Abstract
Interactions between proteins and glycosaminoglycans (GAGs) of the extracellular matrix are important to the regulation of cellular processes including growth, differentiation and migration. Understanding these processes can benefit greatly from the study of protein–GAG interactions using GAG oligosaccharides of well-defined structure. Materials for such studies have, however, been difficult to obtain because of challenges in synthetic approaches and the extreme structural heterogeneity in GAG polymers. Here, it is demonstrated that diversity in structures of oligosaccharides derived by limited enzymatic digestion of materials from natural sources can be greatly curtailed by a proper selection of combinations of source materials and digestive enzymes, a process aided by an improved understanding of the specificities of certain commercial preparations of hydrolases and lyases. Separation of well-defined oligosaccharides can then be accomplished by size-exclusion chromatography followed by strong anion-exchange chromatography. We focus here on two types of chondroitin sulfate (CS) as starting material (CS-A, and CS-C) and the use of three digestive enzymes with varying specificities (testicular hyaluronidase and bacterial chondroitinases ABC and C). Analysis using nuclear magnetic resonance and mass spectrometry focuses on isolated CS disaccharides and hexasaccharides. In all, 15 CS hexasaccharides have been isolated and characterized. These serve as useful contributions to growing libraries of well-defined GAG oligosaccharides that can be used in further biophysical assays.
Keywords: chondroitin sulfate, chondroitinase, enzymatic specificity, hyaluronidase, oligosaccharides
Introduction
Complexes involving glycosaminoglycans (GAGs) of the extracellular matrix and various proteins are important in regulating cell–cell interactions and cell-signaling events (Lindahl and Hook 1978; Handel et al. 2005; Raman et al. 2005; Sasisekharan et al. 2006; Imberty et al. 2007; Gandhi and Mancera 2008). Those interactions have innumerable physiological consequences including organogenesis/growth control (Cohn et al. 1976; Beaulieu et al. 1991), cell adhesion (de Aguiar et al. 2005), coagulation/thrombosis (De Mattos et al. 2008; He et al. 2008), regeneration/wound healing (Gorio et al. 1997; Cattaruzza and Perris 2005), tumorigenesis/metastasis (Cattaruzza and Perris 2005; Muramatsu and Muramatsu 2008), morphogenesis (Thesleff et al. 1988; Domowicz et al. 2000), inflammation (Kaplan et al. 2002; Handel et al. 2005; Koninger et al. 2006; Doodes et al. 2009) and neural development/regeneration (Perris et al. 1996; Pettway et al. 1996; Inatani et al. 2001; Tully et al. 2004). They also play an important role in infection by pathogens (vanPutten et al. 1997) and in mediation of prion internalization (Warner et al. 2002; Ben-Zaken et al. 2003; Horonchik et al. 2005). Involvement of GAGs in such a variety of processes is perhaps not surprising given the diversity of structures and their extracellular location (Handel et al. 2005; Sasisekharan et al. 2006; Gandhi and Mancera 2008).
There are many different types of GAGs: heparan sulfate, heparin (Hp), chondroitin sulfate (CS), dermatan sulfate, keratan sulfate and hyaluronic acid (HA), all of them physiologically active and biomedically important. They differ in terms of composing units (uronic acids, or galactose, and hexosamines). These units occur in disaccharides of one uronic acid/galactose and one hexosamine linked to make a linear polymer. In all except HA extensive sulfation adds to their highly anionic character and structural diversity.
This structural diversity in GAGs is essential to the specificity and variety of interactions with proteins mediating the processes listed above. For example, a specific Hp pentasaccharide with a rare 3-sulfation is clinically exploited due to its high affinity for antithrombin and consequent action in controlling the clotting process (Jin et al. 1997; Richard et al. 2009). Specific sequences of CS having a mix of non- and 4-sulfated N-acetylgalactosamine (GalNAc) residues are believed to mediate the binding of erythrocytes infected by the pathogen responsible for placental malaria (Achur et al. 2008; Singh et al. 2008). Understanding these GAG–protein interactions can clearly be of importance to human health (Kaplan et al. 2002; Handel et al. 2005; Horonchik et al. 2005; Raman et al. 2005; Imberty et al. 2007; Gandhi and Mancera 2008; Muramatsu and Muramatsu 2008; Singh et al. 2008; Richard et al. 2009). However, studies leading to these understandings require the availability of GAG oligosaccharides with well-defined structure (Jin et al. 1997; Muthusamy et al. 2004; Singh et al. 2008; Richard et al. 2009). Studies presented here on enzymatically digested products from natural sources of CS polysaccharides illustrate a means of producing these well-defined oligosaccharides. CS is the most abundant GAG in the body with numerous biological functions (Sugahara et al. 2003). We have chosen to initially explore the production of CS oligosaccharides, since CS is the most homogeneous-sulfated GAG type composed of just alternating GalNAc and glucuronic acid (GlcA) units that are differentially sulfated. This facilitates separation and structure determination.
Among many combinations of enzymes and CS substrates available for study (Ernst et al. 1995), here we choose three commonly used commercially available CS degrading enzyme preparations, the CS lyases, chondroitinase ABC from Proteus vulgaris and chondroitinase C from Flavobacterium heparinum and the hydrolase, testicular hyaluronidase from sheep testes. We choose two CS standards for investigation, bovine tracheal CS-A (btCS-A, mostly 4-sulfated) and shark cartilage CS-C (scCS-C, predominantly 6-sulfated). Neither enzyme nor substrate preparations are particularly pure, but they are readily available in quantities suitable for preparative work, justifying a systematic investigation of the digestive properties of these systems. A comparison of yields and structures of the major disaccharide and hexasaccharide products from the six digestion combinations is presented. After proper isolation by a combination of size-exclusion chromatography (SEC) and strong-anion exchange (SAX) chromatography as successfully performed in previous works (Deepa et al. 2007; Pothacharoen et al. 2007), all oligosaccharide species were characterized by a combination of nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). The number of distinct species produced for analysis is strikingly small and point to specificities in the enzyme preparations that can be exploited in devising approaches to production of well-defined oligosaccharides.
Results
Disaccharide analysis of extensively digested btCS-A and scCS-C
In order to set a standard for further analysis of disaccharides released under more limited digestion conditions, a nearly complete digestion of btCS-A and scCS-C was attempted (Supplementary data, Figure S1A and B). After a week-long digestion of btCS-A polymers (8–50 kDa, Supplementary data, Figure S2) during which enzymes were replaced periodically, high-pressure liquid chromatography (HPLC) analysis showed disaccharide content to be 8% ΔC0S, 42% ΔC6S and 50% ΔC4S (Supplementary data, Figure S1C and Tables I and II). This is in reasonable agreement with previous works (Mucci et al. 2000; Muthusamy et al. 2004; 10% ΔC0S, 40% ΔC6S and 50% ΔC4S). Small deviations depending on sources are not unexpected.
Table I.
Structure and respective abbreviations used for disaccharides and hexasaccharides obtained from six different digestions
| Structural description | Abbreviationa | |
|---|---|---|
| Disaccharides | ΔUA(β1 → 3)GalNAc | ΔC0S |
| ΔUA(β1 → 3)GalNAc4S | ΔC4S | |
| ΔUA(β1 → 3)GalNAc6S | ΔC6S | |
| ΔUA2S(β1 → 3)GalNAc6S | ΔC2,6S | |
| Hexasaccharides | ΔUA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc4S-ol | ΔC6;6;4S-ol |
| ΔUA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc4S(β1 → 4)GlcA(β1 → 3)GalNAc4S-ol | ΔC6;4;4S-ol | |
| ΔUA(β1 → 3)GalNAc4S(β1 → 4)GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc4S-ol | ΔC4;6;4S-ol | |
| ΔUA(β1 → 3)GalNAc4S(β1 → 4)GlcA(β1 → 3)GalNAc4S(β1 → 4)GlcA(β1 → 3)GalNAc4S-ol | ΔC4;4;4S-ol | |
| ΔUA2S(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc(β1 → 4)GlcA(β1 → 3)GalNAc6S | ΔC2,6;0;6S | |
| ΔUA2S(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc(β1 → 4)GlcA(β1 → 3)GalNAc4S | ΔC2,6;0;4S | |
| ΔUA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc6S-ol | ΔC6;6;6S-ol | |
| ΔUA(β1 → 3)GalNAc4S(β1 → 4)GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc6S-ol | ΔC4;6;6S-ol | |
| ΔUA(β1 → 3)GalNAc4S(β1 → 4)GlcA2S(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc6S-ol | ΔC4;2,6;6S-ol | |
| GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc-ol | C6;6;0S-ol | |
| GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc4S(β1 → 4)GlcA(β1 → 3)GalNAc-ol | C6;4;0S-ol | |
| GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc4S(β1 → 4)GlcA(β1 → 3)GalNAc4S-ol | C6;4;4S-ol | |
| GlcA(β1 → 3)GalNAc4S(β1 → 4)GlcA(β1 → 3)GalNAc4S(β1 → 4)GlcA(β1 → 3)GalNAc4S-ol | C4;4;4S-ol | |
| GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc4S-ol | C6;6;4S-ol | |
| GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA2S(β1 → 3)GalNAc6S-ol | C6;6;2,6S-ol | |
| GlcA(β1 → 3)GalNAc6S(β1 → 4)GlcA2S(β1 → 3)GalNAc4S6S(β1 → 4)GlcA(β1 → 3)GalNAc6S-ol | C6;2,6;6S-ol | |
| GlcA2S(β1 → 3)GalNAc6S(β1 → 4)GlcA(β1 → 3)GalNAc4S(β1 → 4)GlcA2S(β1 → 3)GalNAc6S-ol | C2,6;4;2,6S-ol |
Nomenclature: ΔUA, Δ4,5unsaturated uronic acid; GalNAc, N-acetylgalactosamine; GlcA, glucuronic acid; S, sulfation group, where the numbers before “S” represent the ring position; -ol stands for reduced sugars (open rings at the reducing-ends).
aIn the structural codes, the comma (,) was used to separate hexoses, whereas semicolon (;) was used to separate disaccharide units. The digits 0, 4 and 6 denote respective positions of sulfation in the GalNAc units, whereas the 2 denotes 2-sulfation at uronic acid units.
