Abstract
Background & objectives:
Considerable efforts are being made to develop new, more effective drugs for osteoporosis, including novel forms of bisphosphonates. The present study was carried out to compare the effect of a novel agent glucosamine alendronate (GA) alone and is combination with dihydroquercetin (DHQ) against the effect of a known drug alendronate (ALN) in the senescence-accelerated OXYS rats as model of osteoporosis.
Methods:
Male OXYS and Wistar (control) rats were randomized across four experimental groups (n=15), which received 1.26 mg GA, 0.84 mg ALN, or 1.26 mg GA + 5.06 mg DHQ per kg of body weight. Untreated rats were used as control groups. At the end of treatment, the bone mineral density (BMD), bone biomechanical properties, and the levels of serum osteocalcin, αC-terminal crosslinked telopeptides of type I collagen (α-CTx) and calcium were measured.
Results:
All treatments increased BMD in rats of both strains, but the improvement was more pronounced in OXYS rats: GA+DHQ increased both the strength of the femur by 20 per cent (P<0.01) and BMD by 7.6 per cent (P<0.023). GA+DHQ and ALN reduced serum α-CTx in OXYS rats. Only GA increased the level of osteocalcin in OXYS rats (P<0.05). ALN increased the cross-sectional area of the femur by 9 per cent (P<0.04) in OXYS and by 12 per cent (P<0.05) in Wistar rats.
Interpretation & conclusions:
The combined treatment with GA+DHQ appears to be more effective at maintaining strength of the femur and BMD in OXYS rats, when compared to the individual drugs GA and ALN.
Keywords: Alendronate, glucosamine, osteoporosis, OXYS rats, strength of bone
Osteoporosis generally develops without symptoms and has a progressive course, with an increasing risk of fractures, at which point it requires medical treatment. Timely diagnosis and the selection of optimal treatment at the appropriate stages of the disease are important for effective management of osteoporosis.
Bisphosphonates (BPs) currently are the best-validated class of antiresorptive agents used in the treatment of metabolic bone diseases, including osteogenesis imperfecta and osteoporosis1,2. These drugs cause a higher bone density increase compared to other antiresorptive drugs such as alfacalcidol or raloxifene3,4. Owing to their high affinity for the bone matrix, these drugs are widely prescribed in medical conditions associated with increased bone resorption. Bisphosphonates are preferentially incorporated into sites of active bone remodeling, as commonly occurs in conditions characterized by accelerated skeletal turnover. Bisphosphonates inhibit osteoclastic bone resorption, and act on mature osteoclasts by inhibiting their attachment to the bone surface5 as well as the formation the ruffled border6. These drugs also inhibit growth of osteoclast precursor cells7. The mechanism of action depends on the presence of one or more amine groups. Nitrogen-containing BPs (pamidronate, alendronate, and risedronate) have a stronger antiresorptive effect. Alendronate (ALN) is one of the oldest and most widely used aminobisphosphonates8.
The therapeutic use of a combination of drugs seems to be promising because, in some cases, it can increase the effectiveness of treatment9,10 and is now the subject of extensive investigation. Dihydroquercetin (DHQ), also known as taxifolin, is a flavonoid from the wood of the larch (Larix sibirica Lebed.) and has strong antioxidant properties12. DHQ activates the formation of collagen type 1 fibers12, which are an important component of the bone structure. Thus, it would be interesting to estimate the therapeutic benefit antiresorptive agents in combination with DHQ in the treatment of osteoporosis.
The correct assessment of the effects of bisphosphonates in humans is difficult due to many factors, e.g., age-dependent effects of these drugs, financial costs of randomized controlled trials, ethical issues, as well as quality of life, individual differences in nutrition and age-related changes in bone density. Among the animal models, the laboratory rodents are the most convenient animals osteoporosis researchers13. However, there are only a few examples of genetic rodent models of osteoporosis, such as the murine model with inherited features of premature ageing, senescence-accelerated mouse (SAM)14. It has been shown that the senescence-accelerated mouse strain P6 (SAMP6) is a good model of senile osteoporosis since it has many features of senile human osteoporosis15. Over the last few years, a large amount of experimental data accumulated which demonstrated that the accelerated senescent OXYS rats are suitable model of osteoporosis16–19. The main diagnostic criterion for osteoporosis, i.e., decrease in bone mineral density is recorded at the age of 6 months in OXYS rats. These features make it possible to use OXYS rats for evaluating the efficacy of osteoporosis treatments.
