevacizumab (Table 2) [51–55].
Table 2.
Key and ongoing trials investigating BV as maintenance therapy for mCRC
Abbreviations: BV, bevacizumab; CAIRO, capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer; CT, chemotherapy; DREAM, double inhibition reintroduction erlotinib avastin in metastatic colorectal cancer; FOLFIRI, irinotecan with infusional 5-fluorouracil and leucovorin; MACRO, Maintenance in Colorectal Cancer; mFOLFOX, modified oxaliplatin with infusional 5-fluorouracil and leucovorin; XELOX, capecitabine and oxaliplatin.
Bevacizumab Beyond First Progression
Data from the observational BRiTE study were used to compare clinical outcomes for patients with mCRC who received bevacizumab beyond progression after first-line treatment [56]. Compared with the group receiving additional treatment without bevacizumab (median OS time, 19.9 months), patients receiving bevacizumab beyond first progression had a significantly longer median OS time (31.8 months; HR, 0.48; p < .001). The ARIES study also provided valuable information regarding the clinical outcomes of patients in community practice receiving bevacizumab beyond first progression. The median survival duration beyond progression for patients continuing bevacizumab was 14.1 months, compared with 7.5 months for patients receiving additional treatment without bevacizumab (HR, 0.52; p < .001) [39]. Because both the ARIES and BRiTE studies were not randomized, these findings may be a result of biases related to patient selection and management (patients with more indolent disease stay on treatment longer) or a true treatment effect. Randomized clinical trials designed to follow up these findings and formally test the clinical benefit of bevacizumab beyond progression are ongoing [57–59].
Bevacizumab and Resection of Metastatic Disease With Curative Intent
Approximately one third of patients with mCRC have disease confined to the liver, and surgical metastatic resection is an important option for a subset of these patients [60]. Of the patients who receive liver metastasectomy with curative intent, up to 40% are alive at 5 years and 25% are alive at 10 years [61]. Preoperative conversion chemotherapy may be needed to convert patients with borderline-resectable disease to resectable disease. Currently, there are no data to suggest that one conversion chemotherapy regimen is superior to another. In addition, cross-study comparisons are particularly problematic because resectability can depend on local surgical expertise as well as the exact size, number, and location of the liver metastases.
The clinical benefit of adding bevacizumab to preoperative chemotherapy is not defined. The safety and efficacy of preoperative bevacizumab were assessed in a post hoc analysis of the NO16966 and First BEAT clinical trials [62]. In the subset of patients with metastases limited to the liver, 12.3% of patients receiving chemotherapy plus bevacizumab (26 of 211) eventually received a R0 resection, compared with 11.6% of patients (24 of 207) treated with chemotherapy plus placebo (p = .81). Additional phase II studies and case series have evaluated preoperative bevacizumab with chemotherapy for patients with liver-only disease, with encouraging rates of conversion to resectability (Table 3) [61, 63, 64]. Practice patterns for the use of preoperative bevacizumab vary. When patients receive preoperative bevacizumab, the treatment is typically stopped 6–8 weeks before surgery to minimize bleeding and wound-healing complications.
Table 3.
Studies evaluating bevacizumab in patients with CRC liver metastases
aDefined as <25% residual viable tumor cells.
Abbreviations: 5-FU, 5-fluorouracil; BV, bevacizumab; CAPEOX, capecitabine with oxaliplatin; CI, confidence interval; CRC, colorectal cancer; FOLFOX, oxaliplatin with infusional 5-FU and leucovorin; NA, not applicable, NR, not reported; OR, objective response; pCR, pathologic complete response.
Toxicity
Initial phase III clinical trials studying bevacizumab compared with placebo noted slightly higher rates of bleeding, arterial thromboembolic events (i.e., cerebral vascular events, myocardial infarction, transient ischemic attack, and angina), gastrointestinal perforation, altered wound healing, proteinuria, and hypertension. These adverse events are now largely considered anti-VEGF class toxicities. In the AVF2107g clinical trial, grade 3–5 (severe, life-threatening, and lethal) adverse events were more common in the bevacizumab arm than in the placebo arm (85% versus 74%), but most of these additional toxicities were readily manageable [23]. The most common bevacizumab-associated adverse event was hypertension, with 11% of patients developing grade 3 hypertension, compared with 2% of patients receiving placebo. Grade 3 hypertension was defined as blood pressure requiring adjustment with an antihypertensive medication. No grade 4 or 5 hypertensive events were seen in that study. Many of the adverse events attributable to bevacizumab in the AVF2107g trial occurred infrequently. The use of bevacizumab was associated with a 3.1% risk for severe bleeding (versus 2.5% for placebo) and a 1.5% rate of gastrointestinal perforation (versus none for placebo). Other grade ≥3 toxicities attributed to bevacizumab included arterial thromboembolic events (2% versus 1%), wound-healing complications (1.3% versus 0.5), and proteinuria (any proteinuria, 26% versus 21%, but no difference in grade 2 or 3 proteinuria) [1, 65, 66]. The incidence rates of adverse events were similar in the NO16966 clinical trial (Table 4 and Fig. 1).
