The scientific review of the application leading to the approval, in the European Union, of cabazitaxel for the treatment of patients with hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing regimen is summarized.
Keywords: Cabazitaxel, Jevtana, Hormone-refractory metastatic prostate cancer, EMA, European Medicines Agency
Learning Objectives:
After completing this course, the reader will be able to:
Summarize the efficacy outcomes of cabazitaxel pivotal trials in the treatment of hormone-refractory metastatic prostate cancer.
Describe the safety profile and most common adverse effects of cabazitaxel in patients with hormone-refractory metastatic prostate cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
On March 17, 2011 the European Commission issued a marketing authorization valid throughout the European Union for Jevtana® (Sanofi-Aventis, Paris, France) for the treatment of patients with hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing regimen.
The active substance of Jevtana® is cabazitaxel acetone solvate, an antineoplastic agent that acts by disrupting the microtubular network in cells. The recommended dose of cabazitaxel is 25 mg/m2 administered as a 1-hour i.v. infusion every 3 weeks in combination with oral prednisone or prednisolone, 10 mg, administered daily throughout treatment.
In the main study submitted for this application, a 2.4-month longer median overall survival time and a 30% lower risk for death were observed for cabazitaxel, compared with mitoxantrone. The most common side effects with cabazitaxel were anemia, leukopenia, neutropenia, thrombocytopenia, and diarrhea.
This paper summarizes the scientific review of the application leading to approval in the European Union. The detailed scientific assessment report and product information, including the summary of product characteristics, are available on the European Medicines Agency Web site (http://www.ema.europa.eu).
Introduction
Treatment of patients with metastatic hormone-refractory prostate cancer (mHRPC) who progress following docetaxel as first-line therapy has included low-dose prednisone and mitoxantrone administered with either prednisone or hydrocortisone [1–3]. Supportive care, with various nonapproved agents with limited activity, is currently used in this setting, with palliation being the main goal of therapy [4].
The applicant, Sanofi-Aventis (Paris, France), submitted, on April 20, 2010, an application for marketing authorization to the European Medicines Agency (EMA) for cabazitaxel (Jevtana®).
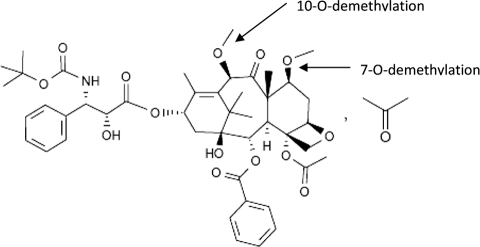
Cabazitaxel is a semisynthetic derivative of 10-deacetyl baccatin III, which is extracted from European yew needles. Cabazitaxel is the 7,10-dimethoxy analog of docetaxel (Fig. 1). It acts by disrupting the microtubular network in cells. Cabazitaxel binds to tubulin and promotes the assembly of tubulin into microtubules while simultaneously inhibiting their disassembly. This leads to the stabilization of microtubules, which results in the inhibition of mitotic and interphase cellular functions.
Figure 1.
Structural formula of cabazitaxel.
Cabazitaxel has a broad spectrum of antitumor activity in murine tumors, including prostate, colon, and mammary adenocarcinomas. It has shown cytotoxic activity in vitro similar to that of docetaxel, and its toxicity profile is similar to those of other taxanes. Clinical activity has been reported in prostate and taxane-resistant metastatic breast cancer patients [5, 6].
The review of this drug application was conducted by the Committee of Human Medicinal Products (CHMP). The CHMP recommended the granting of a marketing authorization for Jevtana based on a positive benefit-risk balance. Following this review the European Commission issued a marketing authorization for cabazitaxel on March 17, 2011. Cabazitaxel in combination with prednisone or prednisolone is indicated for the treatment of patients with mHRPC previously treated with a docetaxel-containing regimen.
This paper summarizes the scientific review of the application leading to approval of cabazitaxel in the European Union. Another recent addition to the approved drug list for docetaxel-resistant tumors is abiraterone (Zytiga®; Centocor Ortho Biotech, Inc., Horsham, PA). The detailed scientific assessment reports and product information for these products are available on the EMA Web site (http://www.ema.europa.eu).