The purpose of the present study was thus to compare the effects of glucosamine alendronate (GA), alone or in combination with DHQ against the effects of ALN, a commonly prescribed aminobisphosphonate, on bone tissue in Wistar and OXYS rats.
Material & Methods
Experimental design: The study was conducted in the Institute of Cytology and Genetics of the Siberian Branch of Russian Academy of Sciences, Russia Federation, Novosibirsk, included male OXYS (60) and Wistar as control (60) rats obtained from the Experimental Animal Facility (Institute of Cytology and Genetics, Novosibirsk, Russia). Rats were housed five in each cage (57×36×20 cm) and kept under standard laboratory conditions (at 22±2°C, 60% relative humidity, and natural light), provided with a standard rodent feed, PK-120-1, Ltd. (Laboratorsnab, Russia), and given water without restrictions.
GA and ALN were synthesized at the Mendeleyev University of Chemical Technology of Russia from alendronic acid and glucosamine in water. DHQ 98 per cent was procured from (DZOD Marker of Eco-friendly Equipment and Nutrition OJSC, Russia, Moscow). To study of drugs action, 8-month old male OXYS and Wistar rats were randomly assigned to one of the four groups separately (n=15 rats/group): (1) GA 1.26 mg, (2) ALN 0.84 mg, (3) GA 1.26 mg + DHQ 5.06 mg per kilogram of body weight per day, and (4) no-treatment control. The weight gain was measured in the course of the experiment.
The dosing regimen was carried out for two month. Two weeks after the end of treatment bone mineral density (BMD) was measured in all animals (under easy narcosis) and blood samples were collected. The preparation of the samples of femur took place after the rats were euthanized.
Bone density measurement: BMD (including both cortical and cancellous bone compartments) of lumbar vertebral bodies, femur and humerus of rats were measured by dual energy X-ray absorptiometry (DEXA) on a Hologic Discovery-A QDR Bone Densitometer (USA) with software adapted to small animals (g/cm2).
Serum assays: Blood cells were pelleted by centrifugation (4°C) at 1500 × g for 20 min and the serum was stored at -70°C until the analysis. Serum calcium was determined by an automatic chemistry analyzer (Biolis 24 i) (Tokyo Boeki Medical System, Japan). The αC-terminal crosslinked telopeptides of type I collagen (RatLaps™ EIA, Immunodiagnostic Systems, UK) and osteocalcin (Rat Gla-OC Competitive EIA Kit Manual, Takara Bio. Inc., Japan) were measured on Multiskan EX (ThermoLabsystems, Finland).
Biomechanical bone strength testing: Femurs were cleaned of soft tissue and stored at -20°C until biomechanical strength testing was performed. The samples of bone tissue had a length of 8 mm including both cortical and cancellous bone compartments. Mechanical testing to assess bone strength of the femoral distal metaphysis was carried out in all groups of rats. The cross-sectional area of the femur was measured using software Scion Image 4.0.2 (Scion Corp., USA). Femurs were placed under a gradually increasing load until fracture (ZWICK TC-FR100TL.A4K, Germany). This apparatus is equipped with a 100 N load cell. The mechanical strength of the femur was measured by applying a vertical load to the femur at a deformation rate of 0.1 mm/min. The load was measured while the load cell was connected to a computer via an amplifier and the load-deformation curves were recorded online. Maximum force (H) - Fmax (the force applied at the failure point) was obtained directly from the force-deformation curves. Maximum stress - strength - was found as the maximum force divided by the cross-sectional area of the femur (mm2). Young's modulus (E) was found as the maximum slope of the force-deformation curve multiplied by the specimen length (8 mm) and divided by the cross-sectional area of the femur. The Young's modulus is the maximum slope of the stress-strain curve.
Statistical methods: The data were analyzed using repeated measures ANOVA and non parametric tests with the statistical package Statistica 6.0, StatSoft (USA). Two-way ANOVA was used to evaluate the differences between OXYS and Wistar rats across different ages (age × genotype) as well as to evaluate effects of treatment (bisphosphonates and genotype). One-way ANOVA was used for individual group comparisons. Comparisons between means were analyzed correlation analysis, as appropriate.