Table II.
Yields of the disaccharides and the major hexasaccharides obtained from different digestion types within different time courses
| Digestion |
Disaccharides |
Hexasaccharides |
|||
|---|---|---|---|---|---|
| Type | Time | Absolute yield (%)a | Structure and relative yieldb | Absolute yield (%)a | Structure and relative yieldb |
| Near-complete digested btCS-A | 7 days | ∼80 | ΔC0S (8%); ΔC4S (50%); ΔC6S (42%) | <1 | Not determined |
| Near-complete digested scCS-C | 7 days | ∼85 | ΔC0S (3%); ΔC4S (26%); ΔC6S (49%); ΔC2,6S (22%) | <1 | Not determined |
| ABC lyase + btCS-A | 2 h 30 min | ∼70 | ΔC0S (7%); ΔC4S (60%); ΔC6S (33%) | ∼3 | ΔC6;6;4S-ol (20%); ΔC6;4;4S-ol or ΔC4;6;4S-ol (35%); ΔC4;4;4S-ol (46%) |
| ABC lyase + scCS-C | 2 h 30 min | ∼ 70 | ΔC0S (6%); ΔC4S (37%); ΔC6S (41%); ΔC2,6S (16%) | ∼3 | ΔC2,6;0;4S (34%); ΔC2,6;0;6S (33%) |
| C lyase + btCS-A | 2 days | ∼8 | ΔC0S (12%); ΔC4S (43%); ΔC6S (45%) | ∼10 | ΔC6;6;6S-ol (8%); ΔC4;6;6S-ol (20%); ΔC4;4;4S-ol (57%) |
| C lyase + scCS-C | 2 days | ∼8 | ΔC0S (14%); ΔC4S (15%); ΔC6S (68%); ΔC2,6S (3%) | ∼10 | ΔC6;6;6S-ol (22%); ΔC4;2,6;6S-ol (39%) |
| Hyaluronidase + btCS-A | 2 days | <1 | Not determined | ∼15 | C6;6;0S-ol (10%); C6;4;0S-ol (8%); C6;4;4S-ol (30%); C4;4;4S-ol (19%); C6;6;4S-ol (32%) |
| Hyaluronidase + scCS-C | 2 days | <1 | Not determined | ∼10 | C6;6;4S-ol (23%); C6;4;4S-ol (18%); C6;6;2,6S-ol (20%); C6;2,6;6S-ol (19%); C2,6;4;2,6S-ol (7%) |
aThe absolute yield was estimated as the percentage of weight (mg of sample) recovered from the peaks 2 (disaccharides) and 6 (hexasaccharides) from the SEC (Bio-Gel P-10 column) of each digestion type. The values are relative to ∼75 mg of loaded material into the column.
bThe relative yield was determined as the percentage of weight (mg of material) recovered as pure isomers fractionated by SAX-HPLC chromatography of the heterogeneous mixture of disaccharides or hexasaccharides from each digestion type. The values are compared with ∼5 mg of material loaded into the column.
HPLC disaccharide analysis of similarly digested scCS-C polymers (∼10–50 kDa, Supplementary data, Figure S2) showed disaccharide content to be 3% ΔC0S, 49% ΔC6S, 26% ΔC4S and 22% ΔC2,6S (Supplementary data, Figure S1D and Table II). Again this is in reasonable agreement with the literature (2% ΔC0S, 49% ΔC6S, 29% ΔC4S, 17% ΔC2,6S and 3% ΔC4,6S; Sorrell et al. 1993; Mucci et al. 2000). Data confirm the heterogeneity of both CS standards. However, quantities of recovered mono- (>5%) and disaccharides (∼80–85%) show the digestions to be only 85–90% complete (Table II), suggesting that both CS polymers may contain sequences resistant to even very extensive digestion conditions.
Limited chondroitinase ABC digestion of btCS-A
SEC profiles of btCS-A digestion products from shorter periods of ABC lyase treatment are shown in Figure 1. MS has been used to determine the size of oligosaccharides eluted in representative peaks (Supplementary data, Figure S3), and the degree of polymerization is indicated above each peak in Figure 1. Disaccharides are the major products for short periods of digestion, consistent with the previously suggested presence of substantial exolytic cleavage activity (Hardingham et al. 1994). HPLC analysis of disaccharide content of samples digested for 2.5 h (Figure 1, open triangles) showed composition to be: 7% ΔC0S, 33% ΔC6S and 60% ΔC4S (Supplementary data, Figure S4 and Table II). In comparison with the extensively digested sample (Supplementary data, Figure S1C), more ΔC4S disaccharide is clearly generated suggesting a preference for cleavage at a 4-sulfated GalNAc.
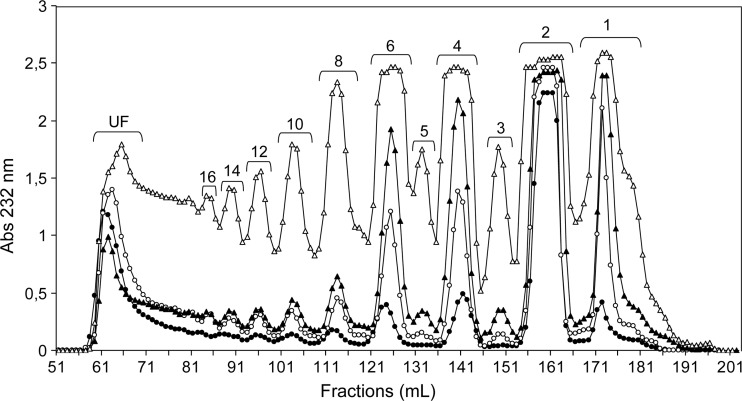
Fig. 1.
Size fractionation on the Bio-Gel P-10 column of the products from btCS-A digested with a commercial preparation of chondroitinase ABC from P. vulgaris. Data from different digestion times are shown: 10 min (filled circles), 30 min (open circles), 1 h (filled triangles) and 2.5 h (open triangles). At the top of each peak, the degree of polymerization is indicated. UF stands for unfractionated material.
Although dominant production of disaccharides in early periods of digestion is in full agreement with the expected high level of exolytic activity (Hardingham et al. 1994), tetrasaccharides and hexasaccharides are significant at the initial periods as well (Figure 1, filled and open circles) and become progressively more abundant with longer digestions (Figure 1, filled and open triangles). Higher order oligosaccharides (longer than 6-mers) become gradually measurable as well. The commercial preparation of ABC lyase is in fact known to be a mixture of two enzymes suspected to differ in endolytic and exolytic activities, lyases I and II (Hamai et al. 1997; Zhang et al. 2009). Therefore, it is reasonable to consider the production profile as a result of their combined and competing activities (see Materials and methods). If the exolytic activity dominated, the high-order oligosaccharides could be simply end-products of multiple exolytic cleavages of chains with different lengths in the starting material. To test this possibility, a digestion of a size-selected fraction of btCS-A, ∼39 kDa or ∼164 residues for short (10 min) and long (2.5 h) periods, was performed (Supplementary data, Figure S5A). Although large amounts of disaccharides were still observed in both time courses, significant amounts of tetrasaccharides and hexasaccharides were seen (24% each for the 10-min digestion) and the amounts of larger oligosaccharides were small. If only exolytic digestion had occurred in the 10 min digestion and only ∼38% disaccharides had been produced, the average length of the remaining fragments should have averaged 24.1 kDa or ∼100 residues in length. Hence, there is significant endolytic activity in the mixture (Jandik et al. 1994) forming mostly tetrasaccharides and hexasaccharides (Hardingham 1994). As the time of digestion proceeds (4.5 h in Supplementary data, Figure S5B), the amount of high-order oligosaccharides progressively decreases, likely due to continued exolytic action, until massive disaccharide amounts are formed as final products (Supplementary data, Figure S1A).
There is a general consensus that the ABC lyase exolytic activity proceeds from the non-reducing end (Hardingham et al. 1994). This directionality was confirmed through the MS characterization of digestion products from a size-selected CS fraction modified at its reducing end (Supplementary data, Figure S6). Hence, the existence of sites less prone to exolytic activity may be reflected in the non-reducing end structure of some oligosaccharide products. The hexasaccharide fraction from limited ABC lyase digestion of unfractionated btCS-A was, therefore, further analyzed by separation on a SAX column followed by NMR and MS analysis. Prior to SAX-HPLC, the mixture of hexasaccharides (Figure 1, peak 6, open triangles) was reduced via treatment with NaBH4 to avoid possible complications from α-/β-anomeric mutarotation. This reaction was only used to facilitate structural characterization and can be skipped when oligosaccharide production is the objective. Only three major peaks appeared in the SAX chromatogram (Figure 2A). Among the three fractions, only the two leading and trailing peaks gave 13C-gradient heteronuclear single-quantum coherence (gHSQC) spectra sufficiently free of contamination from species eluting at either side to allow structure determination. The first peak was characterized as ΔC6;6;4S-ol (Table I). Its 13C-gHSQC spectrum showed two 1H/13C-signals characteristic of 6-sulfation and one cross-peak typical of 4-sulfation (Supplementary data, Figure S7A and Supplementary data, Table SI). This last 4-sulfate-related signal is connected in the correlation spectroscopy (COSY) spectrum to a resonance of a far upfield methylene group (δH = 3.69 ppm), as opposed to an anomeric resonance (Supplementary data, Figure S7B). This methylene derives from the open sugar ring coming from the reduction reaction, thus proving that the 4-sulfated GalNAc unit is located at the reducing terminus of the hexasaccharide. The last hexasaccharide from the SAX-HPLC was identified as the entirely 4-sulfated hexasaccharide (ΔC4;4;4S-ol, Table I) based on intense 1H4-13C4 downfield cross-peaks characteristic of 4-sulfated GalNAc residues (Supplementary data, Figure S8A and Supplementary data, Table S1). The central elution fraction, which could not be completely characterized by 13C-gHSQC, was further subjected to analysis using 15N-gHSQC. This has previously proven to be very diagnostic for sulfation types in CS oligosaccharides (Pomin et al. 2010). Besides the three cross-peaks showing 4-sulfation from the contaminating entirely 4-sulfated hexasaccharides (Supplementary data, Figure S8B), an additional intense cross-peak typical of a non-reducing end or middle 6-sulfated GalNAc residue is observed. This suggests that the middle SAX-HPLC fraction (Figure 2A) is either ΔC6;4;4S-ol or ΔC4;6;4S-ol.