Nonclinical aspects
In vitro, cabazitaxel has demonstrated antitumor activity in sensitive murine and human cell lines. Cabazitaxel showed cytotoxic activity similar to that of docetaxel. In vitro, cabazitaxel has demonstrated activity in several docetaxel-resistant cell lines, including cell lines expressing the multidrug resistance gene (mdr-1) and tumor cell lines resistant to selected chemotherapeutic agents. However, no studies were presented with docetaxel-resistant prostate tumor cell lines.
There were similarities in the toxicological profile of cabazitaxel between preclinical and clinical studies, and the major expected effects observed in the clinic are hematological findings (mainly neutropenia and its complications) and gastrointestinal disorders (mainly diarrhea, nausea, and vomiting).
Clinical Pharmacology
Pharmacokinetics
In clinical trials, cabazitaxel exhibited a long terminal half-life of 95 hours. Cabazitaxel was extensively metabolized in the liver (>95%). Cabazitaxel was mainly metabolized by cytochrome P450 (CYP)3A4 and CYP3A5 (the contribution of CYP3A estimated to be in the range of 80%–90%) and to a lesser extent by CYP2C8. The metabolism of cabazitaxel may be modified by the concomitant administration of compounds that are known to be potent inhibitors (e.g., ketoconazole) or inducers (e.g., rifampicin, carbamazepine, phenobarbital, and phenytoin) of CYP3A. Likewise, coadministration of cabazitaxel with medicinal products that are known to be primarily metabolized through CYP3A may increase the exposure of these medicinal products. Cabazitaxel is also a substrate of P-glycoprotein (P-gp). CYP3A4, CYP3A5, and P-gp are subject to genetic polymorphism. In a population pharmacokinetic analysis in 70 patients aged ≥65 years (57 were aged 65–75 years and 13 were aged >75 years), no age effect on the pharmacokinetics of cabazitaxel was observed. The safety and efficacy of cabazitaxel have not been established in children and adolescents aged <18 years, and cabazitaxel is not indicated for use in these age groups. Interindividual variability in cabazitaxel clearance was significantly related to body surface area.
Cabazitaxel is contraindicated in patients with hepatic impairment (bilirubin ≥1× the upper limit of normal [ULN] or aspartate aminotransferase and/or alanine aminotransferase ≥1.5× ULN). Mild to moderate renal impairment did not have meaningful effects on the pharmacokinetics of cabazitaxel. No data are available for patients with severe renal impairment (creatinine clearance <30 mL/minute) or end-stage renal disease; therefore, these patients should be treated with caution and monitored carefully during treatment.
Dose Finding
Initially, a dose of 20 mg/m2 administered every 3 weeks as a 1-hour i.v. infusion was determined in a phase I study in patients with advanced solid tumors and was recommended for further clinical development [7]. The dose-limiting toxicity of cabazitaxel was neutropenia and its infectious complications at the highest dose tested.
In a phase II study with metastatic breast cancer patients (Study ARD6191), the safety and antitumor activity were assessed at a dose of 20 mg/m2 every 3 weeks for the first cycle, with possible intrapatient escalation to 25 mg/m2 for cycle 2 allowed in the absence of any toxicity of grade >2 during cycle 1. In 21 of 71 patients, the dose of cabazitaxel could be escalated to 25 mg/m2 i.v. after the first cycle [8]. Thus, the 25-mg/m2 dose was selected for the pivotal phase III study (EFC6193).
The optimal dose of cabazitaxel is still to be further explored. A phase III study (EFC11785) is under preparation with the primary objective of demonstrating noninferiority in terms of overall survival (OS) for cabazitaxel at a dose of 20 mg/m2 (arm A) versus a dose of 25 mg/m2 (arm B) in combination with prednisone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel. Approximately 1,200 patients are planned to be enrolled.
Clinical Efficacy and Safety
Clinical Efficacy
The pivotal study for this application was trial EFC6193 (TROPIC; ClinicalTrials.gov identifier, NCT00417079) [5]. The TROPIC study was a multicenter, multinational, randomized, open-label phase III study comparing the efficacy and safety of cabazitaxel plus prednisone or prednisolone with those of mitoxantrone plus prednisone or prednisolone in patients with mHRPC previously treated with a docetaxel-containing regimen.
The trial included patients with histologically or cytologically proven prostate adenocarcinoma refractory to hormone therapy and previously treated with a docetaxel-containing regimen. Patients needed to have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0–2 and documented progression according to the Response Evaluation Criteria in Solid Tumors (measurable disease) or based on a rising prostate-specific antigen (PSA) level or the appearance of new lesions (nonmeasurable disease). Patients with previous treatment with a <225 mg/m2 cumulative dose of docetaxel were not eligible (following protocol amendment).