Results
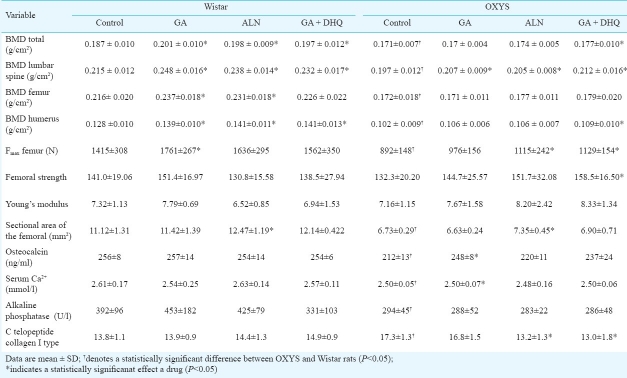
The total BMD in the control group (group 4) of OXYS rats was 8.6 per cent lower than that in control Wistar rats (P<0.001). The response to treatment was plotted in a graph for both Wistar and OXYS rats. All three pharmacological interventions significantly increased total BMD in Wistar rats compared to the control group: GA increased it by 7.5 per cent (P<0.001), ALN by 5.9 per cent (P<0.001) and GA+DHQ by 5 per cent (P<0.01) (Table). Lumbar spine BMD was increased in the GA+DHQ group by 8 per cent (P<0.001), in the ALN group by 10.7 per cent (P<0.001) and in the GA group by 15 per cent (P<0.004). BMD in the femur was increased in the GA group (10%, P<0.006) and in the ALN group (7%, P<0.04). Compared to baseline, BMD in the humerus increased significantly in all three treatment groups (P<0.006, P<0.003, and P<0.004, respectively).
Table.
Bone mineral density (BMD), femur area, and biomechanical strength properties at the femur, serum Ca2+ and ALP after treatment with GA, ALN, or GA with DHQ in OXYS and Wistar rats (n=15/group)
The increases of BMD were different in pairwise comparisons of the treatment groups between Wistar and OXYS rats. Total BMD was increased only in the GA+DHQ group of OXYS rats by 3.5 per cent (P<0.062) and humerus BMD by 20 per cent (P<0.034) compared to controls. All drug formulations increased lumbar spine BMD in OXYS rats compared to the control group: in the GA+DHQ group it was increased by 7.6 per cent (P<0.01), in the GA group by 5 per cent (P<0.05), and the ALN group by 4 per cent (P<0.01). No changes were seen in BMD of the femur. Total BMD correlated significantly with serum osteocalcin (r=0.39; P=0.045), with Fmax (r=0.59; P=0.001), and with the cross-sectional area of the femur (r=0.59; P=0.001) in all rats.
The Fmax of femur in the rats was dependent on genotype (P<0.001) and, in Wistar rats, it was 50 per cent higher than in OXYS rats (P<0.0005). As shown in the Table, the Fmax was increased in the ALN and GA+DHQ groups of OXYS rats (P<0.04 and P<0.006, respectively) and in the GA group of Wistar rats (P<0.02).
The cross-sectional area of the femur in control rats was dependent on genotype and, in Wistar rats, was higher by 70 per cent compared to OXYS rats (P<0.001). In groups treated with ALN, the cross-sectional area of the femur was increased by 12 per cent (P<0.05) in Wistar rats and by 9 per cent (P<0.004) in the OXYS strain (Table). ANOVA did not show significant differences in femoral strength between Wistar and OXYS rats. A strong positive correlation was found between Fmax and bone strength in both Wistar (r=0.73; P=0.001) and OXYS rats (r=0.91; P=0.001). None of the three drug formulations changed femur strength in Wistar rats, but the GA+DHQ treatment increased femur strength in OXYS rats (P<0.001).
Serum αC-terminal crosslinked telopeptides of type I collagen (α-CTx), a marker of bone resorption, in the control OXYS rats was significant higher than in Wistar (P<0.05). After the treatment this level decreased, but only in rats OXYS and only in ALP and GA+DHQ groups (P<0.05).
Serum osteocalcin was influenced by genotype (P<0.026), but not the drug treatments. Osteocalcin in control groups was lower in OXYS rats compared to the Wistar strain (P<0.01). The treatment with GA increased the level of osteocalcin only in OXYS rats (P<0.05).
The serum Ca2+ in OXYS rats was significantly lower than in Wistar rats (P<0.002). None of the drugs affected serum Ca2+ in Wistar rats. The increase of Ca2+ was found only in the GA group of OXYS rats (P<0.01). This increase eliminated the difference in serum Ca2+ level between this group of OXYS rats and the control Wistar rats.
