Abstract
Background & objectives:
Coriandrum sativum (CS), has been widely used in traditional systems of medicine for treatment of rheumatoid arthritis. However, the mechanism of action for its antiarthritic effects is not clearly known. Therefore, the present study was carried out to evaluate the antiarthritic activity of CS in rats in two experimental models.
Methods:
The antiarthritic activity of CS seed hydroalcoholic extract (CSHE) was evaluated in adult Wistar rats by using two experimental models, viz. formaldehyde and Complete Freund's adjuvant (CFA) induced arthritis. The expression of pro-inflammatory cytokines (predominantly contributed by macrophages) was also evaluated. TNF-α level was estimated in serum by ELISA method. TNF-R1, IL-1 β and IL-6 expression in the synovium was analysed by immunohistochemistry.
Results:
CSHE produced a dose dependent inhibition of joint swelling as compared to control animals in both, formaldehyde and CFA induced arthritis. Although there was a dose dependent increase in serum TNF-α levels in the CSHE treated groups as compared to control, the synovial expression of macrophage derived pro-inflammatory cytokines/cytokine receptor was found to be lower in the CSHE treated groups as compared to control.
Interpretation & conclusions:
Our results demonstrate that the antiarthritic activity of CSHE may be attributed to the modulation of pro-inflammatory cytokines in the synovium. In further studies CSHE could be explored to be developed as a disease modifying agent in the treatment of RA.
Keywords: Adjuvant arthritis, Coriandrum sativum, formaldehyde induced arthritis, pro-inflammatory cytokines, TNF-alpha
Coriandrum sativum L. (Apiaceae) is a small, perennial herb widely distributed in India, Pakistan, West Asia, Mediterranean, and America. All parts of the plant are edible, but the fresh leaves and the dried seeds are commonly used in cooking. The chemical constituents of C. sativum seed (CS) are essential oil, sugars (glucose, fructose and sucrose), alkaloids, flavones, resins, tannins, anthraquinones, sterols (beta-sitosterol and beta-sitosteroline), and fixed oils1.
In the traditional systems of medicine, formulations containing CS seed extract have been used as stimulants, carminatives, antispasmodics, diuretic and anti-rheumatic2–4. CS seeds have been shown to reduce serum cholesterol levels in experimental hyperlipidaemia5 and have also been reported to be potent antioxidants in in vitro testing systems6. The anti-inflammatory activity of this plant extract has been demonstrated in carrageenan induced paw oedema in experimental animals7,8.
The anti-inflammatory activity of this plant has been evaluated in detail, but it is still not clear whether CS primarily contributes toward the antiarthritic activity or just acts as an adjuvant to the principal drug. This is because in addition to the articular and periarticular inflammation mediated by arachidonic acid derived autacoids, an immunological hyper-reactive state is maintained in the synovium that predominantly fuels this inflammatory process9,10. The inflammatory environment in the synovium is primarily conditioned by macrophage cytokines like tumour recrosis factor-α (TNF-α), interleukins (IL-6, IL-1), granulocyte macrophage-colong stimulating factor (GM-CSF), etc11. This is the reason why non steroidal anti-inflammatory drugs (NSAIDs), though effective in reducing the articular and periarticular inflammation, are less effective in attenuating disease progression. Therefore, the present study was carried out to scientifically evaluate the traditional use of CS in arthritis with the aim to elucidate the probable mechanism of antiarthritic action.
Material & Methods
Animals: Adult male Wistar albino rats (150-180 g) from the institutional breeding stock were used in the study. Animals were housed at 25±2°C in clean polypropylene cages in groups of three with access to food and water ad libitum throughout the duration of the study (17 days for formaldehyde induced arthritis and 28 days for complete Freund's Adjuvant induced arthritis). They were acclimatized for a during of 7 days before experimentation. The study protocol was approved by the Institutional Animal Ethics Committee. All India Institute of Medical Sciences (AIIMS), New Delhi.