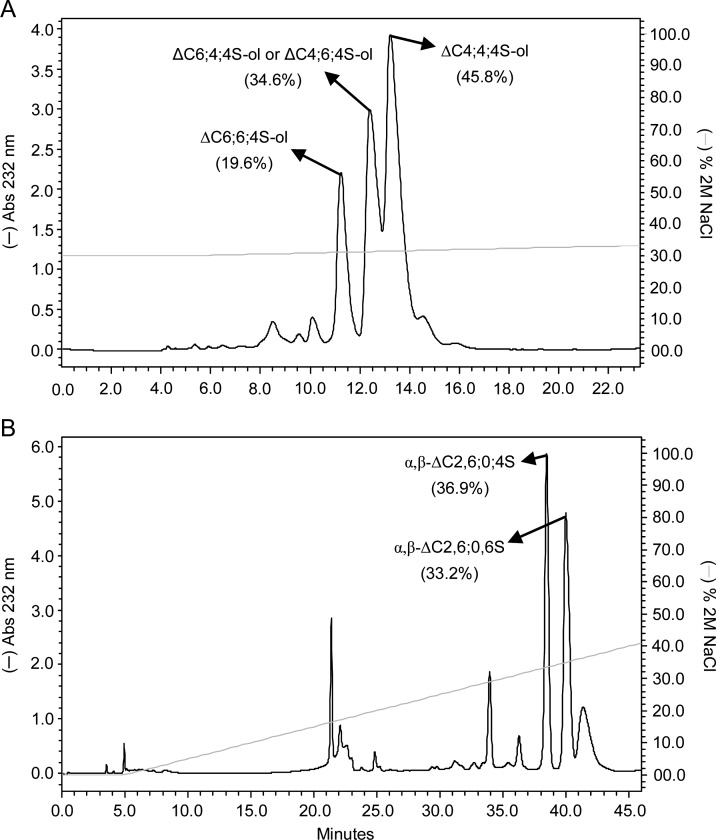
Fig. 2.
SAX-HPLC fractionation of unsaturated hexasaccharides from digestion of (A) btCS-A and (B) scCS-C with a commercial preparation of chondroitinase ABC from P. vulgaris. Mixtures of hexasaccharides (reduced and unreduced for btCS-A and scCS-C, respectively) were obtained from peak 6 of their respective Bio-Gel P-10 chromatograms of 2.5 h digestions. The two fractionated isomers as characterized by NMR and MS spectroscopy were (A) ΔC6;6;4S-ol, and ΔC4;4;4S-ol for btCS-A and (B) α,β-ΔC2,6;0;6S and α,β-ΔC2,6;0;4S for scCS-C. (A) The middle peak could be either ΔC6;4;4S-ol or ΔC4;6;4S-ol. The percentage of material in each peak is indicated in parentheses. The NaCl gradient is shown with the continuous light grey line.
The appearance of hexasaccharides with 6-sulfation at central sites and the non-reducing terminus, and 4-sulfation at the reducing terminus, is expected based on a lower preference for exolytic cleavage at 6-sulfated sites. Exolytic cleavage beginning at the non-reducing end would have been slow at the 6-sulfated sites allowing more time for endolytic cleavage at an upstream 4-sufated site. The high yield of a ΔC4;4;4S-ol oligosaccharide (Table II) is likely a simple consequence of the higher percentage of 4-sulfation in the starting material.
Limited chondroitinase ABC digestion of scCS-C
The oligosaccharide distribution using chondroitinase ABC to digest the atypical scCS-C substrate over a 2.5-h digestion period (Supplementary data, Figure S9) is similar to that seen for btCS-A at 1 h (Figure 1, filled triangles). The slower progression of the digestion is consistent with a preference of the ABC lyase for 4-sulfation sites and the smaller percentage of 4-sulfation in the scCS-C substrate. scCS-C is 26% 4-sulfated as opposed to 50% in btCS-A (Supplementary data, Figures S1C vs D and Table II).
Analyses of the disaccharide fractions were distinctive in two respects. First, a new HPLC peak was detected (Supplementary data, Figure S10A). Its MS spectrum (Supplementary data, Figure S10B) indicated an MW of 539 Da, typical of a disulfated unsaturated CS-derived disaccharide. The 13C-gHSQC spectrum confirmed the presence of 6-sulfation on GalNAc, with additional 2-sulfation on GlcA (Supplementary data, Figure S10C). Second, even though native scCS-C possesses only ∼ 26% 4-sulfation (Supplementary data, Figure S1D), larger amounts (37%) of 4-sulfated disaccharides were produced (Supplementary data, Figure S10A and Table II). This is consistent with a preference for cleavage in 4-sulfated regions of CS molecules by the exolytic component of ABC lyase preparation.
The presence of hexasaccharides, although comprising <5% of the digested material after 2.5 h digestion (Table II), is again consistent with significant endolytic activity of the commercial preparation of the ABC lyase from P. vulgaris. Fractionation by SAX-HPLC of the unreduced hexasaccharides revealed two well-separated major peaks (Figure 2B); both were further characterized by 13C-gHSQC spectra (Supplementary data, Figure S11A and B). Positions of sulfate-related 1H/13C cross-peaks suggest that the first fraction to be ΔC2,6;0;4S (Table I) and the second fraction to be ΔC2,6;0;6S (Table I).
The high representation of 2-sulfated GlcAs and non-sulfated GalNAcs in the hexasaccharide fraction is in sharp contrast to the lower amounts of disaccharides containing these sulfation patterns under near-complete digestion conditions (22 and 3%, respectively; Table II). This sulfation pattern, ΔC2,6;0, may slow the exolytic action of the 4-sulfation-prefering ABC lyase allowing endolytic activity to produce hexasaccharides with an unexpectedly small number of isomers. 4-sulfation at the reducing end is more prevalent as expected based on preference for cleavage at a 4-sulfated site. The appearance of a hexasaccharide with 6-sulfation at this position likely reflects the abundance of these sites in the starting material.
Limited chondroitinase C digestion of scCS-C
The commercial preparation of chondroitinase C is expected to display enhanced activity toward CS-C substrates, but both the chondroitinase C preparation used here and the related chondroitinase AC preparation are known to display activity toward CS-A products as well (Michelacci and Dietrich 1976; Aguiar et al. 2003). The AC preparation is well characterized and activities toward 4-sulfated and 6-sulfated regions reside in a single enzyme (Gu et al. 1995). The mixed activities in the chondroitinase C preparation reflect a similar activity profile, but one that may result from the presence of multiple enzyme components (see Materials and methods). The activity of chondroitinase C was observed to be lower than that of chondroitinase ABC and hence longer digestion times were used (48 h in Figure 3 vs 2.5 h in Figure 1, open triangles). Only the results after 2-day digestion are presented in Figure 3, and this plot is used to compare the size distribution results on digesting the preferred 6-sulfation-rich substrate, scCS-C, to the less preferred 4-sulfation-rich substrate, btCS-A. The distribution of oligosaccharide sizes appears similar for both substrates as confirmed by the quantitation of amounts of disaccharides and hexasaccharides presented in Table II. This suggests a substantial amount of 4-sulfation-dependent activity. The higher amounts of hexasaccharide and other oligosaccharides when compared with disaccharides for both digestions suggest a dominant endolytic activity for this enzyme preparation.
Fig. 3.
Size fractionation on the Bio-Gel P-10 column of the products from 2-day digestions of scCS-C (open circles) and btCS-A (filled circles) with a commercial preparation of chondroitinase C from F. heparinum. The degree of polymerization is given at the top of each peak. UF stands for unfractionated material.
Disaccharide fractions after 2-day digestion of scCS-C showed 68% of the structures to have a single 6-type sulfation (Supplementary data, Figure S12A and Table II), whereas the starting material has ∼49% (Supplementary data, Figure S1D). Some 6-sulfation preference therefore exists. The specificity of the C lyase preparation as seen in scCS-C digestions can be better assessed by the examination of hexasaccharides isolated by SAX-HPLC combined with analysis by NMR and MS (Figure 4A and Supplementary data, Figures S13 and S14A and B). The large amount of hexasaccharide bearing 6-sulfated units at reducing ends, such as ΔC6;6;6S-ol and ΔC4;2,6;6S-ol (Table I) would support specificity for cleavage at a 6-sulfated site, but may also be explained as a consequence of the preponderance of 6-sulfation in the starting material. A comparison with a C lyase digestion of btCS-A, as presented below, helps to differentiate these possibilities. The different numbers of sulfates in oligosaccharide products from scCS-C when 2-sulfation of the GlcA occurs along with sulfation of the GalNAc are distinctive and facilitate the isolation of homogeneous hexasaccharides from this digestion type.