Table 4.
Incidence of selected bevacizumab-related adverse events observed in the BRiTE, BEAT, and ARIES trials and in the BV-containing arm of the AVF2107g and NO16966 trials
aIncludes ischemic cardiac events.
bNew or worsened hypertension (requiring medication).
Abbreviations: 5-FU, 5-fluorouracil; ARIES, Avastin® Regimens: Investigation of Effects and Safety; ATE, arterial thromboembolism; BEAT, Bevacizumab Expanded Access Trial; BRiTE, Bevacizumab Regimens' Investigation of Treatment Effects; BV, bevacizumab; CT, chemotherapy; FOLFOX, leucovorin, 5-FU, and oxaliplatin; GI, gastrointestinal; IFL, irinotecan, bolus 5-FU, and leucovorin; NR, not reported; XELOX, capecitabine and oxaliplatin.
Although the AVF2107g study did not show an association between bevacizumab and the risk for venous thromboembolism (VTE), a meta-analysis of four placebo-controlled studies of chemotherapy with and without bevacizumab suggested a potential risk for VTE in patients receiving antiangiogenic therapy [67]. However, a large pooled analysis using patient-specific data from 10 placebo-controlled trials of chemotherapy with and without bevacizumab found no difference in the risk for VTE in patients receiving bevacizumab compared with placebo [68]. VTE remains a significant concern for all patients with mCRC [69]. Nonetheless, bevacizumab does not appear to increase the risk for VTE or the risk for bleeding while on anticoagulation. On the basis of these data, bevacizumab is considered an appropriate option in patients with a history of VTE and in patients who are receiving well-monitored anticoagulation.
The incidence rates of adverse events for first-line bevacizumab in observational studies have generally mirrored results from placebo-controlled studies (Table 4). The observed rate of arterial thromboembolic events for patients receiving bevacizumab in the BRiTE study was comparable with the rate observed in other phase III clinical trials [38, 70]. De novo hypertension and worsening hypertension were common adverse events, but most hypertension events were controlled with routine medical management. One case of proteinuria was reported. These clinical outcomes were similar to what was observed in the ARIES, First BEAT, and other observational studies [39–41, 71, 72].
Role of Patient Age and Comorbidities
The role of patient age and other comorbidities in predicting the efficacy and tolerability of bevacizumab is of particular interest given that phase III trials of first-line bevacizumab generally enrolled patients who were younger and had a better performance status than the general population with mCRC. To address this concern, several studies have analyzed the effect of age on clinical outcomes. In a pooled analysis of two placebo-controlled studies, Kabbinavar et al. [73] evaluated clinical outcomes for patients aged ≥65 years treated with first-line chemotherapy plus bevacizumab compared with chemotherapy plus placebo. The OS duration was greater in the group treated with bevacizumab (19.3 months) than in the group treated with placebo (14.3 months; HR, 0.70, p = .006). The rates of adverse events leading to study discontinuation were similar in the two groups (14.8% for bevacizumab versus 12.0% for placebo). A second retrospective analysis of pooled data from four randomized studies evaluated clinical outcomes for patients receiving chemotherapy plus bevacizumab versus chemotherapy plus placebo [74]. The HR for OS was 0.79 (95% CI, 0.66–0.93) for patients aged ≥70 years treated with bevacizumab, compared with 0.77 (95% CI, 0.69–0.86) for patients aged <65 years treated with bevacizumab. For patients who are ≥70 years old, the relative risk for an arterial thromboembolic event was ∼3.2% in the control group and 6.7% in the bevacizumab group. This higher risk is proportionally the same in younger patients (i.e., approximately twofold). It should be noted that the underlying risk for an arterial thromboembolism is higher in older patients, regardless of the treatment received. The survival benefit from bevacizumab is preserved in older patients because, despite the higher risk for an arterial thromboembolic event, the major cause of mortality in older cancer patients is still cancer. Registry studies have further confirmed the safety and efficacy seen in clinical trials [75, 76]. Taken together, these data suggest that the decision of whether or not to use bevacizumab should be made based on factors other than age.
Other Biological and Chemotherapy Combinations With Bevacizumab
New combinations of chemotherapy with bevacizumab have shown encouraging results in early clinical trials. A phase II study of 5-FU, LV, oxaliplatin, and irinotecan (FOLFOXIRI) with bevacizumab in 57 patients with untreated mCRC demonstrated a PFS interval of 13.1 months (95% CI, 10.9–15.2 months), an overall RR of 77%, and a 100% disease control rate [77]. The regimen was associated with high rates of grade 3–4 neutropenia (49%) and diarrhea (14%), but there were no treatment-related deaths. The combination of bevacizumab with FOLFOXIRI remains investigational and is currently the subject of an ongoing phase III trial [78].