Test drug: Dried seeds were obtained and authenticated by Professor Mohammad Ali, pharmacognosist and phytochemist at Jamia Hamdard University, New Delhi, India, as Coriandrum sativum L. (Apiaceac) and a voucher specimen (PRL/JH/08/04) was submitted at the herbarium for future reference. The seeds were coarsely ground and the powder was extracted by cold maceration with 50 per cent v/v methanol for 72 h. The slurry was filtered through cotton wool to separate the suspended particles. The solvent was evaporated under reduced pressure till a resinous extract was obtained. The total quantitative yield of extract was 13.86 per cent w/w. C. sativum hydroalcoholic extract (CSHE) was further subjected to pharmacognostical standardization for detection of secondary plant metabolites12 and was found to contain sugars, alkaloids, tannins, resins, flavonoids, fatty acids and sterols.
Formaldehyde induced arthritis: The experimental protocol and methodology of the present study are similar to our earlier report in which we had evaluated the antiarthritic activity of Terminalia chebula in experimental models13. Animals were divided into five groups (n=6) and the baseline ankle joint diameter was measured by a micrometer screw gauge on the day of the experiment. Group I received the vehicle (2ml/kg, 1% gum acacia) and served as the control. Group II received the standard drug indomethacin (3mg/kg body weight)13, groups III, IV and V received CSHE in doses of (8, 16 and 32 mg/kg body weight), respectively. Thirty minutes after oral administration of vehicle/drugs, arthritis was induced by subplantar administration of 0.1 ml formaldehyde (2% v/v) into the left hind paw of all the animals. This was designated as day 1. Vehicle/drug treatment was continued for the duration of 9 more days. Formaldehyde (0.1 ml 2% v/v) was again injected into the same paw on the third day14,15. Increase in joint diameter was measured on days 8, 9 and 10, 30 min after administration of the respective vehicle/drug treatment.
Complete Freund's adjuvant (CFA) induced arthritis: Animals were divided into five groups as mentioned under formaldehyde induced arthritis. Thirty minutes after oral administration of vehicle/drug, 0.1 ml of CFA (Difco, USA: 0.05% Mycobacterium butyricum in mineral oil) was injected into the subplantar surface of the left hind paw by a 26 gauge needle16. This was designated as day 0. Vehicle/drug treatment was continued for duration of 20 more days. Joint size measurements were carried out on days 3, 7, 14 and 21. On day 21, the animals were sacrificed and terminal blood collection was done. The serum was separated and TNF-α levels were estimated by capture ELISA (U-CyTech Biosciences, The Netherlands). The ankle joints were collected and stored at -80°C for histopathology and immunohistochemistry studies as described by us13.
Toxicity profile of CSHE: Evaluation of acute oral toxicity of CSHE was carried out according to the method described in Organization for Economic Cooperation and Development (OECD) guidelines for testing of chemicals – 42517. To reduce the number of animals, a limit test at 2000 mg/kg body weight was performed using five male Wistar rats (150-180 g). All the animals were observed for behavioural changes and mortality till 14 days after administration of the dose.
Evaluation of 28 day oral toxicity was carried out according to the method described in OECD guidelines for testing of chemicals – 40718. Sixteen male Wistar rats (150-180 g) were divided into two groups (n=8). Group I received the vehicle (2 ml/kg body weight, 1% gum acacia) and served as control and group II received CSHE in a dose of 160 mg/kg body weight (five times the maximum dose tested in anti-arthritic studies). Drug/vehicle administration was continued for the duration of 28 days.
Statistical analysis: Comparison between groups was done by One-way ANOVA followed by Dunnette's Multiple Comparison test, P<0.05 was considered significant.
Results
Effect of CSHE on joint swelling in formaldehyde induced arthritis: An increase in joint diameter was seen in all animals throughout the observation period (Fig. 1). Although all drug treated groups showed a decrease in joint swelling as compared to the control, the difference was significant only in Group V (CSHE 32 mg/kg) on all observation days. Groups II (indomethacin) and IV (CSHE 16 mg/kg) showed significant reduction in joint swelling only on days 9 and 10. CSHE at a dose of 8 mg/kg (Group III) produced a non significant reduction in joint swelling.
Fig. 1.