Cabazitaxel was administered i.v., over 1 hour every 3 weeks, at a starting dose of 25 mg/m2. Mitoxantrone was administered i.v., over 15–30 minutes every 3 weeks, at a starting dose of 12 mg/m2. Prednisone, 10 mg, was administered orally, daily, to all patients.
The primary efficacy endpoint was the OS duration, defined as the time interval from the date of randomization to the date of death resulting from any cause. The primary analysis of the primary efficacy endpoint was performed using the intent-to-treat population. Randomization was stratified according to measurable versus nonmeasurable disease and ECOG PS score (0 or 1 versus 2), and used a dynamic allocation method.
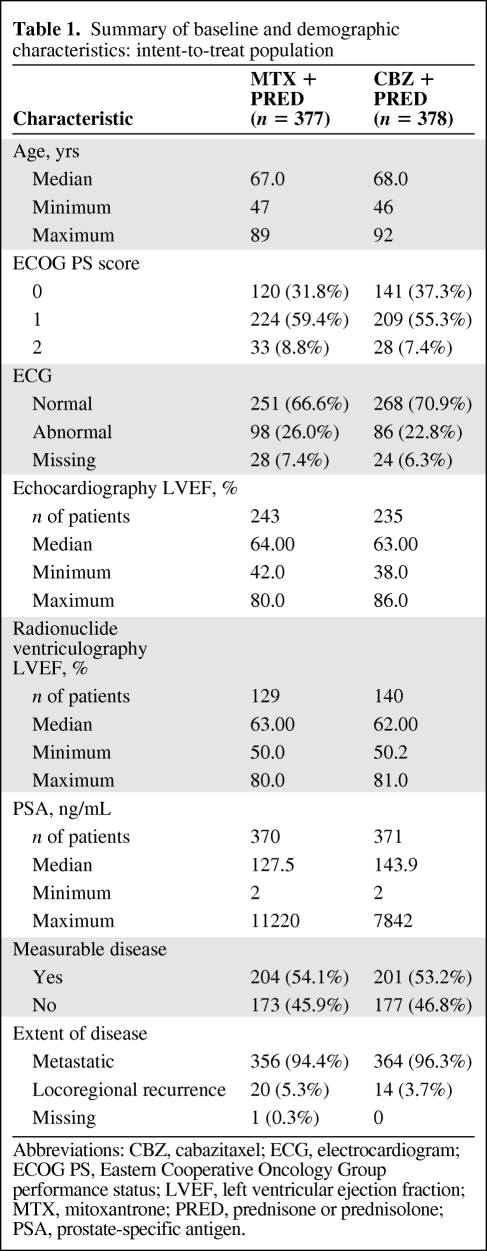
In total, 755 patients were randomized into the study. The baseline characteristics were well balanced between the treatment groups (Table 1). Almost all patients (99.3%) had received hormonal therapy for their prostate cancer, 60.1% of the patients had received radiotherapy, 53.3% of the patients had prior surgery, and 14.9% of the patients had received two or more regimens of prior docetaxel-based chemotherapy. Most of the patients (72%) had progressed during or within 3 months since their last docetaxel dose.
Table 1.
Summary of baseline and demographic characteristics: intent-to-treat population
Abbreviations: CBZ, cabazitaxel; ECG, electrocardiogram; ECOG PS, Eastern Cooperative Oncology Group performance status; LVEF, left ventricular ejection fraction; MTX, mitoxantrone; PRED, prednisone or prednisolone; PSA, prostate-specific antigen.
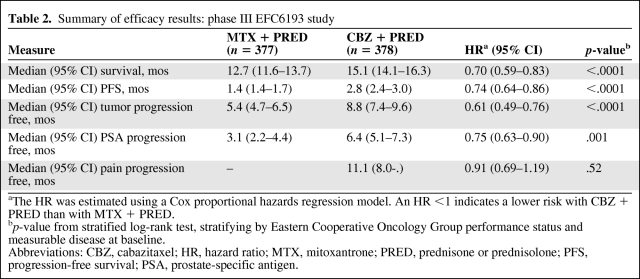
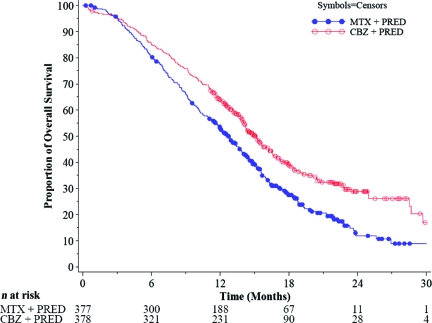
The main efficacy results are summarized in Table 2. In the primary analysis, the superiority of cabazitaxel over mitoxantrone was observed, with a 2.4-month longer median OS time and a 30% lower risk for death (Fig. 2).
