Cancer-related fatigue (CRF) is an underestimated phenomenon and is prevalent in head and neck cancer patients. Results on aspects of the time course of CRF and its correlation with pain as well as the impact of pain on CRF are discussed.
Keywords: Oropharynx, Fatigue, Pain, Radiotherapy, Cancer, Chemotherapy, Head and neck, Pain
Learning Objectives
After completing this course, the reader will be able to:
Discuss the incidence of cancer-related fatigue and the impact it has on cancer patients.
Evaluate clinical correlates of cancer-related fatigue and describe possible interventions.
Explain the time course of cancer-related fatigue before, during, and post-treatment and the effect treatment has on patients for years after treatment.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
Cancer-related fatigue (CRF) is a highly prevalent and underestimated symptom in cancer patients. This study aims to analyze CRF solely in a cohort of oropharyngeal cancer patients who underwent treatment with radiotherapy (RT).
Methods.
In January 2008 to June 2010, 87 consecutive oropharyngeal carcinoma patients underwent definitive RT. Concurrent chemotherapy was used for 94% of patients. The median prescription dose to the planning target volume of the gross or clinical tumor volume was 70 Gy for definitive cases (n = 84) and 66 Gy for postoperative cases (n = 3), both delivered over 6.5 weeks. A normalized 12-point numeric rating scale assessed CRF from patient visits before, during, and after RT.
Results.
The median follow-up of living patients was 14 months. Fatigue peaked 1–2 weeks post-RT and remained higher than baseline for up to 2 years post-RT in 50% of patients. The average fatigue score at the time of completion of therapy or maximum thereafter up to 1 year post-RT was significantly worse than baseline. Patients who experienced pain had a trend toward significance with association for a higher maximum difference in fatigue from baseline. Karnofsky performance status score, weight change, and mood disorders did not correlate with CRF.
Conclusions.
Fatigue was a common treatment-related symptom in this uniform cohort of patients with oropharyngeal cancer. RT was highly correlated with worsening of CRF. Pain control has the potential to help mitigate CRF in patients experiencing pain, and will need to be confirmed using larger datasets.
Introduction
Of the ∼300 million people in the U.S., 11 million currently have or have carried a diagnosis of cancer. Almost 1.5 million people will be diagnosed with cancer annually, and >50% will live >5 years [1, 2]. Strides in extending overall survival have led to an increased focus on improving quality of life (QOL) for these patients. One of the symptoms that has become of increasing interest is fatigue or, more specifically, cancer-related fatigue (CRF) [3, 4]. CRF is experienced by these patients from the time of diagnosis and for an indeterminate number of years after treatment [3–5].
The National Comprehensive Cancer Network (NCCN) defines CRF as “a distressing persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” [2]. Numerous reports cite CRF as one of the most common symptoms experienced by cancer patients [6–11]. However, CRF is often underreported and undertreated [2]. Studies have shown that CRF is present at the time of cancer diagnosis in 50%–75% of patients. This is prior to the intense therapies that are used to prolong patient's overall survival. Furthermore, the prevalence of CRF can increase up to 80%–96% and 60%–93% for patients undergoing chemotherapy and radiotherapy (RT), respectively [5]. A large survey of 1,569 patients found that CRF was experienced by 80% of individuals who underwent chemotherapy and/or RT [10, 11].
Our understanding of CRF is rudimentary because for many years debilitating symptoms of pain and nausea captured oncologists attention [12]. During the past two decades, significant strides have been made in palliative pharmacologic interventions, such as greater use of effective serotonin 5-HT3 receptor antagonist antiemetics and newer formulations of μ-opioid receptor (MOR) agonist pain medications [5]. With better control of these symptoms, CRF, a new phenomenon, arose as one of the most pronounced side effects of cancer itself as well as the therapy these patients receive [3, 4, 13, 14].
A key element in understanding CRF is that it is not relieved by rest or sleep, unlike the commonly confused term of “tiredness” [15]. Its mechanism likely is multifactorial, and cited causes of CRF are anemia, depression, a cancer-induced hypermetabolic state, pain, poor nutrition, and the cancer treatment itself [5]. More specifically, CRF is thought to be partially mediated by proinflammatory cytokines [16–18], circadian rhythm disturbances [19], endocrine dysregulation [16], vagal-afferent activation [20], and genomic disruption [21]. However, the exact mechanism remains unknown and is an area of active research.