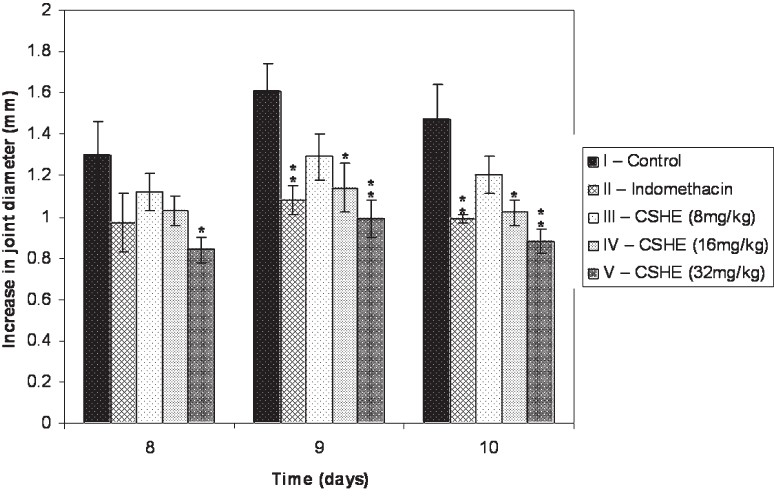
Effect of CSHE treatment on joint swelling in formaldehyde induced arthritis. CSHE = C. sativum hydro-alcoholic extract. All values are Mean±SE. Statistical analysis by One-way ANOVA followed by Dunnett's Multiple Comparison. P*<0.05; **<0.01 as compared to control.
Effect of CSHE on joint swelling in CFA induced arthritis: Administration of CFA produced an increase in the joint diameter of all the animals, which was persistent throughout the observation period (Fig. 2). Maximum joint swelling was observed on day 3, after which there was a gradual decrease except in the control and CSHE (8 mg/kg) treated groups, which showed a slight increase in joint diameter from Day 14 to day 21. At the highest dose (32 mg/kg) CSHE was more efficacious than indomethacin in reducing the joint swelling.
Fig. 2.
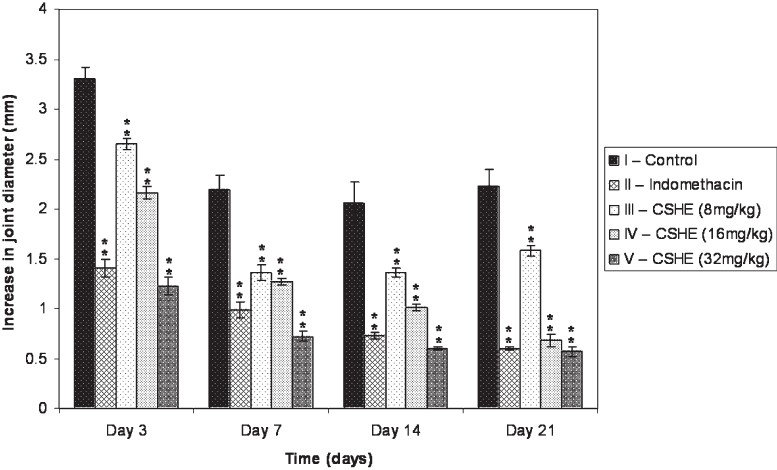
Effect of CSHE treatment on joint swelling in CFA induced arthritis. CSHE = C. sativum hydro-alcoholic extract. All values are Mean±SE. Statistical analysis by One-way ANOVA followed by Dunnett's Multiple Comparison. **P<0.01 as compared to control.
Effect of CSHE on serum TNF-α in CFA induced arthritis: Serum TNF-α level in normal rats was not in the detectable range with the ELISA kit used (data not shown). All the drug/vehicle treated animals showed an increase in serum TNF-α level as compared to normal animals. Indomethacin treated rats showed a significant >2.5 fold increase in the TNF-α levels (173.8± 6.68) as compared to the control (67.25±2.61). Similarly, CSHE produced a dose dependent increase in serum TNF- α levels (95.6±2.9, 130.6±5.6, 153.5±5.8) which was significant (P<0.05) as compared to control.