Table 2.
Summary of efficacy results: phase III EFC6193 study
aThe HR was estimated using a Cox proportional hazards regression model. An HR <1 indicates a lower risk with CBZ + PRED than with MTX + PRED.
bp-value from stratified log-rank test, stratifying by Eastern Cooperative Oncology Group performance status and measurable disease at baseline.
Abbreviations: CBZ, cabazitaxel; HR, hazard ratio; MTX, mitoxantrone; PRED, prednisone or prednisolone; PFS, progression-free survival; PSA, prostate-specific antigen.
Figure 2.
Kaplan–Meier curves of overall survival by treatment group (TROPIC trial, intent-to-treat population).
Abbreviations: CBZ, cabazitaxel; MTX, mitoxantrone; PRED, prednisone or prednisolone.
Clinical Safety
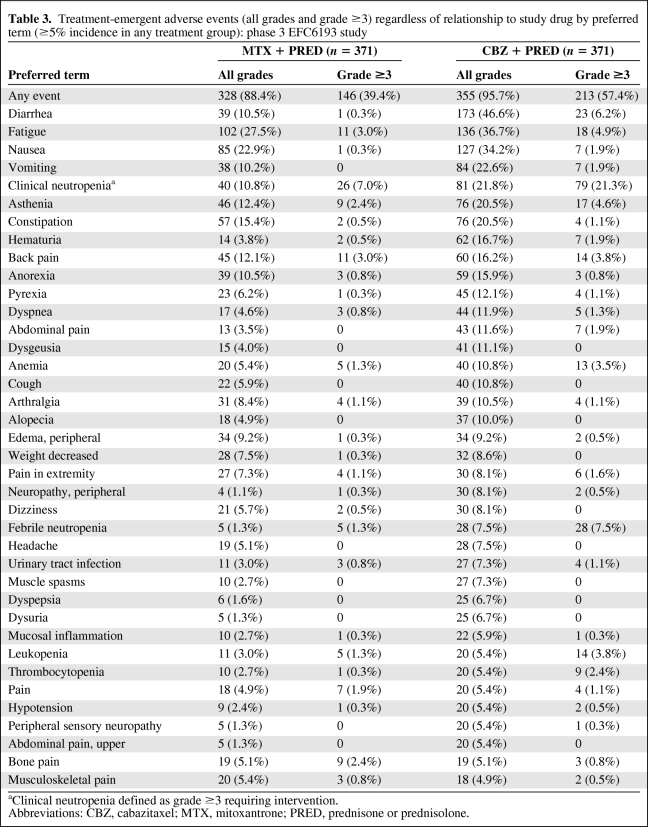
In the EFC6193 pivotal study, grade ≥3 treatment-emergent adverse events occurred in 57.4% of patients in the cabazitaxel group and 39.4% of patients in the mitoxantrone group (Table 3). The most commonly reported treatment-emergent adverse events of all grades were diarrhea (46.6%), fatigue (36.7%), nausea (34.2%), vomiting (22.6%), and neutropenia (21.8%). The most commonly (≥3%) reported grade ≥3 treatment-emergent adverse events were neutropenia (21.3%), febrile neutropenia (7.5%), diarrhea (6.2%), fatigue (4.9%), asthenia (4.6%), back pain (3.8%), leukopenia (3.8%), and anemia (3.5%).
Table 3.
Treatment-emergent adverse events (all grades and grade ≥3) regardless of relationship to study drug by preferred term (≥5% incidence in any treatment group): phase 3 EFC6193 study
aClinical neutropenia defined as grade ≥3 requiring intervention.
Abbreviations: CBZ, cabazitaxel; MTX, mitoxantrone; PRED, prednisone or prednisolone.