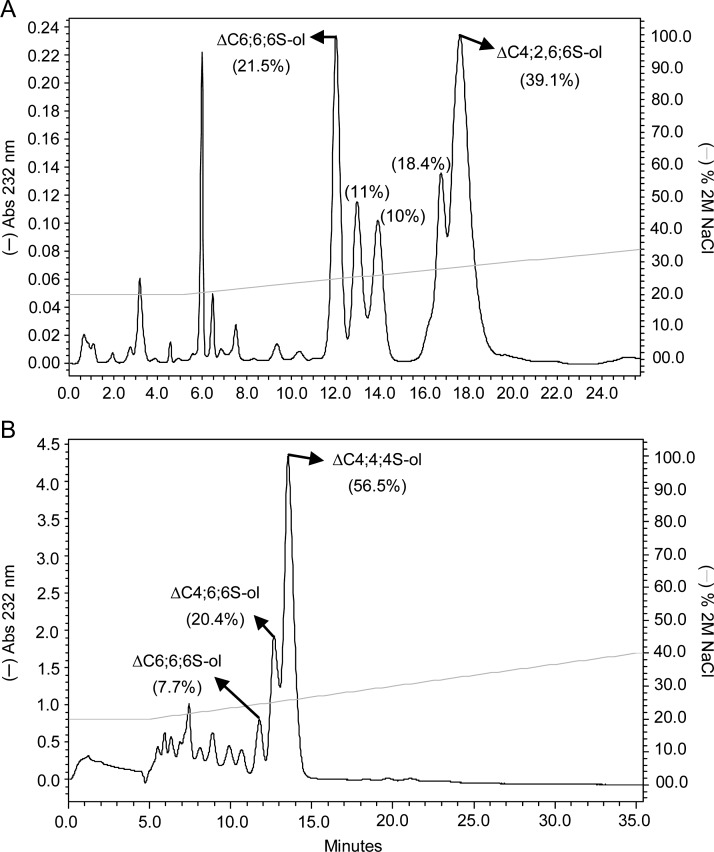
Fig. 4.
SAX-HPLC fractionation of reduced unsaturated hexasaccharides from 2-day digestions of (A) scCS-C and (B) btCS-A with a commercial preparation of C lyase from F. heparinum. Both mixtures of hexasaccharides were obtained from peak 6 of their respective Bio-Gel P-10 chromatograms. The fractionated isomers are (A) ΔC6;6;6S-ol and ΔC4;2,6;6S-ol for scCS-C and (B) ΔC6;6;6S-ol, ΔC4;6;6S-ol and ΔC4;4;4S-ol for btCS-A. The mean the percentage of material in each peak is given in parentheses. The NaCl gradient is shown with the continuous light grey line.
Limited chondroitinase C digestion of btCS-A
The slight activity preference of C lyase for 6-sulfated regions is noticeable when the disaccharides derived from the atypical substrate btCS-A are analyzed. Disaccharide analysis of products from 2-day incubation revealed nearly equivalent amounts of 4- and 6-sulfated disaccharides (43 and 45%; Supplementary data, Figure S12B and Table II), when compared with the excess of 4-sulfated disaccharides (50 vs 42%) seen on near-complete digestion of btCS-A (Supplementary data, Figure S1C and Table II).
As in the case of scCS-C, the amount of hexasaccharide is relatively high in btCS-A digestions showing substantial endolytic activity. Again a small number of isomers (three) were obtained from SAX-HPLC (Figure 4B) of the mixture of reduced hexasaccharides (peak 6, open triangle, in Figure 1). These were characterized by both 13C-gHSQC NMR (Supplementary data, Figure S15) and MS (Supplementary data, Figure S14C and D), giving the following structures: ΔC6;6;6S-ol, ΔC4;6;6S-ol and ΔC4;4;4S-ol (Table I). The ∼57% occurrence of a ΔC4;4;4S-ol hexasaccharide was unexpectedly high (Table II). Occurrence may have been elevated somewhat by the slight preference for cleavage at 6-sulfated sites, but it can also be a consequence of clustering of 4-sulfation in the starting material. The presence of the ΔC6;6;6S-ol hexasaccharide was likewise surprising, even though in small amounts. Random distributions of 6-sulfated sites in even a slightly 4-sulfate-rich sample would make the occurrence of three in a row rare. Evidence supporting the occurrence of sulfation domains rather than random distribution in CS backbones does exist (Sorrell et al. 1993). In any case, preference for cleavage in 6-sulfated regions over 4-sulfated regions is small when btCS-A is used as a substrate for the C lyase preparation.
Hyaluronidase digestion of btCS-A and scCS-C
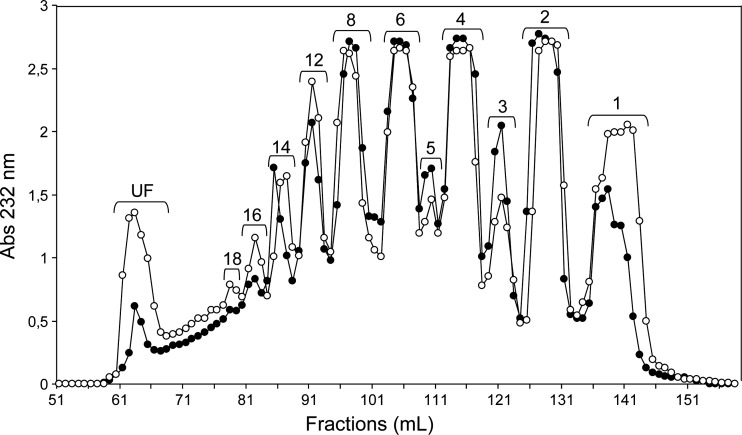
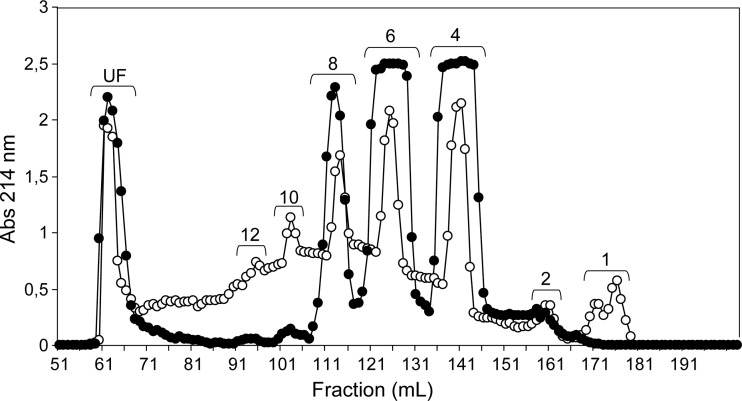
It is clear from the above results that longer oligosaccharides are produced when endolytic activity dominates and production of certain products can be enhanced when non-reducing end processing is reduced by a less preferred sulfation pattern. It is therefore of interest to examine products from digestion with other enzymes for which endolytic activity naturally dominates. Commercial preparations of ovine hyaluronidase, a hydrolase named for its activity toward non-sulfated HA, is known to have such specificity (Takagaki et al. 1994). Preference for cleavage at 4-sulfated or non-sulfated sites (Knudson et al. 1984) should complement observations made using the chondroitinase C preparation where cleavage at 6-sulfated sites is slightly preferred. This hydrolase would also add saturated oligosaccharides to the growing library of unsaturated oligosaccharides produced by the lyases. Sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) of the enzyme shows a number of bands, but these are believed to be proteolysis products of a single enzyme (see Materials and methods). Figure 5 presents gel-permeation results for digestions with hyaluronidase on both CS substrates. The small amounts of disaccharide and heavy distribution toward medium sized-oligosaccharides (ranging from 4 to 12 residues, Figure 5) support the predicted effects of high endolytic activity. Surprisingly, hyaluronidase appears to show some enhanced activity toward a 6-sulfation-rich substrate, as scCS-C appears to have been digested faster than btCS-A (Figure 5). The apparent acceleration of digestion may, however, reflect differences in accessibility due to secondary and tertiary structure as opposed to specificity at the cleavage site. Additional information can again be obtained by separation and characterization of hexasaccharide fractions.
Fig. 5.
Size fractionation on the Bio-Gel P-10 column of the products from 2-day digestion of btCS-A (open circles) and scCS-C (filled circles) with a commercial preparation of ovine hyaluronidase. The degree of polymerization is given at the top of each peak. UF stands for unfractionated material.
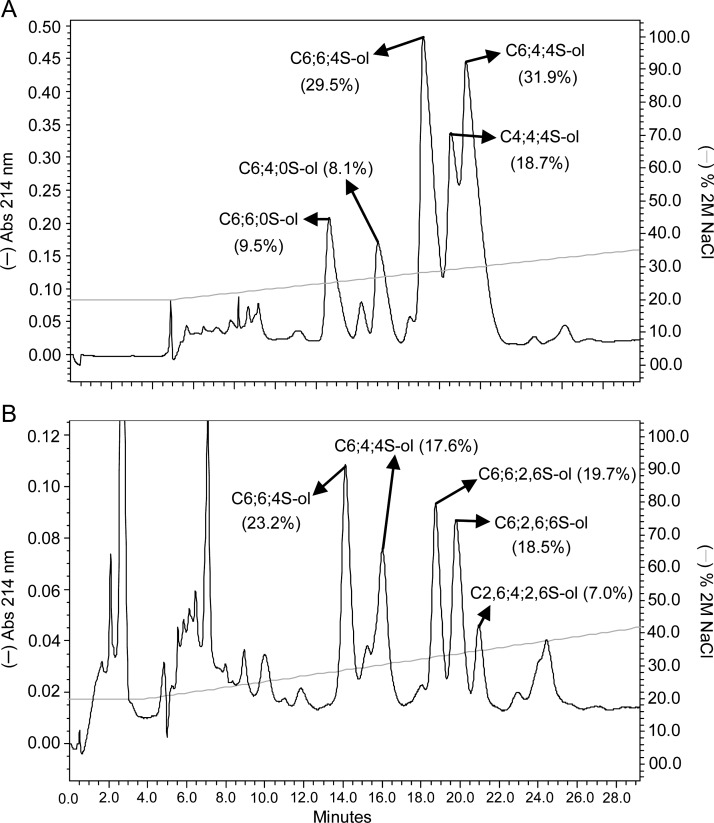
Digestions of both btCS-A and scCS-C showed two well-separated groups of reduced hexasaccharides on SAX-HPLC (Figure 6). In the case of btCS-A, 13C-gHSQC NMR (Supplementary data, Figure S16) and MS spectra (Supplementary data, Figure S17) showed the first group to be C6;6;0S-ol and C6;4;0S-ol, and the second group to be C6;6;4S-ol, C4;4;4S-ol and C6;4;4S-ol (Figure 6A, Table II). The groups of HPLC peaks differ in the extent of sulfation. The hexasaccharides in the first group, with a non-sulfated GalNAc at the reducing end, were in fact anticipated based on the expected ability of hyaluronidase to cleave at non-sulfated sites (Knudson et al. 1984). The relatively high percentage of hexasaccharides bearing 6-sulfation in both groups is consistent with the suggestion that 6-sulfated regions may be more accessible to the enzyme. It is noteworthy, however, that all hexasaccharides of the second group have 4-sufation at the reducing ends, confirming a preference for cleavage between a 4-sulfated GalNAc and a GlcA (Knudson et al. 1984).