Discussion
The main goal of the present study was to compare the effects of a novel pharmacological agent, GA, alone and in combination with DHQ against the effects of the current standard of care in osteoporosis, ALN, on bone tissue in Wistar and OXYS rats. We showed that the effects of these drugs on physiological bone remodeling were different in the senescent-accelerated OXYS rats and in the control strain, Wistar rats. These differences may be associated with some pathological features of bone tissue in OXYS rats and with differences in its quality between the two strains.
The criteria of the effectiveness of treatment and prevention of osteoporosis include an increase and/or stabilization of BMD. This depends on the ability of drugs to suppress bone resorption. As a comparison drug, ALN was used which is widely prescribed for osteoporosis in both men and post-menopausal women20–22. The efficacy of ALN has been investigated in various studies23, including in animal models24,25. Our data showed that ALN increased BMD, but its effect on bone tissue was dependent on genotype: the drug increased total and regional BMD in Wistar rats, but in OXYS rats, it significantly increased BMD only in the lumbar spine. Probably, this is due to the differences in bone formation and development between the two strains. In OXYS rats, the growth of BMD ends at the age of 6 months, while in control rats, it continues until the age of 12 months19. The accrual of low peak bone mass during childhood and adolescence is a major determinant of the risk of osteoporosis. We have shown previously that OXYS rats develop abnormally low peak bone mass and BMD19.
All pharmacological treatments improved total BMD as well as BMD in some regions in Wistar rats. In contrast, only the GA+DHQ increased the total BMD in OXYS rats. ALN, a proven treatment for osteoporosis, did not produce any significant improvement in total BMD in OXYS rats. Importantly, the lumbar spine BMD increased in all treatment groups of Wistar and OXYS rats. Changes in BMD with therapy are a rather weak indicator for bone strength; other factors, such as changes in bone turnover, may be a more robust predictor of fracture risk following anti-resorptive therapy.
Serum Ca2+ was not different between the treatment and control groups in Wistar rats, but in OXYS rats, GA increased the level of serum Ca2+. BMD in the femur of Wistar rats was dependent on the level of serum Ca2+, while in OXYS rats, no such correlation was seen. Moreover, the level of serum Ca2+ in OXYS rats was negatively correlated with the bone Fmax (r=-0.35; P=0.05) and strength (r=- 0.36; P=0.05).
Treatments for osteoporosis are designed to reduce fracture risk by improving bone strength, but the mechanical strength of long bones cannot be assessed in clinical studies. Measurements of BMD during an anti-resorptive therapy cannot serve as a reliable predictor of bone strength25. BMD is a major parameter influencing bone strength, but organization of the trabeculae, the geometry of bones, and the properties of the matrix (mineral and collagen) also contribute to bone strength26. The increase of BMD of the femur did not affect the bone strength in Wistar rats and only treatment with GA produced a significant increase of the Fmax (the force applied at the failure point). It should be noted that at the age of 12 months the Fmax in control Wistar rats was 50 per cent higher than in control OXYS rats. However, this difference was dependent on the cross-sectional area of the femur, which was also higher in Wistar rats (by 70%).
Both the treatment with GA and with ALN increased the Emax of the femur in OXYS rats, but only the combination treatment was effective at increasing bone strength.
ALN, a widely prescribed drug for the prevention of fractures in osteoporosis, had a weak effect on mechanical strength of bone compared to the GA+DHQ combination. ALN increased the cross-sectional area of the femur in both Wistar and OXYS rats, but only in Wistar rats was this accompanied by an increase of femur's BMD. We can hypothesize that this may be due to disturbances of the mineralization in the senescence-accelerated OXYS strain.
In OXYS rats, the combination therapy with GA+DHQ had a greater effect on mechanical strength of femur compared to ALN or GA alone. Further studies will be necessary to elucidate the possible mechanism underlying the effects of DHQ on bone structure. The bioavailability of alendronate can be increased by its combination with glucosamine, which is utilized for biosynthesis of glycoproteins and glycosaminoglycans. DHQ is a flavonoid and a structural analogue of quercetin, but quercetin and DHQ may have different biological properties27. It is known that in vitro, quercetin enhances formation of osteoprogenitor cells in the bone marrow, stimulates osteoblast function by stimulating osteoblast differentiation and mineralization, while it suppresses osteoclast function by inhibiting differentiation of osteoclast precursor cells and osteoclast apoptosis28–30 and by inhibiting osteoclast-induced cytokine production by osteoblasts31. It is not known if DHQ has similar effects, although it has been shown to stimulate fibril formation and promote stabilization of fibrillar forms of collagen type 1 in vitro12. Changes in bone collagen maturation, which is not detected by measurements of BMD, may also be involved in bone strength25. Our results suggest that this may be the case in OXYS rats. As a consequence, the drug combination acts on both collagen structure and bone resorption and thereby may reduce non-vertebral, e.g., hip fractures, which are the most clinically relevant type of fracture in the elderly.