Preclinical and phase II trial data suggested that combining anti-VEGF and anti–epidermal growth factor receptor (EGFR) therapies resulted in greater antitumor activity [79–81]. The phase III PACCE trial randomized patients with untreated mCRC to combination chemotherapy (FOLFOX or FOLFIRI based on physician choice) and bevacizumab, with or without panitumumab [35]. The addition of panitumumab resulted in a shorter PFS interval (10.4 months) than in the control arm (11.4 months) and a statistically significant shorter OS time (HR, 1.27; 95% CI, 1.06–1.52). Surprisingly, no differences in the OS, PFS, or RR outcomes were observed in the subset of patients with wild-type KRAS tumors receiving panitumumab. The CAIRO2 trial randomized patients with untreated mCRC to capecitabine, oxaliplatin, and bevacizumab with and without cetuximab [36]. The median PFS interval was shorter in the cetuximab arm (9.4 months) than in the control arm (10.7 months). In the subset of patients with wild-type KRAS tumors, the addition of cetuximab to chemotherapy and bevacizumab did not alter the PFS or OS outcome, but there was a trend toward a higher RR in the group receiving cetuximab (61%) than in the group receiving placebo (50%; p = .06). It is unclear whether the outcomes in these trials were compromised by antagonistic effects between bevacizumab and anti-EGFR monoclonal antibodies or by the greater toxicity of the combination in the setting of chemotherapy, which may have precluded the otherwise better use of all active agents.
Biomarkers
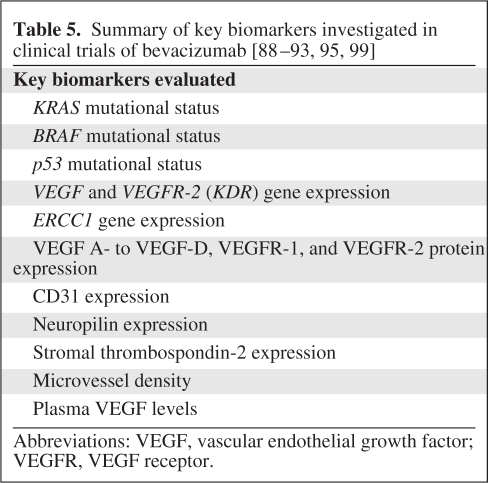
To date, no prospectively validated biomarkers have emerged to include or exclude patients from anti-VEGF therapy. It is unknown whether interactions in the host–tumor microenvironment, biological features unique to the tumor, or features unique to the patient are most likely to yield predictors of responsiveness to treatment (Table 5) [82]. Factors mediating resistance to anti-VEGF therapy have been described in preclinical models [83, 84]. However, several markers that have appeared promising in preclinical models have failed as predictors of response in human trials [83, 85, 86]. Initial human biomarker studies evaluated the effect of the tumor–host microenvironment and tumor genotype on clinical outcomes. A retrospective analysis was performed on 278 tissue samples (bevacizumab, 153; placebo, 125) from the AVF2107g study. Stromal VEGF, stromal thrombospondin-2, and microvessel density were not predictors of a longer survival time for patients receiving bevacizumab, compared with placebo [87]. A related analysis of microdissected tumors from 295 patients enrolled in the same study demonstrated a longer OS time for all patients treated with bevacizumab, compared with placebo, regardless of their KRAS, BRAF, and P53 mutation status [23, 88, 89]. These findings were recently confirmed in the MAX trial, in which the KRAS and BRAF mutation status failed to predict benefit with bevacizumab [90]. Post hoc exploratory analyses were performed on tumor samples from the NO16966 trial [91]. A high CD31 (higher vessel number), high VEGF-A, and low human epidermal growth factor receptor 2 expression level were correlated with a longer duration of response. High levels of neuropilin, which is a cell surface receptor for VEGF-A, VEGF, and placental growth factor, were associated with less benefit from bevacizumab.
Table 5.
Abbreviations: VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
A recent analysis demonstrated an association between intratumoral levels of VEGF-D, which can bind and activate both VEGFR-3 and VEGFR-2, and a benefit from bevacizumab. In the phase III MAX study, the expression levels of VEGF family members A through D and VEGF receptors VEGFR-1 and VEGFR-2 were analyzed using immunohistochemistry from formalin-fixed paraffin-embedded tumor tissue [92]. The expression of VEGF-D emerged as a predictor of response to bevacizumab treatment, and these results remained statistically significant after correction for baseline clinical and pathological factors. For patients treated with bevacizumab, low VEGF-D expression was predictive of a significantly longer PFS interval than with high levels of VEGF-D expression. A separate analysis suggested that VEGF-D levels increased shortly before the development of treatment resistance [93]. Interestingly, circulating VEGF-D also emerged as a predictive biomarker for bevacizumab treatment benefit in the phase III Cancer and Leukemia Group B 80303 trial of gemcitabine with and without bevacizumab for metastatic pancreatic cancer [94]. These results are considered exploratory and need confirmation in additional clinical trials.