Immunohistochemistry of the synovial joint: Localization of large amounts of pro-inflammatory cytokines (IL-1β, IL-6) and TNF-R1 was seen in the synovial membrane of the control animals (Fig. 3). A reduction in the expression of these cytokines/cytokine receptor was observed in the CSHE (32 mg/kg) treated animals as compared to the control, whereas the expression in the indomethacin treated group was intermediate to that of control and CSHE treated groups. CSHE treatment reduced the expression of IL-1β to a greater extent as compared to IL-6 and TNF-R1 expression.
Fig. 3.
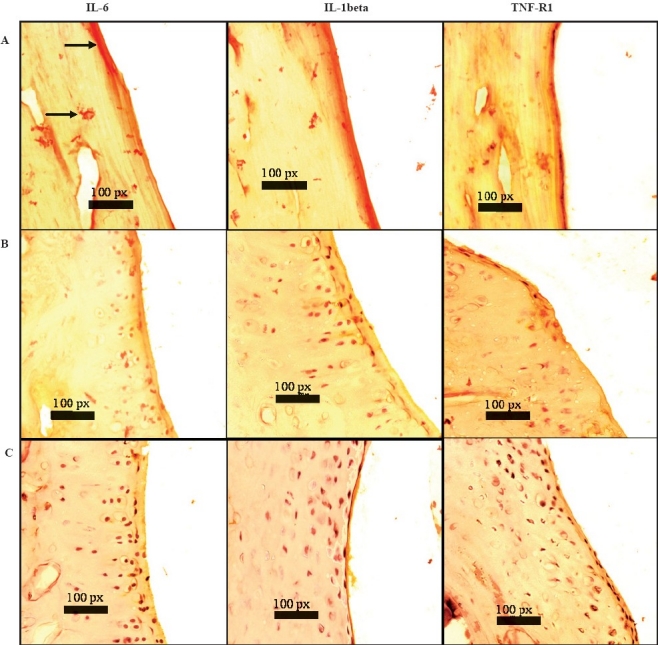
Effect of CSHE treatment on cytokine/cytokine receptor expression in rat synovial joint: Sections were 6 μm thick and photomicrographs were taken at 40X. CSHE = C. sativum hydro-alcoholic extract. A = control animal; B = indomethacin treated animal; C = CSHE (32 mg/kg) treated animal; Bold arrows = DAB staining (yellow-brown) of the synovial membrane depicting presence of respective cytokines.
Toxicity profile of CSHE: CSHE at a dose of 2000 mg/kg did not produce any behavioural abnormalities or mortality till 14 days after drug administration. As all the tested animals survived, the oral LD50 of CSHE was found to be >2000mg/kg body weight in rats.
Chronic administration of CSHE at a dose of 160mg/kg body weight for 28 days did not produce any overt physiological changes as compared to normal vehicle treated control (data not shown). Chronic administration of CSHE produced a statistically significant (P<0.05) increase in bleeding time and WBC count as compared to normal animals, but these changes were also within physiological limits. There was no change in percentage body weight of liver, heart, kidneys and spleen. All other parameters remained unaltered.
Discussion
In the present study, the antiarthritic activity of CSHE was evaluate by using two experimental models of arthritis, viz. formaldehyde and CFA induced arthritis. Formaldehyde induces arthritis by denaturing proteins at the site of administration, which leads to the development of an immunological reaction against the degraded products19. In this model, CSHE at the highest dose tested demonstrated good antiarthritic activity and was superior to 3 mg/kg indomethacin in reducing the joint swelling throughout the observation period. However, one of the limitations of this model is that it does not elicit the cell mediated immunity and is therefore, self limiting. Thus, to further confirm the activity of the test drug, its efficacy in reducing joint inflammation in CFA induced arthritis in rats was evaluated.
The CFA induced arthritis model is widely used for pharmacological evaluation of antiarthritic agents as it shares a number of clinical and immunological features with human arthritis16. Along with measurement of joint swelling, three key cytokines primarily secreted by macrophages, viz. TNF-α, IL-6 and IL-1β were also evaluated. These cytokines have been reported to be expressed at significant levels in chronic state of the disease11. The expression of TNF-R1 was also studied in the synovium as most of the pathophysiological effects of TNF-α are known to be mediated through this receptor subtype20,21.