Fig. 6.
SAX-HPLC fractionation of reduced saturated hexasaccharides from 2-day digestion of (A) btCS-A and (B) scCS-C with a commercial preparation of ovine hyaluronidase. The percentage of material in each peak is given in parentheses. The NaCl gradient is shown with the continuous light grey line.
The major hexasaccharides from the hyaluronidase digestion of scCS-C were characterized as follows: C6;6;4S-ol, C6;4;4S-ol, C6;6;2,6S-ol, C6;2,6;6S-ol and C2,6;4;2,6S-ol (Figure 6B and Supplementary data, Figure S18 for NMR, and Supplementary data, Figure S19 for MS spectra). Curiously, these products do not contain any non-sulfated GalNAc units. However, the occurrence of non-sulfated GalNAc residues is lower than in btCS-A (3%, Supplementary data, Figure S1D). More prevalent are products eluting at higher salt concentrations containing 2,6-disulfated units. This shows some enhanced hyaluronidase activity for regions with 2,6-disulfated sites in scCS-C. Products from hyaluronidase digestion bearing 2-sulfation have been observed previously (Nadanaka and Sugahara 1997). Aside from some enhanced activity toward 2,6-disulfated regions, the large amount of hexasaccharides bearing C-type sulfation is consistent with the abundance in the starting material together with the suggested enzymatic accessibility to 6-sulfation-rich regions.
The occurrence of hexasaccharide products with 2–5 and potentially 6-sulfation sites on digestion with hyaluronidase facilitates separation of discrete products on SAX-HPLC. Caution must be exercised, however, in assuming that hexasaccharide structures observed represent sequences abundant in native polymers. The transglycosidase activity of hyaluronidase can clearly generate sequences not present or abundant in its polymeric substrates (Takagaki et al. 1994, 1999). However, our observations are reproducible and remain relevant to the potential for defined product retrieval from btCS-A and scCS-C.
Discussion
Well-defined, higher-order, GAG oligosaccharides can be of a tremendous value in the investigation of the activities of the numerous proteins involved in GAG metabolism and modulation of cellular function. It would initially seem that digestion of naturally occurring GAG polymers followed by separation on the basis of size and charge would offer a route to the preparation of such oligosaccharides. However, high exolytic activities of certain enzyme preparations lead to the production of primarily disaccharides, and simple statistical predictions of structural diversity, even for oligosaccharides as small as hexasaccharides, would suggest unmanageable numbers of isomers. Work presented here shows that the non-random distribution of sulfation in CS chains, together with cleavage preferences of commercial preparations of digestive enzymes, contribute to a limited diversity in products and enhanced utility of a digest and separate preparation protocol.
The current research was restricted to a study of the activity of just three commonly used commercial enzyme preparations, and their actions on just two readily available CS standards. However, comparative analysis of digestion products from the two differentially sulfated substrates provided some insight into the relationship of cleavage specificities of the enzyme preparations and the limited number of hexasaccharides produced. The hexasaccharide fractions were chosen for further characterization in this work due to a combination of potential biological interest and feasibility in HSQC-based structural determination. Endolytic activity contributes substantially to the production of the biologically interesting medium-sized products in digestions with C lyase and hyaluronidase, whereas the substantial exolytic activity in ABC lyase preparations limit the production of larger oligosaccharides. However, for the small amounts of larger oligosaccharides produced, specificities for exolytic cleavage seem to contribute to the production of limited numbers of isomers, possibly by enhancing opportunities for endolytic cleavage when regions of low exolytic activity are encountered. For example, the commercial preparation of ABC lyase from P. vulgaris, whose activity results from a competition between two enzymes (endolyase and exolyase), produced a very limited set of hexasaccharide structures, mostly with a central and/or non-reducing end 6-sulfated GalNAc or a 2,6;0S pattern penultimate to the reducing end (Table II). This is likely due to its 4-sulfation cleavage preference at the reducing end (Hardingham et al. 1994). Exploiting this preference, ∼2 mg of the uncommon hexasaccharide, ΔC2,6;0;4S was prepared by treating 75 mg of scCS-C with ABC lyase.
The commercial preparation of chondroitinase C from F. heparinum showed predominantly endolytic action. Only 8% disaccharides were produced with limited digestion as opposed to the 70% with limited ABC lyase digestion (Table II), something that could obscure any effect of exolytic stalling on hexasaccharide product distributions. Analysis of the small amount of generated disaccharide products did show effective cleavage at 4-sulfated GalNAc units as well as 6-sulfated units. There is, in fact, only a slight elevation of 6-sulfation in disaccharides isolated after short digestions of btCS-A when compared with disaccharides produced on near-complete digestion. The 4,4,4-hexasaccharide isolated from a btCS-A digestion was also produced in large amounts (57%, Table II) showing cleavage ability at a 4-sulfated site, but all other hexasaccharides from either btCS-A or scCS-C digestion have a 6-sulfated GalNAc unit located at their reducing ends. In addition, the SEC profiles of both substrates were observed to be similar (Figure 3). Hence, specificity for both 4- and 6-sulfated sites does exist. We cannot exclude that this results from contamination of the chondroitinase C preparation with an ABC type enzyme, but both AC and C isolates have previously been shown to have substantial activities toward CS-A and CS-C (Gu et al. 1995; Aguiar et al. 2003). In the former case, the enzyme is quite pure (Gu et al. 1995).
As expected, the commercial hyaluronidase preparation from sheep testes exhibits major endolytic activity producing medium- (4- to 8-mers, Figure 6) and large-sized oligosaccharides (peaks UF in Figure 6) as primary products. As expected, it also displays a slightly enhanced ability to cleave after both 4-sulfated and non-sulfated GalNAc residues (Knudson et al. 1984). For digestion of scCS-C, there is an enhanced abundance of hexasaccharides that carry 2- and 6-disulfated disaccharide units, indicating a possible preference for regions bearing additional sulfation on GlcAs as well. The number of hexasaccharide types is larger than that produced by the lyases studied here, but the number is still below statistical expectations. The occurrence of hexasaccharides with both less and more sulfation than one per GalNAc residue also facilitates the separation of products by SAX in this case.
Using enzyme preparations that have substantial endolytic activities combined with a consideration of enzyme specificities and a judicious choice of substrates can clearly produce significant amounts of well-defined CS oligosaccharides. For example, treatment of btCS-A with chondroitinase C produced more absolute amounts of hexasaccharides than digestions with chondroitinase ABC (Table II). The SAX-HPLC-based separation of hexasaccharides from the chondroitinase C digestion of btCS-A revealed only three major isomers (Figure 4B). High levels of larger oligosaccharides were also obtained from hyaluronidase digestions of either btCS-A or scCS-C (Figure 5). Separation of hexasaccharides from hyaluronidase digestion on SAX-HPLC shows well-separated sets of structures bearing two, three or more sulfates, and again no more than three isomers in each group (Figure 6). The structure, C6;6;4S-ol, represents 32% of all hyaluronidase produced hexasaccharides from btCS-A, and the hexasaccharide amount represents ∼15% of total digested material (Table II). This enabled ready production of ∼3 mg of a very specific hexasaccharide from 75 mg of readily available starting material.
The data reported in Table I is far from a complete survey of production possibilities. But it shows that with a proper choice of enzyme and substrate the diversity in structures produced can be minimized and certain target structures can be produced with reasonable yields. There is some additional supporting data in the literature. A library of CS octasaccharides obtained from the hyaluronidase digestion of scCS-C has, for example, been presented (Deepa et al. 2007). Digestion conditions are different and structures are deduced by additional enzymatic degradation as opposed to NMR/MS analyses. Many of the basic specificities observed in our studies can, however, be seen in the octasaccharide structures as well. Some of the products of such research have already proven useful in development and use of epitope recognition monoclonal antibodies (Caterson et al. 1990; Pothacharoen et al. 2007). Hopefully, the combination of data presented here and elsewhere will suggest procedures for the production of targeted oligosaccharides and guide additional research into other substrate–enzyme combinations for preparation of biologically active GAG oligosaccharides with well-defined structures.