Head and neck cancer patients who undergo definitive treatment experience some of the most dramatic acute side effects while undergoing therapy. The treatment regimens are intense and often include induction chemotherapy followed by concurrent chemoradiotherapy [22, 23]. The acute treatment effects include, but are not limited to, severe mucositis, epidermal ulceration/desquamation of the neck, xerostomia, agusia, and odynophagia. Opioid pain medications, oral analgesics, and proper oral rinses and hygiene can palliate some of these acute side effects. CRF, when coupled with these side effects, can be debilitating and lead to improper ongoing self care, malnutrition, loss of weight, productivity, and reduced overall QOL [24, 25].
We applied a fatigue assessment form created at the Memorial Sloan-Kettering Cancer Center (MSKCC) based on the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0, to help gain further information on the time course, severity, and clinical correlates of CRF in oropharyngeal head and neck cancer patients.
Materials and Methods
Patients
This study was conducted in accordance with ethical standards at the MSKCC and was approved by the institution's ethics committee. The approval process was completed to incorporate our fatigue assessment form to be included during routine consultation, treatment visits, and follow-up appointments. Institutional review board/privacy board approval was under waiver number WA0469–09.
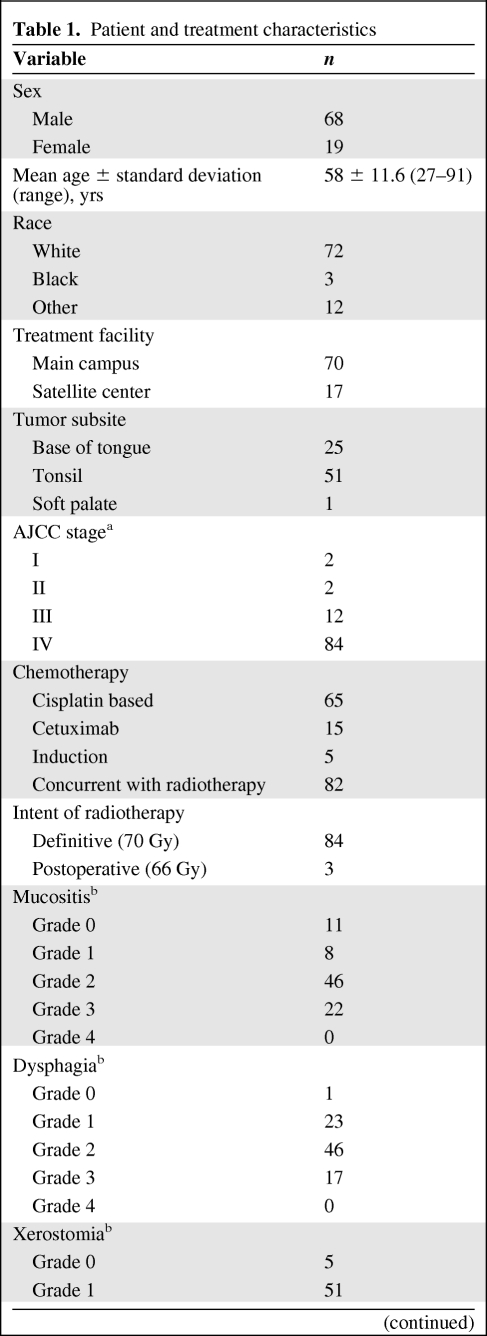
From January 2008 to June 2010, 87 consecutive patients who underwent curative intent treatment at MSKCC for oropharyngeal cancer were analyzed. Ninety-seven percent were definitive cases (n = 84), treated to a total RT dose of 70 Gy, and 3% were postoperative cases (n = 3), treated to a total dose of 66 Gy. All RT plans used intensity-modulated RT with dose painting. Concurrent chemotherapy was used for 94% of patients (n = 82). By the American Joint Committee on Cancer Cancer Staging Manual, Seventh Edition, 84% of patients were stage IV. The majority of patients (n = 65) had cisplatin-based chemotherapy (Table 1).
Table 1.
Patient and treatment characteristics
Table 1a.
(Continued)
aAccording to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, Seventh Edition.
bGrade based on the Common Terminology Criteria for Adverse Events, version 4.0.
Data Collection
A fatigue assessment form created at MSKCC based on the CTCAE, version 4.0, was used to capture the degree of CRF experienced by patients. Two separate physician-completed assessment forms were used to capture CRF. The main campus used a four-point fatigue scale questionnaire whereas satellite centers used a three-point scale. The scales were unified by converting to a 12-point scale. Baseline fatigue levels prior to treatment were imputed using the first observation carried backward (FOCB) method [26, 27]. The assessment was performed at the time of consultation, at weekly treatment visits, and at follow-up appointments at 6–12 weeks, and in 3-month intervals for 1 year and then 4-month intervals for year 2, and biannually thereafter. In addition, weight, Karnofsky performance status (KPS) scores, and pain scores were collected at each appointment. At the time of consultation and follow-up, comorbid conditions, including mood disorders, were evaluated using standard Diagnostic and Statistical Manual of Mental Disorders, version 4, definitions [28]. Comorbid diagnoses were filtered using International Classification of Diseases, Ninth Revision, coding to include only conditions that have been associated with CRF, including anemia, pain disorders, diabetes mellitus (I or II), hypothyroidism, Parkinson's disease, chronic obstructive pulmonary disease, cardiovascular disease, pulmonary hypertension, renal failure, chronic liver disease and cirrhosis, and nutritional deficiencies. Also, the number of and type of CRF-inducing medications were recorded. This was limited to opioids, antihistamines, benzodiazepines, anxiolytics, antipsychotics, antidepressants, and antiepileptics.