Neutropenia was the most common adverse reaction leading to treatment discontinuation (2.4%). Neutropenic complications included neutropenic infections (0.5%), neutropenic sepsis (0.8%), and septic shock (1.1%), which in some cases resulted in a fatal outcome. The dose should be reduced in cases of febrile neutropenia or prolonged neutropenia despite appropriate treatment. Patients should be retreated only when neutrophils recover to a level ≥1,500/mm3.
The use of G-CSF has been shown to limit the incidence and severity of neutropenia. Primary prophylaxis with G-CSF should be considered in patients with high-risk clinical features (age >65 years, poor PS, previous episodes of febrile neutropenia, extensive prior radiation ports, poor nutritional status, and other serious comorbidities) that predispose them to greater complications from prolonged neutropenia.
Hypersensitivity reactions have been reported in patients treated with cabazitaxel. A causal association with any of the excipients or the active substance has not been established. Premedication including an antihistamine, a corticosteroid, and an H2 antagonist should be performed to mitigate the risk for and severity of hypersensitivity. Patients should be observed closely for hypersensitivity reactions, especially during the first and second infusions.
Among the 371 patients treated with cabazitaxel in the prostate cancer study, 240 patients were aged ≥65 years, including 70 patients aged >75 years. The following adverse reactions were reported at rates ≥5% higher in patients aged ≥65 years than in younger patients: fatigue (40.4% versus 29.8%), clinical neutropenia (24.2% versus 17.6%), asthenia (23.8% versus 14.5%), pyrexia (14.6% versus 7.6%), dizziness (10.0% versus 4.6%), urinary tract infection (9.6% versus 3.1%), and dehydration (6.7% versus 1.5%). The incidences of the following grade ≥3 adverse reactions were higher in patients aged ≥65 years than in younger patients: neutropenia based on laboratory abnormalities (86.3% versus 73.3%), clinical neutropenia (23.8% versus 16.8%), and febrile neutropenia (8.3% versus 6.1%).
In study EFC6193, the percentage of patients who died from treatment-emergent adverse events (other than progressive disease) within 30 days of their last infusion was 4.9% in the cabazitaxel group, compared with 1.9% in the mitoxantrone group. Of the 18 patients who died in the cabazitaxel group, seven of these deaths were attributed to neutropenia and its consequences and five deaths were a result of cardiac events.
Benefit–Risk Assessment
Cabazitaxel plus prednisone was associated with a 2.4-month longer median OS duration than with mitoxantrone plus prednisone. Secondary endpoints, such as the progression-free survival (PFS) interval, tumor response rate, and tumor progression were consistent with the primary endpoint. In secondary analyses, a median PFS difference of 1.4 months in favor of cabazitaxel plus prednisone was also observed. Cabazitaxel plus prednisone was also associated with a higher response rate and longer time to PSA progression. However, these parameters have inherent limitations as a result of, among other things, ascertainment bias, interobserver variability, and the fact that no independent review of the PFS assessment was carried out.
There was some uncertainty over the efficacy in patients who had received <225 mg/m2 of docetaxel. Previous treatment with a <225 mg/m2 cumulative dose of docetaxel was introduced as an exclusion criterion after protocol amendment. A subgroup of 59 patients had received a prior cumulative dose of docetaxel <225 mg/m2 (29 patients in the cabazitaxel arm and 30 patients in the mitoxantrone arm). There was no significant difference in the OS time between study arms in this subgroup of patients (hazard ratio, 0.96; 95% confidence interval, 0.49–1.86). This observation may be a result of lower efficacy in this subgroup because of different patient or disease characteristics. However, the low number of patients in this subgroup analysis may also explain the lack of a clear effect.
Neutropenia was the most common adverse reaction with cabazitaxel. In the pivotal trial, cabazitaxel at the dose tested caused life-threatening neutropenia. Investigators were advised to strictly follow the protocol regarding dose delays and modifications and to treat neutropenia in accordance with the American Society of Clinical Oncology guidelines. This appeared to limit the incidence and severity of neutropenia. Monitoring of CBCs is essential on a weekly basis during cycle 1 and before each treatment cycle thereafter so that the dose can be adjusted if needed. No specific dose adjustment recommendation was considered in elderly patients because no age effect on the pharmacokinetics of cabazitaxel was observed, although these patients may be more likely to experience certain adverse reactions.