Blood-based biomarkers have, until now, produced mixed results. A retrospective analysis of 1,816 patients with colon, lung, and renal cell cancers found that plasma VEGF levels were not predictive of a benefit from bevacizumab [95]. Interestingly, when VEGF levels from the phase III Avastin® and Docetaxel (AVADO) breast cancer trial were tested using a novel VEGF assay, an association between plasma VEGF and a benefit from bevacizumab treatment was observed [96]. Analyses using this novel VEGF assay in patients with mCRC have not yet been reported.
The role of hypertension in predicting responsiveness to bevacizumab is controversial. The most comprehensive analysis to date analyzed ∼5,900 patients across six phase III studies in mCRC and breast, lung, and renal cell cancers [97]. This analysis used patient-specific data, including blood pressure values from each visit. Increased blood pressure on treatment was not predictive of treatment response to bevacizumab in five of six clinical trials. Based on these data, strategies to increase blood pressure, by increasing the bevacizumab dose or by avoiding blood pressure treatment, are likely to be of little value. Hypertension, which is a risk factor for more serious cardiovascular and cerebrovascular events, should be regularly monitored and managed.
Summary and Future Directions
Bevacizumab has demonstrated clinical benefit for the first-line treatment of patients with mCRC with a variety of fluoropyrimidine-based regimens. The choice of chemotherapy backbone in combination with bevacizumab is dependent on patient comorbidities, preferences around toxicities, and practical considerations, such as convenience and cost. For patients with a good performance status, initial therapy with bevacizumab and a combination regimen is generally preferred, with the FOLFOX, FOLFIRI, and XELOX regimens having the most data supporting their use. For patients with an impaired performance status, several studies support the benefit of initiating treatment with a fluoropyrimidine (5-FU or capecitabine) plus bevacizumab without a second cytotoxic agent.
When practical, patients should be treated to progression. Proactive symptom management and adjustments in the doses of cytotoxic agents, as well as strategically timed treatment breaks, may allow first-line treatment to become more sustainable, particularly with average treatment durations now approaching 1 year for many first-line patients. The optimal strategy for induction and maintenance therapy is not yet known; however, the results of several important trials evaluating differing maintenance approaches are due in the near future. Ongoing trials are also attempting to determine whether or not the activity of first-line therapy can be augmented even further.
Lastly, efforts to identify biomarkers related to sensitivity and resistance to bevacizumab are now reporting intriguing results. Although these efforts need independent confirmation, they are an important proof of principle for the value of these approaches. Biomarkers to guide which patients should be treated could have a substantial effect on the use of angiogenesis inhibitors for multiple tumor types. In addition, biomarkers can also be used to identify and prioritize which other factors should also be targeted. In turn, this information should greatly accelerate the development of the next generation of treatments for colorectal cancer.
Acknowledgments
The authors take full responsibility for the content of the paper but thank Huzefa Photowala, Ph.D. (CodonMedical, supported by Genentech, Inc.) for his editorial, copyediting, and production assistance.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: John H. Strickler, Herbert I. Hurwitz
Provision of study material or patients: John H. Strickler, Herbert I. Hurwitz
Collection and/or assembly of data: John H. Strickler, Herbert I. Hurwitz
Data analysis and interpretation: John H. Strickler, Herbert I. Hurwitz
Manuscript writing: John H. Strickler, Herbert I. Hurwitz
Final approval of manuscript: John H. Strickler, Herbert I. Hurwitz
References
- 1.Genentech, Inc. Avastin® (bevacizumab) [package insert] [accessed December 14, 2011]. Available at http://www.gene.com/gene/products/information/pdf/avastin-prescribing.pdf.
- 2.Borgström P, Bourdon MA, Hillan KJ, et al. Neutralizing anti-vascular endothelial growth factor antibody completely inhibits angiogenesis and growth of human prostate carcinoma micro tumors in vivo. Prostate. 1998;35:1–10. doi: 10.1002/(sici)1097-0045(19980401)35:1<1::aid-pros1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Borgström P, Gold DP, Hillan KJ, et al. Importance of VEGF for breast cancer angiogenesis in vivo: Implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer Res. 1999;19:4203–4214. [PubMed] [Google Scholar]
- 4.Borgström P, Hillan KJ, Sriramarao P, et al. Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: Novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Res. 1996;56:4032–4039. [PubMed] [Google Scholar]
- 5.Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 6.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 7.Melnyk O, Shuman MA, Kim KJ. Vascular endothelial growth factor promotes tumor dissemination by a mechanism distinct from its effect on primary tumor growth. Cancer Res. 1996;56:921–924. [PubMed] [Google Scholar]
- 8.Warren RS, Yuan H, Matli MR, et al. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest. 1995;95:1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildiers H, Guetens G, De Boeck G, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. 2003;88:1979–1986. doi: 10.1038/sj.bjc.6601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis LM. Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer. Semin Oncol. 2006;33(suppl 10):S1–S7. doi: 10.1053/j.seminoncol.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 12.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih T, Lindley C. Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–1802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 14.PR Newswire. Pharmacia Announces Closing of SU5416 (Semaxanib) Clinical Trials. [accessed December 14, 2011]. Available at http://www.prnewswire.com/news-releases/pharmacia-announces-closing-of-su5416-semaxanib-clinical-trials-75895232.html.