Materials and methods
Materials and reagents
The sodium salt of btCS-A, the sodium salt of scCS-C, hyaluronidase from sheep testes (type V) (EC 3.2.1.35), chondroitin ABC lyase from P. vulgaris (EC 4.2.2.4), chondroitin C lyase from F. heparinum (EC 4.2.2.X), Sephadex G-15 resin (fractionation range of dextrans <1.5 kDa) and 1,9-dimethyl-methylene blue (dye content 80%) were purchased from Sigma-Aldrich Co. (St Louis, MO). A pre-packed Spherisorb S10 SAX column (10 × 250 mm, 5 μm) was from Waters Corporation (Milford, MA). Bio-Gel P-10 gel resin (fractionation range of dextrans from ∼1.5 to ∼20 kDa) in fine polyacrylamide beads, Bio-Gel P-60 (fractionation range of dextrans from 10 to 60 kDa) in medium polyacrylamide beads, the polypropylene chromatographic columns (120 × 1.5 and 120 × 3.0 cm for SEC and 1.0 × 50 cm for desalting) were purchased from Bio-Rad Life Science (Hercules, CA). Deuterium oxide “100%” (D 99.96%) was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA), and HPLC-grade methanol was purchased from Fisher Scientific (Fair Lawn, NJ).
Analysis of commercial hyaluronidase and chondroitinase ABC and C preparations
The purity of enzymes was checked by SDS–PAGE as presented in Supplementary data, Figure S20, and several bands were excised for MS analysis of peptides from a trypsin digest. All enzyme preparations show multiple bands. Partially resolved bands near 120 kDa in the chondroitinase ABC sample are consistent with an expected mixture of two isoforms (endolyase and exolyase), and the presence of the endolyase was confirmed by MS. Although one preparation examined was designated bovine serum albumin free (BSA free), the bands near 55 kDa show peptides consistent with BSA. BSA is frequently used as a stabilizer in these preparations, and when this is done, the amounts are much higher. The chondroitinase C preparation also showed several bands. While not specified as either BSA containing or BSA-free in the product literature, the preparation does contain BSA as a stabilizer, accounting for the intense band near 55 kDa. The additional bands above 100 kDa appear to be associated with BSA (peptides from the distinct band at ∼120 kDa were shown by MS to be consistent with the BSA sequence). The 74-kDa band yields peptides consistent with the 700 amino acid AC lyase, but the band at ∼62 kDa could not be identified.
The commercial preparation of hyaluronidase from sheep testes type V showed a major band at ∼28 kDa an additional band at ∼22 kDa and a minor band at 56 kDa. The bands at 28 and 56 kDa had originally been interpreted as dimers and tetramers (Khorlin et al. 1973). However, recent structural work on homologous hyaluronidases shows single polypeptides of ∼55 kDa having a catalytic domain of ∼35 kDa (Stern and Jedrzejas 2006). Tests for activity on size separated fractions of our material showed activity in both the 56- and 28-kDa materials. Both the 33- and 58-kDa bands from bovine testicular hyaluronidase have been shown to have activity previously (Oettl et al. 2003). Given the lack of unique activities for the separated fractions, commercial preparations of all enzymes were used in the in the following digestions. SDS–PAGE data shown in Supplementary data, Figure S20 can be used in the future to alert investigators to possible variations in commercial preparations and consequent deviations from results reported here.
Limited enzymatic digestions of btCS-A and scCS-C
Five ABC lyase digestions of btCS-A were individually performed incubating 150 mg of the polysaccharide with 1 mg of chondroitinase ABC (0.33 IU) in a 5 mL digesting buffer (50 mM Tris–HCl, pH 8.0, 150 mM sodium acetate, 100 μg/mL of BSA) at 37°C, for 10, 30, 60, 150 or 270 min. After the respective periods of incubation, each digested sample was heated (boiled) at 100°C for 15 min to stop the reaction. One ABC lyase digestion of scCS-C and two C lyase digestions of btCS-A and scCS-C were performed using the same protocol described at the beginning of this section; however, only the period of 150 min for the ABC lyase digestion of scCS-C was executed, whereas 48 h was used for C lyase digestions with 8.7 μg of enzyme (2.5 IU). Hyaluronidase digestions of both btCS-A and scCS-C were performed by incubation of each CS type (150 mg) with 10 mg of hyaluronidase (1.5 × 104 IU) in 3 mL of 50 mM sodium phosphate, 150 mM NaCl (pH 6.0) at 37°C, for 48 h each. The digested samples were then heated (boiled) at 100°C for 15 min.
Size fractionation of CS-digested products
A 2.5-mL sample (∼75 mg) from each digestion was subjected to SEC on a Bio-Gel P-10 column (120 × 1.5 cm) using an elution solution of 10% ethanol in 1 M aqueous NaCl at a flow rate of 1 mL/15 min/fraction. The separations were monitored by ultraviolet absorption at λ = 214 nm or λ = 232 nm for the hydrolase- and lyase-derived products, respectively (Horonchik et al. 2005). Some digestion types were subjected to size separation more than once (Figure 1). The lyase- or hydrolase-digested disaccharide and hexasaccharide fractions were selected for further separation and analyses. Tubes corresponding to their peaks were pooled, concentrated and desalted on a Sephadex G-15 column (1.0 × 50 cm) using distilled water as eluent. The respective desalted fractions were freeze-dried and stored until use. Additional fractionations of native btCS-A and scCS-C samples on Bio-Gel P-60 were carried out under similar conditions, using a larger column (120 × 3.0 cm) with loadings of 120 mg.
Reduction of CS hexasaccharides
To provide samples having terminal sugars reduced to their corresponding N-acetylgalactosaminitol forms (-ol), the desalted and lyophilized hexasaccharides from the Bio-Gel P-10 column (except those from the ABC lyase digestion of scCS-C) were treated with an equivalent weight of sodium borohydride (NaBH4) in 1 mL of water for 3 h. The reduction reactions were then stopped by adding a molar equivalent of acetic acid and stirring for 1 h in an ice-bath. This was followed by desalting on a Sephadex G-15 column (1.0 × 50 cm).
SAX chromatography for isolation of CS isomers
About 5 mg of the different lyase- and hydrolase-digested hexasaccharide or disaccharide fractions were dissolved in 100 μL of water and individually subjected to SAX-HPLC on a 10 mm × 0.25 cm column using a linear NaCl gradient from 0 to 2 M in H2O (pH previously adjusted to 5.0 with HCl). Elution occurred over a 60-min period at a flow rate of 3.0 mL/min. The separations were monitored by UV absorption at λ = 214 nm or λ = 232 nm for the hydrolase- and lyase-derived products, respectively. The peaks were collected separately, concentrated, desalted on a Sephadex G-15 column (1.0 × 50 cm), lyophilized and weighed.
Overdigestion of btCS-A and scCS-C
In order to provide the highest yields of disaccharides for composition analysis, 75 mg of both CS substrates were individually dissolved in 2.5 mL of the digesting buffer described above. Then, both chondroitinases (1.5 mg of 0.5 IU ABC lyase and 3.5 μg of 1 IU C lyase) were added together every 12 h, and the mixtures were kept at 37°C for 7 days. Both overdigested samples were size-fractionated on a Bio-Gel P-10 column and monitored by UV absorbance at 232 nm. The disaccharide peaks were individually pooled, concentrated, desalted, lyophilized and weighed. About 57 and 61 mg of btCS-A and scCS-C disaccharides were recovered. Correcting for water loss in lyase reactions, the best yields of disaccharides for btCS-A and scCS-C were ∼79 and 84%, respectively; less than 5% was recovered as monomers. The disaccharide types from each substrate were subsequently analyzed by SAX-HPLC. The integrals of the peaks were used to determine relative amounts of disaccharide types.
Structure determination and yields
The disaccharides and hexasaccharides were analyzed by a combination of MS and one-dimensional (1D) and two-dimensional (2D) NMR experiments, including 1D 1H (data not shown), 1H/1H double-quantum filtered COSY, 1H/1H total COSY (data not shown) and 1H/13C gHSQC spectroscopy. The locations of sulfate groups were assigned primarily on the basis of characteristic changes in 1H/13C-chemical shifts of cross-peaks in the 1H/13C gHSQC spectra. The identities of the isolated products along with a description of nomenclature used are presented in Table I. The absolute and relative yields of the disaccharides, and hexasaccharides, are tabulated in Table II. A table of 1H- and 13C-chemical shifts of all disaccharides studied, as well as those for the ΔC6;6;4S-ol hexasaccharide as an illustrative example is presented in Supplementary data (Table S2). The parameters and conditions for NMR and MS experiments, including specifications of the instruments, are also described in Supplementary data.
Supplementary data
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
Funding
This work was supported by a grant from the National Center for Research Resources of the NIH, RR005351. V.H.P. was partially supported by a post-doctoral fellowship (PDE #201019/2008-6) from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Conflict of interest
None declared.
Abbreviations
BSA, bovine serum albumin; btCS-A, bovine tracheal CS-A; COSY, correlation spectroscopy; CS, chondroitin sulfate; 1D, one-dimensional; GAG, glycosaminoglycan; GalNAc, N-acetylgalactosamine; gHSQC, gradient heteronuclear single-quantum coherence; GlcA, glucuronic acid; HA, hyaluronic acid; Hp, heparin; HPLC, high pressure liquid chromatography; MS, mass spectrometry; NMR, nuclear magnetic resonance; PAGE, polyacrylamide gel electrophoresis; SAX, strong anion-exchange; scCS-C, shark cartilage CS-C; SDS, sodium dodecyl sulfate; SEC, size-exclusion chromatography.