Statistical Analysis
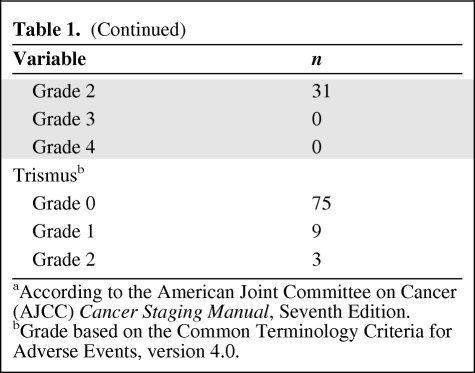
As seen in Table 2, for missing fatigue assessment scores at baseline, prior to any treatment, the FOCB method was used to replace the missing value with the first valid score during RT. This correction factor can alter the baseline pre-RT fatigue scores toward a higher mean than the original data. The resulting effect is to dampen the calculated effect of RT on fatigue.
Table 2.
Time course of CRF
(A) is based on imputation by first observation carried backward. (B) is based on actual fatigue values from all patients within 12 months post-RT.
RT effect: Wilcoxon signed rank test on (B) − (A): p < .0001.
Abbreviations: CRF, cancer-related fatigue; RT, radiotherapy.
The mean effect of RT on fatigue was evaluated by calculating the net difference between the maximum fatigue score over the entire RT period through 1 year post-RT and the baseline score. The Wilcoxon signed rank test was used to assess the statistical significance of this difference. To investigate the association between this maximum difference in fatigue and potential fatigue level predictors, nominal categorical variables were evaluated using the Wilcoxon rank sum or Kruskal-Wallis test for two or multiple groups, respectively, and ordinal or continuous variables were evaluated using the test of nonzero Spearman's rank correlation coefficient. p-values <.05 were considered statistically significant. Because of the nature of this retrospective study, and it being the first of its type to the best of our knowledge to look at a cohort of head and neck subsite cancer patients, we did not perform any multiple testing adjustments. We choose to report and comment on all the p-values directly. We caution here that an enhanced overall (family-wise) type 1 error rate is present.
Results
Time Course of Fatigue
With a median follow-up of 14 months for living patients (range, 6–24 months), the entire 87-patient cohort was able to be evaluated with near uniform completion rates of fatigue assessment at each visit. On average, less than one fatigue score per course of follow-up was not captured per patient. All patients were evaluated at least once per week while on treatment, unless complications arose, in which case they were evaluated more frequently.
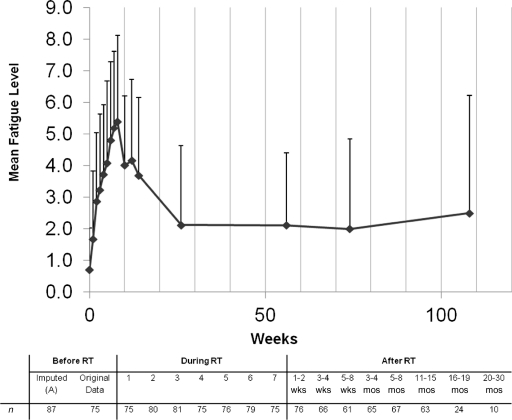
Figure 1 and Table 2 show the trends in fatigue scores over time. The mean baseline fatigue score for the entire cohort was 0.76 (SD, 1.36). The average fatigue score at the time of completion of therapy or maximum thereafter up to 1 year after treatment was 6.89 (SD, 2.44). The difference between these values was statistically significant (p < .0001). Fatigue peaked at week 8–9 from the initiation of RT (post-RT week 1–2), with a mean fatigue score of 5.39 (SD, 2.73).
Figure 1.
Fatigue scores before, during, and after radiotherapy (RT). Data are shown as mean plus standard deviation of the original scores after the scales were unified before any data imputation. The dashed line represents the completion of treatment.