- 15.Sanofi. Zaltrap™ (Aflibercept) Significantly Improved Survival in Previously Treated Metastatic Colorectal Cancer Patients. [accessed December 14, 2011]. Available at http://en.sanofi.com/Images/26167_20110606_Zaltrap_2_en.pdf.
- 16.AstraZeneca. AstraZeneca Announces Results of Recentin HORIZON II Phase III Trial in Metastatic Colorectal Cancer, 2010. [accessed December 14, 2011]. Available at http://www.astrazeneca.com/Media/Press-releases/Article/20100528--AstraZeneca-Announces-Results-of-Recentin-HORIZON-II-
- 17.Phase-3 Sunitinib Trial in Metastatic Colorectal Cancer Stopped. HemOncToday. 2009. Jul 1, [accessed December 14, 2011]. Available at http://www.hemonctoday.com/article.aspx?rid=41305.
- 18.Hecht JR, Trarbach T, Jaeger E, et al. A randomized, double-blind, placebo-controlled, phase III study in patients (Pts) with metastatic adenocarcinoma of the colon or rectum receiving first-line chemotherapy with oxaliplatin/5-fluorouracil/leucovorin and PTK787/ZK 222584 or placebo (CONFIRM-1) J Clin Oncol. 2005;23(16 suppl):3. [Google Scholar]
- 19.Koehne C, Bajetta E, Lin E, et al. Results of an interim analysis of a multinational randomized, double-blind, phase III study in patients (pts) with previously treated metastatic colorectal cancer (mCRC) receiving FOLFOX4 and PTK787/ZK 222584 (PTK/ZK) or placebo (CONFIRM 2) J Clin Oncol. 2006;24(18 suppl):3508. [Google Scholar]
- 20.Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 21.Margolin K, Gordon MS, Holmgren E, et al. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: Pharmacologic and long-term safety data. J Clin Oncol. 2001;19:851–856. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. [accessed December 14, 2011]. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 23.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Updated results from the BICC-C study. J Clin Oncol. 2008;26:689–690. doi: 10.1200/JCO.2007.15.5390. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Results from the BICC-C study. J Clin Oncol. 2007;25:4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 26.Bajetta E, Di Bartolomeo M, Mariani L, et al. Randomized multicenter phase II trial of two different schedules of irinotecan combined with capecitabine as first-line treatment in metastatic colorectal carcinoma. Cancer. 2004;100:279–287. doi: 10.1002/cncr.11910. [DOI] [PubMed] [Google Scholar]
- 27.Borner MM, Bernhard J, Dietrich D, et al. A randomized phase II trial of capecitabine and two different schedules of irinotecan in first-line treatment of metastatic colorectal cancer: Efficacy, quality-of-life and toxicity. Ann Oncol. 2005;16:282–288. doi: 10.1093/annonc/mdi047. [DOI] [PubMed] [Google Scholar]
- 28.Cartwright T, Lopez T, Vukelja SJ, et al. Results of a phase II open-label study of capecitabine in combination with irinotecan as first-line treatment for metastatic colorectal cancer. Clin Colorectal Cancer. 2005;5:50–56. doi: 10.3816/ccc.2005.n.016. [DOI] [PubMed] [Google Scholar]
- 29.Köhne CH, De Greve J, Hartmann JT, et al. Irinotecan combined with infusional 5-fluorouracil/folinic acid or capecitabine plus celecoxib or placebo in the first-line treatment of patients with metastatic colorectal cancer. EORTC study 40015. Ann Oncol. 2008;19:920–926. doi: 10.1093/annonc/mdm544. [DOI] [PubMed] [Google Scholar]
- 30.Patt YZ, Lee FC, Liebmann JE, et al. Capecitabine plus 3-weekly irinotecan (XELIRI regimen) as first-line chemotherapy for metastatic colorectal cancer: Phase II trial results. Am J Clin Oncol. 2007;30:350–357. doi: 10.1097/COC.0b013e31804b40bb. [DOI] [PubMed] [Google Scholar]
- 31.Rea DW, Nortier JW, Ten Bokkel Huinink WW, et al. A phase I/II and pharmacokinetic study of irinotecan in combination with capecitabine as first-line therapy for advanced colorectal cancer. Ann Oncol. 2005;16:1123–1132. doi: 10.1093/annonc/mdi227. [DOI] [PubMed] [Google Scholar]
- 32.Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: Results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 33.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 34.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 35.Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 36.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 37.Schmoll H, Cunningham D, Sobrero A, et al. mFOLFOX6 + cediranib vs mFOLFOX6 + bevacizumab in previously untreated metastatic colorectal cancer (mCRC): A randomized, double-blind, Phase II/III study (HORIZON III) [abstract 5800] Ann Oncol. 2010;21(suppl 8) viii189. [Google Scholar]