There was a dose dependent decrease in joint swelling in the CSHE treated groups. However, in the lowest dose treated group, there was a slight increase in joint swelling from day 14 to 21. This increase was also seen in the control group and is believed to be produced due to the heightened immune response seen during the late phase of this model22,23. Serum TNF-α levels were found to be elevated from normal levels in all animals immunized with CFA, while expression of TNF-R1, IL-1β and IL-6 was found to be reduced in the synovial membrane of the drug treated animals. CSHE produced a greater inhibition of pro-inflammatory cytokine expression as compared to indomethacin. Even though an increase in TNF-α levels would generally produce an increase in disease activity, it can only be hypothesized that the decrease in TNF-R1 expression in the synovium could have accounted for the attenuation of joint damage in the treated groups as this receptor subtype is known to mediate most of the pathophysiological effects of TNF-α20,21.
Although it is difficult to attribute the activity of CSHE to a particular phytochemical present in it, we believe that steroidal compounds (primarily beta-sitosterol and beta-sitosterolin) present in high concentration in coriander seeds24 were responsible for the observed antiarthritic effect. We base this hypothesis on previously published studies in which beta-sitosterol and beta-sitosterolin have been evaluated in inflammation and arthritis. Beta-sitosterol has been shown to be effective in reducing phologistic agent induced paw oedema in experimental animals. Additionally, these sterols have also been shown to inhibit the secretion of pro-inflammatory cytokines including TNF-α by macrophages25–27 which might play an important role in the pathogenesis of RA11.
Results of toxicity studies with CSHE demonstrated its safety on chronic administration. Even though there were marginal changes in the physiological parameters of CSHE treated groups as compared to the normal animals, these changes were well within the normal physiological limits. In conclusion, CSHE significantly reduced the joint swelling and synovial expression of pro-inflammatory cytokines without producing any detectable adverse effects, suggesting that CSHE might have the potential to be developed as a safer alternative to NSAIDs and disease modifying agent for the treatment of RA.
Acknowledgment
Authors acknowledge financial support from the Indian Council of Medical Research, New Delhi, India
References
- 1.Evans WC. Volatile oil and resins. In: Evans WC, editor. Trease and Evans pharmacognosy. New Delhi: Elsevier; 2006. p. 262. [Google Scholar]
- 2.Singh B, Chaurasia OP, Jadhav KL. An ethnobotanical study of Indus valley (Ladakh) J Econ Tax Bot Addl Ser. 1996;12:92–101. [Google Scholar]
- 3.Said M, editor. Indian Medical Science Series, 55. Delhi: Sri Satguru Publications; 1997. Hamdard pharmacopoeia of Eastern medicine. [Google Scholar]
- 4.Khare CP. Indian medicinal plants - an illustrated dictionary. New York: Springer; 2007. [Google Scholar]
- 5.Chithra V, Leelamma S. Hypolilpidemic effect of coriander seeds (Coriandrum sativum): mechanism of action. Plant Foods Hum Nutr. 1997;51:167–72. doi: 10.1023/a:1007975430328. [DOI] [PubMed] [Google Scholar]
- 6.Krishnakantha T, Lokesh BR. Scavenging of superoxide anions by spice principles. Indian J Biochem Biophys. 1993;30:133–4. [PubMed] [Google Scholar]
- 7.Ammar NM, Al-Okbi SY, Mohamed D. Study of the anti-inflammatory activity of some medicinal edible plants growing in Egypt. J Islamic Acad Sci. 1997;10:113–22. [Google Scholar]
- 8.Mascolo N, Autore G, Capasso F. Biological screening of Italian medicinal plants for anti-inflammatory activity. Phytother Res. 1987;1:28–31. [Google Scholar]