Clinical Correlates of CRF
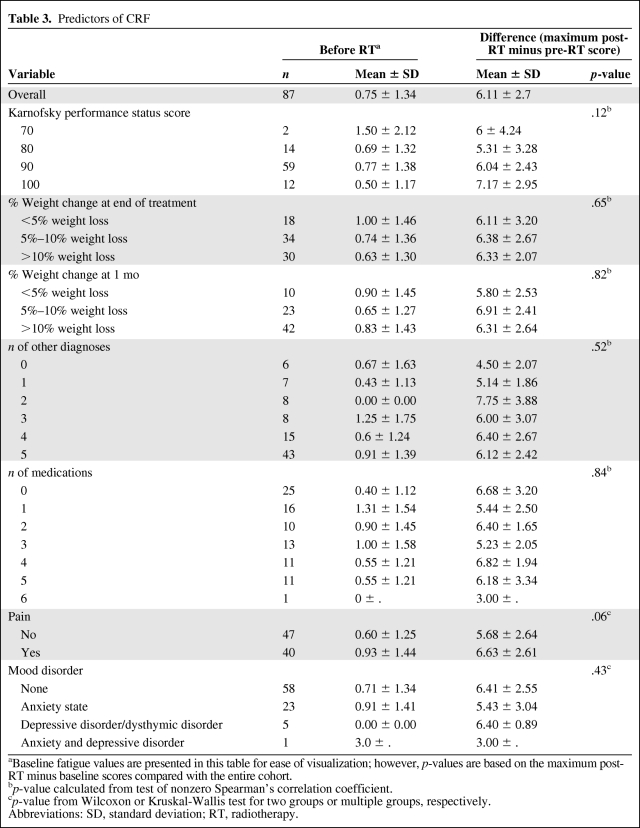
The mean fatigue impairment caused by RT (Table 2) was quantified as 6.13 points (p < .0001). Table 3 shows the results from the univariate analysis of the association between clinical factors and the maximum change in fatigue from baseline. These variables included the KPS score, percent weight change at the end of treatment compared with baseline and at 1 month post-treatment compared with baseline, other comorbid diagnoses that were previously reported to worsen CRF, the number of unique fatigue-inducing prescription medications, the experience of pain, and mood disorders (anxiety, depressive disorders, and the combination of anxiety and depressive disorders).
Table 3.
Predictors of CRF
aBaseline fatigue values are presented in this table for ease of visualization; however, p-values are based on the maximum post-RT minus baseline scores compared with the entire cohort.
bp-value calculated from test of nonzero Spearman's correlation coefficient.
cp-value from Wilcoxon or Kruskal-Wallis test for two groups or multiple groups, respectively.
Abbreviations: SD, standard deviation; RT, radiotherapy.
All patients had KPS scores >70, and performance scores had a mild trend toward predicting CRF (p = .12). Thirty patients had weight loss >10% at 1 month into treatment, with a fatigue score at baseline versus a maximum post-RT minus baseline score of 0.83 ± 1.43 versus 6.31 ± 2.64. Overall both weight change from baseline at the end of treatment and at 1 month post-treatment were not significant predictors of CRF (p = .65 and p = .82, respectively).
The most common comorbid conditions were cardiovascular disease (22%), chronic obstructive pulmonary disease (14%), endocrine dysfunction combining hypothyroidism and diabetes mellitus (12%), and anemia (10%), with the remaining conditions having an incidence of <4% each. Patients with two comorbid diagnoses had a fatigue score at baseline of 0 and a maximum post-RT minus baseline score of 7.75 ± (3.88). Overall, other comorbid diagnoses were not significant as predictors for fatigue (p = .52). Patients with a depressive or dysthymic disorder without an anxiety disorder had a baseline fatigue score of 0 and a maximum post-RT minus baseline score of 6.4 ± 0.89. Mood disorders overall were not predictive of CRF (p = .43).
The most common fatigue-inducing medications used by patients were opioids (93%), anxiolytics and antidepressants (36%), and benzodiazepines (28%), with the remaining medications each with an incidence <7%. Patients taking two fatigue-inducing medications had a fatigue score at baseline of 0.90 and a maximum post-RT minus baseline score of 6.40 ± 1.65. The number of fatigue-inducing medications did not reach significance (p = .84).
Forty patients experienced significant pain, necessitating MOR-agonist pain medications or an increase in the pain medication management regimen. Pain reached a strong trend toward significance for correlation with CRF, with a baseline fatigue score of 0.93 ± 1.44 in patients experiencing pain, compared with a baseline score of 0.6 ± 1.25 in patients without pain. The maximum post-RT minus baseline score for patients with pain was 6.63 ± 2.61, compared with 5.68 ± 2.64 for patients who did not experience pain (p = .06).