- 38.Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: The BRiTE observational cohort study. The Oncologist. 2009;14:862–870. doi: 10.1634/theoncologist.2009-0071. [DOI] [PubMed] [Google Scholar]
- 39.Cohn AL, Bekaii-Saab T, Bendell JC, et al. Clinical outcomes in bevacizumab (BV)-treated patients (pts) with metastatic colorectal cancer (mCRC): Results from ARIES observational cohort study (OCS) and confirmation of BRiTE data on BV beyond progression (BBP) J Clin Oncol. 2010;28(15 suppl):3596. [Google Scholar]
- 40.Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: The BEAT study Ann Oncol. 2009;20:1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 41.Bendell JC, Bekaii-Saab TS, Cohn AL, et al. Similarities in treatment (Tx) patterns and clinical outcomes (CO) in patients (pts) with metastatic colorectal cancer (mCRC) initially treated with FOLFOX/BV or FOLFIRI/BV: Results from ARIES, a bevacizumab (BV) observational study. J Clin Oncol. 2011;29(4 suppl):480. doi: 10.1634/theoncologist.2012-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 43.Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 44.Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: The addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706–3712. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 45.Tebbutt NC, Wilson K, Gebski VJ, et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: Results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28:3191–3198. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]
- 46.Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol. 2009;27:5727–5733. doi: 10.1200/JCO.2009.23.4344. [DOI] [PubMed] [Google Scholar]
- 47.Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer—a GERCOR study. J Clin Oncol. 2006;24:394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 48.Adams RA, Meade AM, Seymour MT, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011;12:642–653. doi: 10.1016/S1470-2045(11)70102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grothey A, Hart LL, Rowland KM, et al. Intermittent oxaliplatin (oxali) administration and time-to-treatment-failure (TTF) in metastatic colorectal cancer (mCRC): Final results of the phase III CONcePT trial. J Clin Oncol. 2008;26(15 suppl):4010. [Google Scholar]
- 50.Tabernero J, Aranda E, Gomez A, et al. Phase III study of first-line XELOX plus bevacizumab (BEV) for 6 cycles followed by XELOX plus BEV or single-agent (s/a) BEV as maintenance therapy in patients (pts) with metastatic colorectal cancer (mCRC): The MACRO Trial (Spanish Cooperative Group for the Treatment of Digestive Tumors [TTD]) J Clin Oncol. 2010;28(15 suppl):3501. [Google Scholar]
- 51.Yalcin S, Uslu R, Dane F, et al. A randomized, multicenter phase III trial of bevacizumab plus capecitabine as maintenance treatment after initial treatment with bevacizumab plus XELOX in previously untreated metastatic colorectal cancer. J Clin Oncol. 2011;11(4 suppl):474. [Google Scholar]
- 52.ClinicalTrials.gov. Combination Chemotherapy and Bevacizumab With or Without Bevacizumab Maintenance Therapy in Treating Patients With Metastatic Colorectal Cancer. [accessed December 15, 2011]. Available at http://www.clinicaltrials.gov/ct2/results?term=NCT00952029.
- 53.ClinicalTrials.gov. Bevacizumab in Treating Patients Who Have Undergone First-Line Therapy for Metastatic Colorectal Cancer. [accessed December 15, 2011]. Available at http://www.clinicaltrials.gov/ct2/results?term=NCT00544700.
- 54.ClinicalTrials.gov. Maintenance Treatment Versus Observation After Induction in Advanced Colorectal Carcinoma. [accessed December 15, 2011]. Available at http://www.clinicaltrials.gov/ct2/results?term=NCT00442637.
- 55.Tournigand C, Scheithauer BSW, Andre CLT, et al. mFOLFOX-bevacizumab or XELOX-bevacizumab then bevacizumab (B) alone or with erlotinib (E) in first-line treatment of patients with metastatic colorectal cancer (mCRC): Interim safety analysis of DREAM study. J Clin Oncol. 2009;27(15 suppl):4077. [Google Scholar]
- 56.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: Results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26:5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 57.ClinicalTrials.gov. Post-First Progression Use of Bevacizumab in Metastatic Colorectal Cancer (mCRC) [accessed December 15, 2011]. Available at http://www.clinicaltrials.gov/ct2/results?term=NCT00862342.