Supplementary Material
References
- Achur RN, Kakizaki I, Goel S, Kojima K, Madhunapantula SV, Goyal A, Ohta M, Kumar S, Takagaki K, Gowda DC. Structural interactions in chondroitin 4-sulfate mediated adherence of Plasmodium falciparum infected erythrocytes in human placenta during pregnancy-associated malaria. Biochemistry. 2008;47:12635–12643. doi: 10.1021/bi801643m. doi:10.1021/bi801643m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar JAK, Lima CR, Berto AGA, Michelacci YM. An improved methodology to produce Flavobacterium heparinum chondroitinases, important instruments for diagnosis of diseases. Biotechnol Appl Biochem. 2003;37:115–127. doi: 10.1042/ba20020089. doi:10.1042/BA20020089. [DOI] [PubMed] [Google Scholar]
- Beaulieu JF, Vachon PH, Chartrand S. Immunolocalization of extracellular-matrix components during organogenesis in the human small-intestine. Anat Embryol. 1991;183:363–369. doi: 10.1007/BF00196837. [DOI] [PubMed] [Google Scholar]
- Ben-Zaken O, Tzaban S, Tal Y, Horonchik L, Esko JD, Vlodavsky I, Taraboulos A. Cellular heparan sulfate participates in the metabolism of prions. J Biol Chem. 2003;278:40041–40049. doi: 10.1074/jbc.M301152200. doi:10.1074/jbc.M301152200. [DOI] [PubMed] [Google Scholar]
- Caterson B, Griffin J, Mahmoodian F, Sorrell JM. Monoclonal-antibodies against chondroitin sulfate isomers—their use as probe for investigating proteoglycan metabolism. Biochem Soc Trans. 1990;18:820–823. doi: 10.1042/bst0180820. [DOI] [PubMed] [Google Scholar]
- Cattaruzza S, Perris R. Proteoglycan control of cell movement during wound healing and cancer spreading. Matrix Biol. 2005;24:400–417. doi: 10.1016/j.matbio.2005.06.005. doi:10.1016/j.matbio.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Cohn RH, Cassiman JJ, Bernfield MR. Relationship of transformation, cell density, and growth-control to cellular distribution of newly synthesized glycosaminoglycan. J Cell Biol. 1976;71:280–294. doi: 10.1083/jcb.71.1.280. doi:10.1083/jcb.71.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aguiar C, Lobao-Soares B, Alvarez-Silva M, Trentin AG. Glycosaminoglycans modulate C6 glioma cell adhesion to extracellular matrix components and alter cell proliferation and cell migration. BMC Cell Biol. 2005;6 doi: 10.1186/1471-2121-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mattos DA, Stelling MP, Tovar AMF, Mourao PAS. Heparan sulfates from arteries and veins differ in their antithrombin-mediated anticoagulant activity. J Thromb Haemost. 2008;6:1987–1990. doi: 10.1111/j.1538-7836.2008.03145.x. doi:10.1111/j.1538-7836.2008.03145.x. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Yamada S, Fukui S, Sugahara K. Structural determination of novel sulfated octasaccharides isolated from chondroitin sulfate of shark cartilage and their application for characterizing monoclonal antibody epitopes. Glycobiology. 2007;17:631–645. doi: 10.1093/glycob/cwm021. doi:10.1093/glycob/cwm021. [DOI] [PubMed] [Google Scholar]
- Dodgson KS, Lloyd AG. Degradation of cartilage chondroitin sulphate by the chondroitinase of Proteus vulgaris. Biochem J. 1958;68:88–94. doi: 10.1042/bj0680088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domowicz M, Mangoura D, Schwartz NB. Cell specific-chondroitin sulfate proteoglycan expression during CNS morphogenesis in the chick embryo. Int J Dev Neurosci. 2000;18:629–641. doi: 10.1016/s0736-5748(00)00039-3. doi:10.1016/S0736-5748(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Doodes PD, Cao YX, Hamel KM, Wang YM, Rodeghero RL, Kobezda T, Finnegan A. CCR5 is involved in resolution of inflammation in proteoglycan-induced arthritis. Arthritis Rheum. 2009;60:2945–2953. doi: 10.1002/art.24842. doi:10.1002/art.24842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst S, Langer R, Cooney CL, Sasisekharan R. Enzymatic degradation of glycosaminoglycans. Crit Rev Biochem Mol Biol. 1995;30:387–444. doi: 10.3109/10409239509083490. doi:10.3109/10409239509083490. [DOI] [PubMed] [Google Scholar]
- Gandhi NS, Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des. 2008;72:455–482. doi: 10.1111/j.1747-0285.2008.00741.x. doi:10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- Gorio A, Lesma E, Vergani L, DiGiulio AM. Glycosaminoglycan supplementation promotes nerve regeneration and muscle reinnervation. Eur J Neurosci. 1997;9:1748–1753. doi: 10.1111/j.1460-9568.1997.tb01532.x. doi:10.1111/j.1460-9568.1997.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Gu K, Linhardt RJ, Laliberté M, Gu k, Zimmerman J. Purification, characterization and specificities of chondroitin lyases and glycuronidase from Flavobacterium heparinum. Biochem J. 1995;312:569–577. doi: 10.1042/bj3120569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamai A, Hashimoto N, Mochizuki H, Kato F, Makigushi Y, Horie K, Suzuki S. Two distinct chondroitin sulfate ABC lyases: An endoeliminase yielding tetrasaccharides and an exoeliminase preferentially acting on oligosaccharides. J Biol Chem. 1997;272:9123–9130. doi: 10.1074/jbc.272.14.9123. doi:10.1074/jbc.272.14.9123. [DOI] [PubMed] [Google Scholar]
- Handel TM, Johnson Z, Crown SE, Lau EK, Sweeney M, Proudfoot AE. Regulation of protein function by glycosaminoglycans—as exemplified by chemokines. Ann Rev Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. doi:10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- Hardingham TE, Fosang AJ, Hey NJ, Hazell PK, Kee WJ, Ewins RJF. The sulfation pattern in chondroitin sulfate chains investigated by chondroitinase ABC and ACIL digestion and reactivity with monoclonal antibodies. Carbohydr Res. 1994;255:241–254. doi: 10.1016/s0008-6215(00)90982-0. doi:10.1016/S0008-6215(00)90982-0. [DOI] [PubMed] [Google Scholar]
- He L, Giri TK, Vicente CP, Tollefsen DM. Vascular dermatan sulfate regulates the antithrombotic activity of heparin cofactor II. Blood. 2008;111:4118–4125. doi: 10.1182/blood-2007-12-127928. doi:10.1182/blood-2007-12-127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horonchik L, Tzaban S, Ben-Zaken O, Yedidia Y, Rouvinski A, Papy-Garcia D, Barritault D, Vlodavsky I, Taraboulos A. Heparan sulfate is a cellular receptor for purified infectious prions. J Biol Chem. 2005;280:17062–17067. doi: 10.1074/jbc.M500122200. doi:10.1074/jbc.M500122200. [DOI] [PubMed] [Google Scholar]
- Imberty A, Lortat-Jacob H, Perez S. Structural view of glycosaminoglycan-protein interactions. Carbohydr Res. 2007;342:430–439. doi: 10.1016/j.carres.2006.12.019. doi:10.1016/j.carres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Inatani M, Haruta M, Honjo M, Oohira A, Kido N, Takahashi M, Honda Y, Tanihara H. Upregulated expression of N-syndecan, a transmembrane heparan sulfate proteoglycan, in differentiated neural stem cells. Brain Res. 2001;920:217–221. doi: 10.1016/s0006-8993(01)02856-6. doi:10.1016/S0006-8993(01)02856-6. [DOI] [PubMed] [Google Scholar]
- Jandik KA, Gu KA, Linhardt RJ. Action pattern of polysaccharide lyases on glycosaminoglycans. Glycobiology. 1994;4:289–296. doi: 10.1093/glycob/4.3.289. doi:10.1093/glycob/4.3.289. [DOI] [PubMed] [Google Scholar]
- Jin L, Abrahams JP, Skinner R, Petitou M, Pike RN, Carrell RW. The anticoagulant activation of antithrombin by heparin. Proc Natl Acad Sci USA. 1997;94:14683–14688. doi: 10.1073/pnas.94.26.14683. doi:10.1073/pnas.94.26.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan CD, O'Neill SK, Koreny T, Czipri M, Finnegan A. Development of inflammation in proteoglycan-induced arthritis is dependent on Fc gamma R regulation of the cytokine/chemokine environment. J Immunol. 2002;169:5851–5859. doi: 10.4049/jimmunol.169.10.5851. [DOI] [PubMed] [Google Scholar]
- Khorlin AY, Vikha IV, Milishnikov AN. Subunit structure of testicular hyaluronidase. FEBS Lett. 1973;31:107–110. doi: 10.1016/0014-5793(73)80084-5. doi:10.1016/0014-5793(73)80084-5. [DOI] [PubMed] [Google Scholar]
- Koninger J, Giese NA, Bartel M, di Mola FF, Berberat PO, di Sebastiano P, Giese T, Buchler MW, Friess H. The ECM proteoglycan decorin links desmoplasia and inflammation in chronic pancreatitis. J Clin Pathol. 2006;59:21–27. doi: 10.1136/jcp.2004.023135. doi:10.1136/jcp.2004.023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson W, Gundlach MW, Schimid TM, Conrad HE. Selective hydrolysis of chondroitin sulfates by hyaluronidase. Biochemistry. 1984;23:368–375. doi: 10.1021/bi00297a028. doi:10.1021/bi00297a028. [DOI] [PubMed] [Google Scholar]