Discussion
To our knowledge, the present study is the first report, and with the longest follow-up, describing, in detail, the time course of CRF and predictive factors for CRF in a uniform cohort of patients undergoing treatment for oropharyngeal cancer. CRF is experienced by the vast majority of cancer patients undergoing treatment [5, 13, 27]. Hickok et al. [29] found that patients undergoing treatment for head and neck, gastrointestinal, and lung cancer experienced some of the greatest levels of CRF. Fatigue was experienced by the entire 87-patient cohort in our study, and RT was significantly associated with worsening of CRF (Table 2). Worsening of CRF throughout treatment is consistent with other series, and at 2 years, 50% of the patients continued to have clinically significant levels of CRF.
Two of the largest studies on CRF stem from national surveys from The Fatigue Coalition, a multidisciplinary team whose goal is to gain an understanding of fatigue in cancer patients specifically [3, 4]. The Fatigue-1 study included 419 cancer patients, 59% of whom had been treated with chemotherapy and 63% of whom had been treated with RT. Survey results from that study found that 78% of patients suffered from fatigue during their treatment; the most common malignancy included in that study was breast cancer [4]. The Fatigue-2 study included 379 cancer patients and found results similar to those of the Fatigue-1 study, with 76% of patients being affected by CRF at least monthly while on treatment, and 30% suffering with fatigue daily [3]. When the two studies' data were combined with other reports, it was estimated that 50% of cancer patients suffer from CRF at the time of diagnosis, and this is greater, at 75%, if they have bone metastasis. While on chemotherapy and RT, 93% and 96% of patients, respectively, experience fatigue [30]. In our study, ∼92% of patients were treated with concurrent chemoradiotherapy, and 6% were treated with induction chemotherapy followed by concurrent chemoradiotherapy. The combination of these differences likely explains the greater incidence of CRF in our cohort.
The peak in CRF in our study occurred 1–2 weeks after RT was completed. This phenomenon mimics the common complaint of worsening mucositis and dysphagia in the weeks after completing RT. It is known that the side effects of RT accumulate through therapy secondary to the DNA-damaging nature of the treatment. Whether it is the accumulation of acute side effects that is the cause for the worsening of CRF during treatment or RT itself innately worsens CRF, the causality is difficult to isolate. Other disease sites show a similar worsening trend in CRF, whereby fatigue rapidly peaks while on treatment and is slow to return to baseline [1, 7, 26, 31]. In our study, even at 100 weeks after treatment patients still had not returned to baseline levels of fatigue. The only other report in the literature specifically dedicated to head and neck cancer patients, a study that analyzed numerous subsites of the head and neck, found that the peak in CRF occurred at 6 weeks into RT, decreased slightly at week 7, and had a second peak at 2 weeks post-RT [1]. Our study did not exhibit this effect, and CRF continued to climb every week from the initiation of treatment until 2 weeks post-RT.
A unique aspect of our study, unlike most series, is that we analyzed a specific head and neck subsite (oropharynx). The importance of this fact is that the RT fields and the relatively similar anatomic primary tumor site among all 87 patients allowed fewer confounding variables to interfere with the associated factors of CRF. Therefore, the organs at risk for receiving high bystander doses of radiation are comparable. This would not be true if one was comparing subglottic larynx or nasopharyngeal cancer, let alone including breast or prostate tumor sites. In addition, the dose prescribed to the primary tumor and involved lymph nodes was uniformly 70 Gy, unless it was postoperative (three patients), in which case the dose was lower at 66 Gy. Other series often have more widely varied treatment approaches [14, 26, 29]. There is conflicting reports on whether or not CRF correlates with tumor-related factors or RT parameters (dose, duration of treatment, and volume) [1, 29, 32–35]. Our study did not analyze these factors because of the homogeneity of the treatment groups, which is a strength of our study in identifying correlates of CRF.
Numerous studies have correlated physiologic findings with RT-related fatigue. These include weight change, concurrent symptoms (nausea, mucositis), use of analgesics, interleukin levels, and anemia [1, 31, 36, 37]. The NCCN identifies several factors believed to be paramount in CRF—pain, malnutrition, and other comorbidities, including other medical conditions [2]. Our study did not find a significant difference in CRF based on weight change, fatigue-associated comorbid diagnoses, or the number of medications with a known side effect of fatigue or somnolence. The fact that nearly everyone loses weight during the treatment of oropharyngeal cancer likely explains why our cohort did not show a difference in CRF related to weight loss. An additional study to help further elucidate this could measure percent lean body mass change rather than gross total body weight loss.