In conclusion, our results showed that the combined treatment with GA+ DHQ was more effective at maintaining strength of the femur and BMD in OXYS rats, when compared to the individual drugs GA and ALN.
Acknowledgment
This work was supported by grants from RFBR 08-04-00722.
References
- 1.Castillo H, Samson-Fang L. American Academy for Cerebral Palsy and Developmental Medicine Treatment Outcomes Committee Review Panel.Effects of bisphosphonates in children with osteogenesis imperfecta: an AACPDM systematic review. Dev Med Child Neurol. 2009;51:17–29. doi: 10.1111/j.1469-8749.2008.03222.x. [DOI] [PubMed] [Google Scholar]
- 2.Russell RGG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–59. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 3.Iwamoto J, Sato Y, Uzawa M, Takeda T, Matsumoto H. Comparison of effects of alendronate and raloxifene on lumbar bone mineral density, bone turnover, and lipid metabolism in elderly women with osteoporosis. Yonsei Med J Feb. 2008;49:119–28. doi: 10.3349/ymj.2008.49.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Iwamoto J, Sato Y, Uzawa M, Takeda T, Matsumoto H. Comparison of the effects of alendronate and alfacalcidol on hip bone mineral density and bone turnover in Japanese men having osteoporosis or osteopenia with clinical risk factors for fractures. Yonsei Med J Aug. 2009;50:474–81. doi: 10.3349/ymj.2009.50.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Colucci S, Minielli V, Zambonin G, Cirulli N, Mori G, Serra M, et al. Alendronate reduces adhesion of human osteoclast-like cells to bone and bone protein-coated surfaces. Calcif Tissue Int. 1998;63:230–5. doi: 10.1007/s002239900519. [DOI] [PubMed] [Google Scholar]
- 6.Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, et al. Bisphosphonate action.Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88:2095–105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Amelio P, Grimaldi A, Isaia GC. Alendronate reduces osteoclast precursors in osteoporosis. Osteoporos Int. 2010;21:1741–50. doi: 10.1007/s00198-009-1129-1. [DOI] [PubMed] [Google Scholar]
- 8.Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev Jan. 2008;(1):CD001155. doi: 10.1002/14651858.CD001155.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki H, Miyakoshi N, Kasukawa Y, Maekawa S, Noguchi H, Kamo K, et al. Effects of combination treatment with alendronate and vitamin K(2) on bone mineral density and strength in ovariectomized mice. J Bone Miner Metab. 2010;28:403–9. doi: 10.1007/s00774-009-0148-5. [DOI] [PubMed] [Google Scholar]
- 10.Ringe JD. Combination treatment in osteoporosis.Basic treatment plus specific osteoporosis medication. Med Monatsschr Pharm. 2009;32:137–40. [PubMed] [Google Scholar]
- 11.Vladimirov YA, Proskurnina EV, Demin EM, Matveeva NS, Lubitskiy OB, Novikov AA, et al. Dihydroquercetin (taxifolin) and other flavonoids as inhibitors of free radical formation at key stages of apoptosis. Biochemistry (Mosc) 2009;74:301–7. doi: 10.1134/s0006297909030092. [DOI] [PubMed] [Google Scholar]
- 12.Tarahovsky YS, Selezneva II, Vasilieva NA, Egorochkin MA, Kim YA. Acceleration of fibril formation and thermal stabilization of collagen fibrils in the presence of taxifolin (dihydroquercetin) Bull Exp Biol Med. 2007;144:791–4. doi: 10.1007/s10517-007-0433-z. [DOI] [PubMed] [Google Scholar]
- 13.Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA, et al. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58:424–30. [PMC free article] [PubMed] [Google Scholar]