- Lindahl U, Hook M. Glycosaminoglycans and their binding to biological macromolecules. Ann Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. doi:10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Lunin VV, Li YG, Linhardt RJ, Miyazono H, Kyogashima M, Kaneko T, Bell AW, Cygler M. High-resolution crystal structure of Arthrobacter aurescens chondroitin AC lyase: An enzyme-substrate complex defines the catalytic mechanism. J Mol Biol. 2004;337:367–386. doi: 10.1016/j.jmb.2003.12.071. doi:10.1016/j.jmb.2003.12.071. [DOI] [PubMed] [Google Scholar]
- Michel G, Pojasek K, Li YG, Sulea T, Linhardt RJ, Raman R, Prabhakar V, Sasisekharan R, Cygler M. The structure of chondroitin B lyase complexed with glycosaminoglycan oligosaccharides unravels a calcium-dependent catalytic machinery. J Biol Chem. 2004;279:32882–32896. doi: 10.1074/jbc.M403421200. doi:10.1074/jbc.M403421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelacci YM, Dietrich CP. Chondroitinase C from Flavobacterium heparinum. J Biol Chem. 1976;251:1154–1158. [PubMed] [Google Scholar]
- Mucci A, Schenetti L, Volpi N. H-1 and C-13 nuclear magnetic resonance identification and characterization of components of chondroitin sulfates of various origin. Carbohydr Polym. 2000;41:37–45. doi:10.1016/S0144-8617(99)00075-2. [Google Scholar]
- Muramatsu T, Muramatsu H. Glycosaminoglycan-binding cytokines as tumor markers. Proteomics. 2008;8:3350–3359. doi: 10.1002/pmic.200800042. doi:10.1002/pmic.200800042. [DOI] [PubMed] [Google Scholar]
- Muthusamy A, Achur RN, Valiyaveettil M, Madhunapantula SV, Kakizaki I, Bhavanandan VP, Gowda CD. Structural characterization of the bovine tracheal chondroitin sulfate chains and binding of Plasmodium falciparum-infected erythrocytes. Glycobiology. 2004;14:635–645. doi: 10.1093/glycob/cwh077. doi:10.1093/glycob/cwh077. [DOI] [PubMed] [Google Scholar]
- Nadanaka S, Sugahara K. The unusual tetrasaccharide sequence GlcA β1-3GalNAc(4-sulfate)β1-4GlcA(2-sulfate)β1-3GalNAc(6-sulfate) found in the hexasaccharides prepared by testicular hyaluronidase digestion of shark cartilage chondroitin sulfate D. Glycobiology. 1997;7:253–263. doi: 10.1093/glycob/7.2.253. doi:10.1093/glycob/7.2.253. [DOI] [PubMed] [Google Scholar]
- Oettl M, Hoechstetter J, Asen I, Bernhardt G, Buschauer A. Comparative characterization of bovine testicular hyaluronidase and a hyaluronate lyase from Streptococcus agalactiae in pharmaceutical preparations. Eur J Pharm Sci. 2003;18:267–277. doi: 10.1016/s0928-0987(03)00022-8. doi:10.1016/S0928-0987(03)00022-8. [DOI] [PubMed] [Google Scholar]
- Ototani N, Yosizawa Z. Purification of chondroitinase-B and chondroitinase-C using glycosaminoglycan-bound AH-Sepharose-4B. Carbohydr Res. 1979;70:295–306. doi: 10.1016/s0008-6215(00)87109-8. doi:10.1016/S0008-6215(00)87109-8. [DOI] [PubMed] [Google Scholar]
- Perris R, Perissinotto D, Pettway Z, BronnerFraser M, Morgelin M, Kimata K. Inhibitory effects of PG-H/aggrecan and PG-M/versican on avian neural crest cell migration. FASEB J. 1996;10:293–301. doi: 10.1096/fasebj.10.2.8641562. [DOI] [PubMed] [Google Scholar]
- Pettway Z, Domowicz M, Schwartz NB, BronnerFraser M. Age-dependent inhibition of neural crest migration by the notochord correlates with alterations in the S103L chondroitin sulfate proteoglycan. Exp Cell Res. 1996;225:195–206. doi: 10.1006/excr.1996.0170. doi:10.1006/excr.1996.0170. [DOI] [PubMed] [Google Scholar]
- Pomin VH, Sharp JS, Li XY, Wang LC, Prestegard JH. Characterization of glycosaminoglycans by N-15 NMR spectroscopy and in vivo isotopic labeling. Anal Chem. 2010;82:4078–4088. doi: 10.1021/ac1001383. doi:10.1021/ac1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothacharoen P, Kalayanamitra K, Deepa SS, Fukui S, Hattori T, Fukushima N, Hardingham T, Kongtawelert P, Sugahara K. Two related but distinct chondroitin sulfate mimetope octasaccharide sequences recognized by monoclonal antibody WF6. J Biol Chem. 2007;282:35232–35246. doi: 10.1074/jbc.M702255200. doi:10.1074/jbc.M702255200. [DOI] [PubMed] [Google Scholar]
- Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol. 2005;12:267–277. doi: 10.1016/j.chembiol.2004.11.020. doi:10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Richard B, Swanson R, Olson ST. The signature 3-O-sulfo group of the anticoagulant heparin sequence is critical for heparin binding to antithrombin but is not required for allosteric activation. J Biol Chem. 2009;284:27054–27064. doi: 10.1074/jbc.M109.029892. doi:10.1074/jbc.M109.029892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigden DJ, Jedrzejas MJ. Structures of Streptococcus pneumoniae hyaluronate lyase in complex with chondroitin and chondroitin sulfate disaccharides—insights into specificity and mechanism of action. J Biol Chem. 2003;278:50596–50606. doi: 10.1074/jbc.M307596200. doi:10.1074/jbc.M307596200. [DOI] [PubMed] [Google Scholar]
- Sasisekharan R, Raman R, Prabhakar V. Glycomics approach to structure–function relationships of glycosaminoglycans. Ann Rev Biomed Eng. 2006;8:181–231. doi: 10.1146/annurev.bioeng.8.061505.095745. doi:10.1146/annurev.bioeng.8.061505.095745. [DOI] [PubMed] [Google Scholar]
- Shaya D, Hahn BS, Bjerkan TM, Kim WS, Park NY, Sim JS, Kim YS, Cygler M. Composite active site of chondroitin lyase ABC accepting both epimers of uronic acid. Glycobiology. 2008;18:270–277. doi: 10.1093/glycob/cwn002. doi:10.1093/glycob/cwn002. [DOI] [PubMed] [Google Scholar]
- Singh K, Gittis AG, Nguyen P, Gowda DC, Miller LH, Garboczi DN. Structure of the DBL3x domain of pregnancy-associated malaria protein VAR2CSA complexed with chondroitin sulfate A. Nat Struct Mol Biol. 2008;15:932–938. doi: 10.1038/nsmb.1479. doi:10.1038/nsmb.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell JM, Carrino DA, Caplan AI. Structural domains in chondroitin sulfate identified by anti-chondroitin sulfate monoclonal-antibodies—immunosequencing of chondroitin sulfates. Matrix. 1993;13:351–361. doi: 10.1016/s0934-8832(11)80040-5. [DOI] [PubMed] [Google Scholar]
- Stern R, Jedrzejas MJ. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara K, Mikami T, Uyama T, Mizugushi S, Nomura K, Kitagawa H. Recent advances in structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struct Biol. 2003;13:612–620. doi: 10.1016/j.sbi.2003.09.011. doi:10.1016/j.sbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Takagaki K, Munakata H, Majima M, Endo M. Enzymatic reconstruction of a hybrid glycosaminoglycan containing 6-sulfated, 4-sulfated, and unsulfated N-acetylgalactosamine. Biochem Biophys Res Commun. 1999;258:741–744. doi: 10.1006/bbrc.1999.0697. doi:10.1006/bbrc.1999.0697. [DOI] [PubMed] [Google Scholar]
- Takagaki K, Nakamura T, Izumi J, Saitoh H, Endo M, Kojima K, Kato I, Majima M. Characterization of hydrolysis and transglycosylation by testicular hyaluronidase using ion-spray mass-spectrometry. Biochemistry. 1994;33:6503–6507. doi: 10.1021/bi00187a017. doi:10.1021/bi00187a017. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Jalkanen M, Vainio S, Bernfield M. Cell-surface proteoglycan expression correlates with epithelial mesenchymal interaction during tooth morphogenesis. Dev Biol. 1988;129:565–572. doi: 10.1016/0012-1606(88)90401-0. doi:10.1016/0012-1606(88)90401-0. [DOI] [PubMed] [Google Scholar]
- Tully SE, Mabon R, Gama CI, Tsai SM, Liu XW, Hsieh-Wilson LC. A chondroitin sulfate small molecule that stimulates neuronal growth. J Am Chem Soc. 2004;126:7736–7737. doi: 10.1021/ja0484045. doi:10.1021/ja0484045. [DOI] [PubMed] [Google Scholar]
- vanPutten JPM, Hayes SF, Duensing TD. Natural proteoglycan receptor analogs determine the dynamics of Opa adhesin-mediated gonococcal infection of Chang epithelial cells. Infect Immun. 1997;65:5028–5034. doi: 10.1128/iai.65.12.5028-5034.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi N. Fractionation of heparin, dermatan sulfate, and chondroitin sulfate sequential precipitation—a method to purify a single glycosaminoglycan species from a mixture. Anal Biochem. 1994;218:382–391. doi: 10.1006/abio.1994.1196. doi:10.1006/abio.1994.1196. [DOI] [PubMed] [Google Scholar]
- Warner RG, Hundt C, Weiss S, Turnbull JE. Identification of the heparan sulfate binding sites in the cellular prion protein. J Biol Chem. 2002;277:18421–18430. doi: 10.1074/jbc.M110406200. doi:10.1074/jbc.M110406200. [DOI] [PubMed] [Google Scholar]
- Xie HX, Nie P, Chang MX, Liu Y, Yao WJ. Gene cloning and functional analysis of glycosaminoglycan-degrading enzyme chondroitin AC lyase from Flavobacterium columnare G(4) Arch Microbiol. 2005;184:49–55. doi: 10.1007/s00203-005-0009-0. doi:10.1007/s00203-005-0009-0. [DOI] [PubMed] [Google Scholar]
- Zhang ZQ, Park Y, Kemp MM, Zhao WJ, Im AR, Shaya D, Cygler M, Kim YS, Linhardt RJ. Liquid chromatography-mass spectrometry to study chondroitin lyase action pattern. Anal Biochem. 2009;385:57–64. doi: 10.1016/j.ab.2008.10.014. doi:10.1016/j.ab.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.