The correlation between psychological disorders and CRF has also been studied by many. Our study did not find a correlation between CRF and mood disorders. Reports from others are conflicting, and it appears that there is a complex interplay among CRF, pain, and mood disorders such as depression [1, 5, 26].
The number of medications a patient uses can be a marker of not only comorbid conditions, such as hypertension or diabetes, but also symptoms of treatment. A prospective study by Jereczek-Fossa et al. [27] analyzing CRF found that patients who needed cortisone during treatment had higher levels of CRF. They concluded that the need for medications to palliate RT-induced side effects may be a marker for RT-induced toxicity. In our cohort, we did not find a correlation between RT-induced toxicities and CRF. The correlation between medication use and its mechanism of association with CRF remains to be fully understood.
Other than RT itself, pain was also associated with fatigue in our study, with borderline significance (p = .06). This was an interesting observation because >90% of the patients in this study required opioid pain medications, and of these an additional 70% required escalation of their pain medication regimen during or after treatment. It is difficult to accurately capture morphine-equivalent dosing because most patients are prescribed opioid medications on an as-needed basis. The approximate mean level of oral morphine-equivalent dose during the first 3 weeks of treatment for patients on opioid medications was 15 mg/day. This increased by the end of treatment to a mean value of 37.5 mg/day. The maximum morphine-equivalent oral dose at the end of treatment was 90 mg/day.
Pain in and of itself is debilitating; however, the side effects from opioid use are also significant. Common side effects of MOR-agonist medications include constipation, nausea, vomiting, hypotension, histamine release, and lethargy or lack of energy [38–40]. It would appear from this study, as well as others, that pain plays an important role in contributing to CRF [3]. Furthermore, there are data to also suggest that patients with greater levels of CRF have greater symptoms of pain [5, 7, 41, 42]. Interestingly, data from The Fatigue Coalition studies found that oncologists perceive pain to be more debilitating to cancer patients than CRF; however, patients felt just the opposite, with CRF negatively affecting their daily lives more than pain [4].
Interestingly, it appears from out study that, despite the sedative effects of analgesic use, fatigue is lower in patients whose pain is controlled. Pain management in cancer patients is another challenge that is highly prevalent [43–45]. Oxycodone, a semisythetic opioid, has been in use for >90 years and is one of the mainstays in treating the pain head and neck cancer patients experience [46]. Numerous other MOR-agonist–based formulations have been created and are well studied for cancer-related pain [44–48]. Heiskanen et al. [46] performed a trial looking at opioid pain medications in 45 patients with chronic cancer pain. The study began with an open-label, randomized titration phase to achieve stable pain control for at least 48 hours, followed by a double-blind, randomized, crossover phase. During the titration period, seven patients withdrew off the medication secondary to nausea, vomiting, sweating, or dry mouth. Only one patient withdrew as a result of sedation complaints, and, furthermore, patients also reported nausea, dizziness, and headaches as reasons they withdrew from the study. This highlights that, by controlling cancer-related pain, it appears that CRF mitigation may offset the sedative nature of this class of medications.
In conclusion, our study presents a detailed depiction of the time course of CRF with a median follow-up >1 year post-treatment specifically for a uniform cohort of patients with oropharyngeal squamous cell cancer. Numerous previously reported variables, including change in weight, comorbid conditions, number of medications, KPS score, and mood disorders, failed to predict with statistical significance which patients suffered from worse CRF in our patient population. Correlation between CRF and the experience of pain appeared to strongly trend toward significance, and this highlights a potential therapeutic target to not only help increase the QOL of these patients, by reducing their symptom of pain, but also to potentially reduce the deleterious effects of CRF. Further studies aimed at addressing not only specific contributors of CRF for each tumor subsite but also methods to quantifiably reduce CRF would be of tremendous value for future researh.
Acknowledgments
Daniel E. Spratt and Mayuko Sakae contributed equally to the manuscript.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Mayuko Sakae, Meier Hsu, Karen Schupak, Nancy Y. Lee
Provision of study material or patients: Samuel Essandoh, Karen Schupak, Jeremy Setton, Nancy Y. Lee
Collection and/or assembly of data: Daniel E. Spratt, Mayuko Sakae, Benjamin H. Lok, Jeremy Setton
Data analysis and interpretation: Daniel E. Spratt, Nadeem Riaz, Benjamin H. Lok, Samuel Essandoh, Meier Hsu, Zhigang Zhang, Nancy Y. Lee
Manuscript writing: Daniel E. Spratt, Mayuko Sakae, Benjamin H. Lok, Zhigang Zhang, Nancy Y. Lee
Final approval of manuscript: Daniel E. Spratt, Mayuko Sakae, Nadeem Riaz, Benjamin H. Lok, Samuel Essandoh, Meier Hsu, Zhigang Zhang, Karen Schupak, Jeremy Setton, Nancy Y. Lee
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network (NCCN) Cancer-Related Fatigue, Version 1.2011. [accessed November 5, 2011]. Available at http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf.