The numbers of patients with any treatment-emergent adverse event, serious treatment-emergent adverse event, or grade ≥3 treatment-emergent adverse event and withdrawals resulting from any treatment-emergent adverse event were significantly higher in the cabazitaxel group than in the mitoxantrone group. In study EFC6193, the percentages of patients who died from treatment-emergent adverse events (other than progressive disease) within 30 days of their last infusion were 4.9% in the cabazitaxel group and 1.9% in the mitoxantrone group. The hematological toxicity was also higher with cabazitaxel plus prednisone than with mitoxantrone plus prednisone. Even with prophylactic or therapeutic G-CSF, cabazitaxel was associated with a higher rate of neutropenia leading to infections and sepsis.
Fatigue, clinical neutropenia, asthenia, pyrexia, dizziness, urinary tract infection, and dehydration were reported at rates ≥5% higher in patients aged ≥65 years than in younger patients. Currently, there are no specific dose recommendations for elderly patients. It is unclear whether or not the <25-mg/m2 dose would have activity similar to that of the ≥25-mg/m2 dose but with a more acceptable side-effect profile. The company has submitted the synopsis of a randomized, open-label study comparing a 20-mg/m2 dose of cabazitaxel with a 25-mg/m2 dose in second-line mHRPC patients.
The use of cabazitaxel should be confined to units specialized in the administration of cytotoxics, and it should only be administered under the supervision of a physician experienced in the use of anticancer chemotherapy.
The CHMP concluded that there was a clear benefit in terms of the OS time associated with cabazitaxel and prednisone. The effect in terms of the OS duration is similar to what has been observed with other late-line cancer therapies, for which dramatic effects in terms of OS times are rare because of the advanced stage of the disease. Because of the poor prognosis, high unmet clinical need, and lack of alternative therapies, the observed OS benefit was considered to outweigh the risks. Therefore, on March 14, 2011 the European Commission granted marketing authorization valid throughout the European Union for cabazitaxel. The EMA will review new information about cabazitaxel on a regular basis. Up-to-date information on this medicinal product is available on the EMA Web site (http://www.ema.europa.eu).
Acknowledgments
The scientific assessment as summarized in this report is based on the marketing authorization application submitted by the applicant company and on important contributions from, among others, the rapporteur and corapporteur assessment teams, CHMP members, and additional experts.
This publication is a summary of the European Public Assessment Report (EPAR) available in the public domain, together with the summary of product characteristics (SmPC), and other product information on the EMA website (http://www.ema.europa.eu). The authors remain solely responsible for the opinions expressed therein.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Data analysis and interpretation: Francesco Pignatti, Eric Abadie, Terry Shepard, David Brown, Daniel O'Connor, Robert James Hemmings, Pierre Demolis, Alexandre Moreau
Manuscript writing: Elias Pean
References
- 1.Tannock I, Gospodarowicz M, Meakin W, et al. Treatment of metastatic prostatic cancer with low-dose prednisone: Evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol. 1989;7:590–597. doi: 10.1200/JCO.1989.7.5.590. [DOI] [PubMed] [Google Scholar]
- 2.Osoba D, Tannock IF, Ernst DS, et al. Health-related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and prednisone. J Clin Oncol. 1999;17:1654–1663. doi: 10.1200/JCO.1999.17.6.1654. [DOI] [PubMed] [Google Scholar]
- 3.Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: Results of the Cancer and Leukemia Group B 9182 study. J Clin Oncol. 1999;17:2506–2513. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 4.Colloca G, Venturino A, Checcaglini F. Patient-reported outcomes after cytotoxic chemotherapy in metastatic castration-resistant prostate cancer: A systematic review. Cancer Treat Rev. 2010;36:501–506. doi: 10.1016/j.ctrv.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 6.Villanueva C, Awada A, Campone M, et al. A multicentre dose-escalating study of cabazitaxel (XRP6258) in combination with capecitabine in patients with metastatic breast cancer progressing after anthracycline and taxane treatment: A phase I/II study. Eur J Cancer. 2011;47:1037–1045. doi: 10.1016/j.ejca.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Mita AC, Denis LJ, Rowinsky EK, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:723–730. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 8.Pivot X, Koralewski P, Hidalgo JL, et al. A multicenter phase II study of XRP6258 administered as a 1-h i.v. infusion every 3 weeks in taxane-resistant metastatic breast cancer patients. Ann Oncol. 2008;19:1547–1552. doi: 10.1093/annonc/mdn171. [DOI] [PubMed] [Google Scholar]