- 58.ClinicalTrials.gov. Second-Line Combination Chemotherapy With or Without Bevacizumab in Treating Patients With Metastatic Colorectal Cancer Who Have Received First-Line Chemotherapy and Bevacizumab. [accessed December 15, 2011]. Available at http://www.clinicaltrials.gov/ct2/results?term=NCT00720512.
- 59.ClinicalTrials.gov. A Study of Avastin® (Bevacizumab) Plus Crossover Fluoropyrimidine-Based Chemotherapy in Patients With Metastatic Colorectal Cancer. [accessed December 15, 2011]. Available at http://www.clinicaltrials.gov/ct2/results?term=NCT00700102.
- 60.Chua YJ, Cunningham D. Neoadjuvant treatment of unresectable liver metastases from colorectal cancer. Clin Colorectal Cancer. 2006;5:405–412. doi: 10.3816/CCC.2006.n.011. [DOI] [PubMed] [Google Scholar]
- 61.Bertolini F, Malavasi N, Scarabelli L, et al. FOLFOX6 and bevacizumab in non-optimally resectable liver metastases from colorectal cancer. Br J Cancer. 2011;104:1079–1084. doi: 10.1038/bjc.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okines A, Puerto OD, Cunningham D, et al. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br J Cancer. 2009;101:1033–1038. doi: 10.1038/sj.bjc.6605259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong R, Saffery C, Barbachano Y, et al. BOXER: A multicentre phase II trial of capecitabine and oxaliplatin plus bevacizumab as neoadjuvant treatment for patients with liver-only metastases from colorectal cancer unsuitable for upfront resection. Eur J Cancer. 2009;7:344–345. [Google Scholar]
- 64.Ribero D, Wang H, Donadon M, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761–2767. doi: 10.1002/cncr.23099. [DOI] [PubMed] [Google Scholar]
- 65.Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173–180. doi: 10.1002/jso.20301. [DOI] [PubMed] [Google Scholar]
- 66.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 67.Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA. 2008;300:2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 68.Cassidy J, Saltz L, Van Cutsem E, et al. Venous thromboembolic events with chemotherapy plus bevacizumab: A pooled analysis of over 6,000 patients in randomized phase II and III studies. J Clin Oncol. 2010;28(15 suppl):3604. doi: 10.1200/JCO.2010.32.3220. [DOI] [PubMed] [Google Scholar]
- 69.Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: Incidence and effect on survival. J Clin Oncol. 2006;24:1112–1118. doi: 10.1200/JCO.2005.04.2150. [DOI] [PubMed] [Google Scholar]
- 70.Tebbutt NC, Murphy F, Zannino D, et al. Risk of arterial thromboembolic events in patients with advanced colorectal cancer receiving bevacizumab. Ann Oncol. 2011;22:1834–1838. doi: 10.1093/annonc/mdq702. [DOI] [PubMed] [Google Scholar]
- 71.Berry SR, Cutsem EV, Kretzschmar A, et al. Final efficacy results for bevacizumab plus standard first-line chemotherapies in patients with metastatic colorectal cancer: First BEAT. J Clin Oncol. 2008;26:4025. [Google Scholar]
- 72.Sobrero A, Ackland S, Clarke S, et al. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology. 2009;77:113–119. doi: 10.1159/000229787. [DOI] [PubMed] [Google Scholar]
- 73.Kabbinavar FF, Hurwitz HI, Yi J, et al. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: Pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol. 2009;27:199–205. doi: 10.1200/JCO.2008.17.7931. [DOI] [PubMed] [Google Scholar]
- 74.Cassidy J, Saltz LB, Giantonio BJ, et al. Effect of bevacizumab in older patients with metastatic colorectal cancer: Pooled analysis of four randomized studies. J Cancer Res Clin Oncol. 2010;136:737–743. doi: 10.1007/s00432-009-0712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kozloff M, Bekaii-Saab TS, Bendell JC, et al. Effectiveness of first- or second-line bevacizumab (BV) treatment (tx) in elderly patients (pts) with metastatic colorectal cancer (mCRC) in ARIES, an observational cohort study (OCS) J Clin Oncol. 2011;29(15 suppl):3625. [Google Scholar]
- 76.Kozloff MF, Berlin J, Flynn PJ, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: Results from the BRiTE observational cohort study. Oncology. 2010;78:329–339. doi: 10.1159/000320222. [DOI] [PubMed] [Google Scholar]
- 77.Masi G, Loupakis F, Salvatore L, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: A phase 2 trial. Lancet Oncol. 2010;11:845–852. doi: 10.1016/S1470-2045(10)70175-3. [DOI] [PubMed] [Google Scholar]
- 78.ClinicalTrials.gov. A Phase III Randomized Trial of FOLFOXIRI + Bevacizumab Versus FOLFIRI + Bevacizumab as First- Line Treatment for Metastatic Colorectal Cancer. [accessed December 15, 2011]. Available at http://clinicaltrials.gov/ct2/show/NCT00719797?term=folfoxiri+bevacizumab&rank=7.