- 3.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. The Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 4.Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: Results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol. 1997;34(suppl 2):4–12. [PubMed] [Google Scholar]
- 5.Stasi R, Abriani L, Beccaglia P, et al. Cancer-related fatigue: Evolving concepts in evaluation and treatment. Cancer. 2003;98:1786–1801. doi: 10.1002/cncr.11742. [DOI] [PubMed] [Google Scholar]
- 6.Ahlberg K, Ekman T, Gaston-Johansson F, et al. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362:640–650. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- 7.Irvine D, Vincent L, Graydon JE, et al. The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy. A comparison with the fatigue experienced by healthy individuals. Cancer Nurs. 1994;17:367–378. [PubMed] [Google Scholar]
- 8.Jacobsen PB, Hann DM, Azzarello LM, et al. Fatigue in women receiving adjuvant chemotherapy for breast cancer: Characteristics, course, and correlates. J Pain Symptom Manage. 1999;18:233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 9.Wagner LI, Cella D. Fatigue and cancer: Causes, prevalence and treatment approaches. Br J Cancer. 2004;91:822–828. doi: 10.1038/sj.bjc.6602012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry DH, Viswanathan HN, Elkin EP, et al. Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16:791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 11.Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: The scale of the problem. The Oncologist. 2007;12(suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 12.Hinds PS, Quargnenti A, Bush AJ, et al. An evaluation of the impact of a self-care coping intervention on psychological and clinical outcomes in adolescents with newly diagnosed cancer. Eur J Oncol Nurs. 2000;4:6–17. doi: 10.1054/ejon.1999.0051. discussion 18–19. [DOI] [PubMed] [Google Scholar]
- 13.Smets EM, Visser MR, Willems-Groot AF, et al. Fatigue and radiotherapy: (A) experience in patients undergoing treatment. Br J Cancer. 1998;78:899–906. doi: 10.1038/bjc.1998.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smets EM, Visser MR, Willems-Groot AF, et al. Fatigue and radiotherapy: (B) experience in patients 9 months following treatment. Br J Cancer. 1998;78:907–912. doi: 10.1038/bjc.1998.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. Eur J Cancer Care (Engl) 1996;5(2 suppl):8–23. doi: 10.1111/j.1365-2354.1996.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 16.Bower JE. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain Behav Immun. 2007;21:863–871. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schubert C, Hong S, Natarajan L, et al. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Miller AH, Ancoli-Israel S, Bower JE, et al. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger AM, Wielgus K, Hertzog M, et al. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2010;18:105–114. doi: 10.1007/s00520-009-0636-0. [DOI] [PubMed] [Google Scholar]
- 20.Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. J Auton Nerv Syst. 1993;45:41–50. doi: 10.1016/0165-1838(93)90360-7. [DOI] [PubMed] [Google Scholar]
- 21.Rich TA. Symptom clusters in cancer patients and their relation to EGFR ligand modulation of the circadian axis. J Support Oncol. 2007;5:167–174. discussion 176–177. [PubMed] [Google Scholar]
- 22.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 23.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 24.Crom DB, Hinds PS, Gattuso JS, et al. Creating the basis for a breast health program for female survivors of Hodgkin disease using a participatory research approach. Oncol Nurs Forum. 2005;32:1131–1141. doi: 10.1188/05.ONF.1131-1141. [DOI] [PubMed] [Google Scholar]
- 25.Janda M, Gerstner N, Obermair A, et al. Quality of life changes during conformal radiation therapy for prostate carcinoma. Cancer. 2000;89:1322–1328. doi: 10.1002/1097-0142(20000915)89:6<1322::aid-cncr18>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 26.Engels JM, Diehr P. Imputation of missing longitudinal data: A comparison of methods. J Clin Epidemiol. 2003;56:968–976. doi: 10.1016/s0895-4356(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 27.Jereczek-Fossa BA, Santoro L, Alterio D, et al. Fatigue during head-and-neck radiotherapy: Prospective study on 117 consecutive patients. Int J Radiat Oncol Biol Phys. 2007;68:403–415. doi: 10.1016/j.ijrobp.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Text Revision Edition. Fourth Edition. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders; pp. 1–943. [Google Scholar]