- 14.Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of senescence. Exp Gerontol. 1997;32:105–9. doi: 10.1016/s0531-5565(96)00036-8. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Zhou X, Emura S, Shoumura S. Site-specific bone loss in senescence-accelerated mouse (SAMP6): a murine model for senile osteoporosis. Exp Gerontol. 2009;44:792–8. doi: 10.1016/j.exger.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Kolosova NG, Kutorgin GD, Safina AF. Bone mineralization in senescence-accelerated OXYS rats. Bull Exp Biol Med. 2002;133:171–4. doi: 10.1023/a:1015507107763. [DOI] [PubMed] [Google Scholar]
- 17.Gonchar A, Kolmogorov U, Gladkih E, Shuvaeva O, Beisel N, Kolosova N. The estimation of the possibilities of synchrotron radiation X-ray fluoresent analysis and atomic spectrometry for the bone's elemental composition determination. Nucl Instr Meth Phys Res A. 2005;543:271–3. [Google Scholar]
- 18.Falameeva OV, Sadovoi OV, Khrapova YuV Kolosova NG. (2006) Structural and functional changes in bone tissue of the spine and limbs in rats OXYS. Khirurgiya Pozvonochnika. 2006;1:88–94. [Google Scholar]
- 19.Muraleva NA, Sadovoi MA, Kolosova NA. Features of development osteoporosis in accelerated senescent OXYS rats. Adv Gerontol. 2010;23:233–43. [PubMed] [Google Scholar]
- 20.Adami S, Prizzi R, Colapietro F. Alendronate for the treatment of osteoporosis in men. Calcif Tissue Int. 2001;69:239–41. doi: 10.1007/s00223-001-1066-2. [DOI] [PubMed] [Google Scholar]
- 21.Lopes R, Coeli CM, Vaisman M, de Farias MLF. Additional beneficial effects of recombinant growth hormone in alendronate-treated patients with idiopathic osteoporosis. Endocrine Journal. 2009;56:851–8. doi: 10.1507/endocrj.k09e-048. [DOI] [PubMed] [Google Scholar]
- 22.Olszynski WP, Davison KS, Ioannidis G, Brown JP, Hanley DA, Josse RG, et al. Effectiveness of alendronate and etidronate in the treatment of osteoporosis in men: a prospective observational study. Osteoporos Int. 2006;17:217–41. doi: 10.1007/s00198-005-1965-6. [DOI] [PubMed] [Google Scholar]
- 23.Abbaspour A, Takahashi M, Sairyo K, Takata S, Yukata K, Inui A, et al. Optimal increase in bone mass by continuous local infusion of alendronate during distraction osteogenesis in rabbits. Bone. 2009;44:917–23. doi: 10.1016/j.bone.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, JiangY , Genant HK. Preclinical studies of alendronate. J Muscul Res. 1991;3:209–16. [Google Scholar]
- 25.Leeming DJ, Henriksen K, Byrjalsen I, Qvist P, Madsen SH, Garnero P, et al. Is bone quality associated with collagen age? Osteoporos Int. 2009;20:1461–70. doi: 10.1007/s00198-009-0904-3. [DOI] [PubMed] [Google Scholar]
- 26.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17:319–36. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Deuster P. Comparison of quercetin and dihydroquercetin: antioxidant-independent actions on erythrocyte and platelet membrane. Chem Biol Interact. 2009;182:7–12. doi: 10.1016/j.cbi.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Wattel A, Kamel S, Mentaverri R, Lorget F, Prouillet C, Petit JP, et al. Potent inhibitory effect of naturally occurring flavonoids quercetin and kaempferol on in vitro osteoclastic bone resorption. Biochem Pharmacol. 2003;65:35–42. doi: 10.1016/s0006-2952(02)01445-4. [DOI] [PubMed] [Google Scholar]
- 29.Wattel A, Kamel S, Prouillet C, Petit JP, Lorget F, Offord E, et al. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF kappa B and AP-1. J Cell Biochem. 2004;92:285–95. doi: 10.1002/jcb.20071. [DOI] [PubMed] [Google Scholar]
- 30.Woo JT, Nakagawa H, Notoya M, Yonezawa T, Udagawa N, Lee IS. Quercetin suppresses bone resorption by inhibiting the differentiation and activation of osteoclasts. Biol Pharm Bull. 2004;27:504–9. doi: 10.1248/bpb.27.504. [DOI] [PubMed] [Google Scholar]
- 31.Trivedi R, Kumar S, Kumar A, Siddiqui JA, Swarnkar G, Gupta V, et al. Kaempferol has osteogenic effect in ovariectomized adult Sprague-Dawley rats. Mol Cell Endocrinol. 2008;289:85–91. doi: 10.1016/j.mce.2008.02.027. [DOI] [PubMed] [Google Scholar]