- 9.Li R, Li J, Cai L, Hu CM, Zhang L. Suppression of adjuvant arthritis by hesperidin in rats and its mechanisms. J Pharmacy Pharmacol. 2008;60:221–8. doi: 10.1211/jpp.60.2.0011. [DOI] [PubMed] [Google Scholar]
- 10.Shingu M, Nagai Y, Isayama T, Naono T, Nobunaga M, Nagai Y. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin Exp Immunol. 1993;94:145–9. doi: 10.1111/j.1365-2249.1993.tb05992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulfgren AK, Lindblad S, Klareskog S, Anderson J, Anderson U. Detection of cytokine producing cells in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:654–61. doi: 10.1136/ard.54.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dey PM, Harborne J, editors. Methods in plant biochemistry. London: Academic Press; 1987. [Google Scholar]
- 13.Nair V, Singh S, Gupta YK. Anti-arthritic and disease modifying activity of Terminalia chebula Retz. in experimental models. J Pharmacy Pharmacol. 2010;62:1801–6. doi: 10.1111/j.2042-7158.2010.01193.x. [DOI] [PubMed] [Google Scholar]
- 14.Brownlee G. Effect of deoxycortone and ascorbic acid on formaldehyde-induced arthritis in normal and adrenalectomised rats. Lancet. 1950;1:157. doi: 10.1016/s0140-6736(50)90259-5. [DOI] [PubMed] [Google Scholar]
- 15.Gujral ML, Sareen KN, Tangri KK, Roy AK, Gupta GP, Amma MKP. Antiarthritic effect of Glycyrrhiza glabra Linn. Indian J Physiol Pharmacol. 1959;3:39–47. [PubMed] [Google Scholar]
- 16.Newbould BB. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br J Pharmacol. 1963;21:127–36. doi: 10.1111/j.1476-5381.1963.tb01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidelines for the testing of chemicals. Paris: OECD; 2001. Organization for Economic Cooperation and Development (OECD) p. 425. [Google Scholar]
- 18.Guidelines for the testing of chemicals. Paris: OECD; 2008. Organization for Economic Cooperation and Development (OECD) p. 407. [Google Scholar]
- 19.Gardner DL. The experimental production of arthritis, A review. Ann Rheum Dis. 1960;19:297–317. doi: 10.1136/ard.19.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldmann M, Maini RN. Anti-TNFalpha therapy of rheumatoid arthritis: What have we learned? Annu Rev Immunol. 2001;19:163–96. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 21.Shibata H, Yoshioka Y, Abe Y, Ohkawa A, Nomura T, Minowa K, et al. The treatment of established murine collagen-induced arthritis with a TNFR1-selective antagonistic mutant TNF. Biomaterials. 2009;30:6638–47. doi: 10.1016/j.biomaterials.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 22.Billiau A, Matthys P. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukocyte Biol. 2001;70:849–60. [PubMed] [Google Scholar]
- 23.Pearson CM, Wood FD. Studies of polyarthritis and other lesions induced in rats by injection of mycobacterial adjuvant, General clinical and pathological characteristics and some modifying factors. Arthritis Rheum. 1959;2:440–59. [Google Scholar]
- 24.Ramadan MF, Jörg-Thomas M. Oil composition of coriander (Coriandrum sativum L.) fruit-seeds. Eur Food Res Tech. 2002;215:204–9. [Google Scholar]
- 25.Gupta MB, Nath R, Srivastava N, Shanker K, Kishor K, Bhargava KP. Anti-inflammatory and antipyretic activities of ß-sitosterol. Planta Medica. 1980;39:157–63. doi: 10.1055/s-2008-1074919. [DOI] [PubMed] [Google Scholar]
- 26.Bouic PJ, Etsebeth S, Liebenberg RW, Albrecht CF, Pegel K, Van Jaarsveld PP. Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: implications for their use as an immunomodulatory vitamin combination. Int J Immunopharmacol. 1996;18:693–700. doi: 10.1016/s0192-0561(97)85551-8. [DOI] [PubMed] [Google Scholar]
- 27.Bouic PJD. Sterols/Sterolins, the natural, nontoxic immunomodulators and their role in the control of rheumatoid arthritis. Arthritis Trust Am. 1998;summer:3–6. [Google Scholar]