- 29.Hickok JT, Roscoe JA, Morrow GR, et al. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005;104:1772–1778. doi: 10.1002/cncr.21364. [DOI] [PubMed] [Google Scholar]
- 30.Flechtner H, Bottomley A. Fatigue and quality of life: Lessons from the real world. The Oncologist. 2003;8(suppl 1):5–9. doi: 10.1634/theoncologist.8-suppl_1-5. [DOI] [PubMed] [Google Scholar]
- 31.Wratten C, Kilmurray J, Nash S, et al. Fatigue during breast radiotherapy and its relationship to biological factors. Int J Radiat Oncol Biol Phys. 2004;59:160–167. doi: 10.1016/j.ijrobp.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Beard CJ, Propert KJ, Rieker PP, et al. Complications after treatment with external-beam irradiation in early-stage prostate cancer patients: A prospective multiinstitutional outcomes study. J Clin Oncol. 1997;15:223–229. doi: 10.1200/JCO.1997.15.1.223. [DOI] [PubMed] [Google Scholar]
- 33.Kiebert GM, Curran D, Aaronson NK, et al. Quality of life after radiation therapy of cerebral low-grade gliomas of the adult: Results of a randomised phase III trial on dose response (EORTC trial 22844). EORTC Radiotherapy Co-operative Group. Eur J Cancer. 1998;34:1902–1909. doi: 10.1016/s0959-8049(98)00268-8. [DOI] [PubMed] [Google Scholar]
- 34.Biswal BM, Kumaraswamy N, Mukhtar F. Prevalence of fatigue among cancer patients undergoing external radiotherapy. Southeast Asian J Trop Med Public Health. 2004;35:463–467. [PubMed] [Google Scholar]
- 35.Smets EM, Garssen B, Cull A, et al. Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br J Cancer. 1996;73:241–245. doi: 10.1038/bjc.1996.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jereczek-Fossa BA, Marsiglia HR, Orecchia R. Radiotherapy-related fatigue. Crit Rev Oncol Hematol. 2002;41:317–325. doi: 10.1016/s1040-8428(01)00143-3. [DOI] [PubMed] [Google Scholar]
- 37.Portenoy RK, Itri LM. Cancer-related fatigue: Guidelines for evaluation and management. The Oncologist. 1999;4:1–10. [PubMed] [Google Scholar]
- 38.McNicol ED, Boyce D, Schumann R, et al. Mu-opioid antagonists for opioid-induced bowel dysfunction. Cochrane Database Syst Rev. 2008;(2):CD006332. doi: 10.1002/14651858.CD006332.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Vella-Brincat J, Macleod AD. Adverse effects of opioids on the central nervous systems of palliative care patients. J Pain Palliat Care Pharmacother. 2007;21:15–25. [PubMed] [Google Scholar]
- 40.Wang X, Dergacheva O, Kamendi H, et al. 5-Hydroxytryptamine 1A/7 and 4α receptors differentially prevent opioid-induced inhibition of brain stem cardiorespiratory function. Hypertension. 2007;50:368–376. doi: 10.1161/HYPERTENSIONAHA.107.091033. [DOI] [PubMed] [Google Scholar]
- 41.Stone P, Richards M, Hardy J. Fatigue in patients with cancer. Eur J Cancer. 1998;34:1670–1676. doi: 10.1016/s0959-8049(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 42.Meyerowitz BE, Sparks FC, Spears IK. Adjuvant chemotherapy for breast carcinoma: Psychosocial implications. Cancer. 1979;43:1613–1618. doi: 10.1002/1097-0142(197905)43:5<1613::aid-cncr2820430508>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Foley KM. The treatment of cancer pain. N Engl J Med. 1985;313:84–95. doi: 10.1056/NEJM198507113130205. [DOI] [PubMed] [Google Scholar]
- 44.Levy MH. Pharmacologic treatment of cancer pain. N Engl J Med. 1996;335:1124–1132. doi: 10.1056/NEJM199610103351507. [DOI] [PubMed] [Google Scholar]
- 45.Portenoy RK. Treatment of cancer pain. Lancet. 2011;377:2236–2247. doi: 10.1016/S0140-6736(11)60236-5. [DOI] [PubMed] [Google Scholar]
- 46.Heiskanen T, Kalso E. Controlled-release oxycodone and morphine in cancer related pain. Pain. 1997;73:37–45. doi: 10.1016/s0304-3959(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 47.Kalso E, Vainio A. Morphine and oxycodone hydrochloride in the management of cancer pain. Clin Pharmacol Ther. 1990;47:639–646. doi: 10.1038/clpt.1990.85. [DOI] [PubMed] [Google Scholar]
- 48.Hanks GW, Twycross RG, Bliss JM. Controlled release morphine tablets: A double-blind trial in patients with advanced cancer. Anaesthesia. 1987;42:840–844. doi: 10.1111/j.1365-2044.1987.tb04107.x. [DOI] [PubMed] [Google Scholar]