- 79.Riedel F, Gôtte K, Li M, et al. EGFR antisense treatment of human HNSCC cell lines down-regulates VEGF expression and endothelial cell migration. Int J Oncol. 2002;21:11–16. [PubMed] [Google Scholar]
- 80.Saltz LB, Lenz HJ, Kindler HL, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: The BOND-2 study. J Clin Oncol. 2007;25:4557–4561. doi: 10.1200/JCO.2007.12.0949. [DOI] [PubMed] [Google Scholar]
- 81.Viloria-Petit A, Crombet T, Jothy S, et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: A role for altered tumor angiogenesis. Cancer Res. 2001;61:5090–5101. [PubMed] [Google Scholar]
- 82.Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010;11:1172–1183. doi: 10.1016/S1470-2045(10)70232-1. [DOI] [PubMed] [Google Scholar]
- 83.Crawford Y, Kasman I, Yu L, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 84.Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 85.Li JL, Sainson RC, Shi W, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67:11244–11253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 86.Pan Q, Chanthery Y, Liang WC, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 87.Jubb AM, Hurwitz HI, Bai W, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24:217–227. doi: 10.1200/JCO.2005.01.5388. [DOI] [PubMed] [Google Scholar]
- 88.Hurwitz HI, Yi J, Ince W, et al. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: Analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. The Oncologist. 2009;14:22–28. doi: 10.1634/theoncologist.2008-0213. [DOI] [PubMed] [Google Scholar]
- 89.Ince WL, Jubb AM, Holden SN, et al. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst. 2005;97:981–989. doi: 10.1093/jnci/dji174. [DOI] [PubMed] [Google Scholar]
- 90.Price TJ, Hardingham JE, Lee CK, et al. Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J Clin Oncol. 2011;29:2675–2682. doi: 10.1200/JCO.2010.34.5520. [DOI] [PubMed] [Google Scholar]
- 91.Foernzler D, Delmar P, Kockx M, et al. Tumor tissue based biomarker analysis in NO16966: A randomized phase III study of first-line bevacizumab in combination with oxaliplatin-based chemotherapy in patients with mCRC [abstract 374]. Presented at the 2010 Gastrointestinal Cancers Symposium; January 22–24, 2010; Orlando, FL. [Google Scholar]
- 92.Weickhardt AJ, Williams D, Lee C, et al. Vascular endothelial growth factors (VEGF) and VEGF receptor expression as predictive biomarkers for benefit with bevacizumab in metastatic colorectal cancer (mCRC): Analysis of the phase III MAX study. J Clin Oncol. 2011;29(15 suppl):3531. [Google Scholar]
- 93.Lieu CH, Tran HT, Jiang Z, et al. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. J Clin Oncol. 2011;29(15 suppl):3533. doi: 10.1371/journal.pone.0077117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nixon AB, Pang H, Starr M, et al. Prognostic and predictive blood-based biomarkers in patients with advanced pancreatic cancer: Results from CALGB 80303. J Clin Oncol. 2011;29(15 suppl):10508. doi: 10.1158/1078-0432.CCR-13-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bernaards C, Hegde P, Chen D, et al. Circulating vascular endothelial growth factor (VEGF) as a biomarker for bevacizumab-based therapy in metastatic colorectal, non-small cell lung, and renal cell cancers: Analysis of phase III studies. J Clin Oncol. 28(15 suppl):10519. [Google Scholar]
- 96.Miles D, de Haas S, Dirix L, et al. Plasma biomarker analyses in the AVADO phase III randomized study of first-line bevacizumab + docetaxel in patients with human epidermal growth factor receptor (HER) 2-negative metastatic breast cancer [abstract P2–16-04]. Presented at the 33rd Annual San Antonio Breast Cancer Symposium; December 8–12, 2010; San Antonio, TX. [Google Scholar]
- 97.Hurwitz H, Douglas PS, Middleton JP, et al. Analysis of early hypertension (HTN) and clinical outcome with bevacizumab (BV) J Clin Oncol. 2010;28(15 suppl):3039. doi: 10.1634/theoncologist.2012-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guan Z, Xu J, Luo R, et al. Bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: Efficacy and tolerability results from the ARTIST study [abstract PD-0007] Ann Oncol. 2010;21(suppl 6) vi22. [Google Scholar]
- 99.El-Khoueiry AB, Pohl A, Danenberg K, et al. Wt Kras and gene expression levels of VEGFR2, EGFR, and ERCC-1 associated with progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC) treated with first-line 5-FU or capecitabine with oxaliplatin and bevacizumab (FOLFOX/BV or XELOX/BV) J Clin Oncol. 2009;27(15 suppl):4056. [Google Scholar]