Abstract
There are few effective obesity interventions directed towards younger children, particularly young minority children. This paper describes the design, intervention, recruitment methods, and baseline data of the ongoing Positive Lifestyles for Active Youngsters (Team PLAY) study. This randomized controlled trial is designed to test the efficacy of a 6-month, moderately intense, primary care feasible, family-based behavioral intervention, targeting both young children and their parent, in promoting healthy weight change.
Participants are 270 overweight and obese children (ages 4 to 7 years) and their parent, who were recruited from a primarily African American urban population. Parents and children were instructed in proven cognitive behavioral techniques (e.g. goal setting, self-talk, stimulus control and reinforcement) designed to encourage healthier food choices (more whole grains, fruits and vegetables, and less concentrated fats and sugar), reduce portion sizes, decrease sweetened beverages and increase moderate to vigorous physical activity engagement. The main outcome of this study is change in BMI at two years post enrollment.
Recruitment using reactive methods (mailings, TV ads, pamphlets) was found to be more successful than using only a proactive approach (referral through physicians). At baseline, most children were very obese with an average BMI z-score of 2.6. Reported intake of fruits and vegetables and minutes of moderate to vigorous physical activity engagement did not meet national recommendations. If efficacious, Team PLAY would offer a model for obesity treatment directed at families with young children that could be tested and translated to both community and primary care settings.
Keywords: randomized controlled trial, children, obesity
1. Introduction
Childhood obesity is a major public health concern recognized by the medical community as a serious chronic disease.[1–3] Treating childhood obesity is difficult and time-consuming for most health care providers, who cite lack of expertise and/or resources as reasons for not proactively addressing their patients’ overweight or obesity.[4,5] Current recommendations for the identification, evaluation, and treatment of childhood obesity in primary care are based on a mix of evidence of varying quality and expert consensus.[6,7] These guidelines emphasize both parent and child behavioral changes that will result in healthful eating and an active lifestyle. Regular contact of parent and child with the clinician is also emphasized.
Family-based interventions for childhood obesity make intuitive sense, particularly for young children who spend significant time with their parents. Family-based interventions have been effective in the treatment of other childhood illnesses such as asthma.[8] Increasingly common, family-based interventions including children and their parent(s) have shown significant improvement in weight-related measures when compared to controls.[9] A 2009 Cochrane review on treatment of childhood obesity found that family-targeted behavioral lifestyle interventions were more successful in decreasing BMI than standard care at six months after follow-up.[10] Moreover, family-based interventions provide an opportunity to examine family functioning, an understudied factor relevant to success in childhood obesity treatment programs. Family relationships and interactional patterns affect not only how family members respond to the diagnosis of a child’s health condition, but also influence subsequent health outcomes through their impact on disease management.[11–13] Families that function well as units are more likely to cope better with the demands of caring for an overweight child and institute the behavioral and environmental changes required for treatment. Preliminary data from this trial has already shown a positive relationship between better family functioning and greater intervention attendance.[14]
While research on weight management interventions for obese and overweight children has increased in the past several years, generalizable evidence-based approaches to evaluation and treatment remain limited, particularly in younger children.[15,16] Current 2010 United States Preventive Services Task Force recommendations [17], which differ from expert consensus [6,7], do not recommend screening for obesity in children less than 6 years given the lack of sufficient evidence for efficacious treatments directed towards younger children that are available to the primary care provider. A review of 31 family-based interventions published between 1977 and 2004 [18] showed only two studies that focused on very young children as their sample population [19,20], despite the high rates of overweight and obesity in children ages 5 and under.[21,22]
The majority of research in the field has been conducted in motivated, middle class, Caucasian populations [10], but there is some evidence that family-based interventions for childhood obesity are also effective with more diverse populations. For example, results of a recent meta-analysis suggested that comprehensive, lifestyle programs that include parental involvement were more efficacious among minority children.[23]
While comprehensive lifestyle interventions have had success in addressing overweight and obesity in children, the question of intensity (participant contact) and sustainability have not been adequately answered.[24] The USPTF review suggests that moderate to high intensity programs are most effective.[15] The recently published Bright Bodies randomized trial produced a long-term (2 years) treatment effect on anthropometric and metabolic markers after delivery of a family-based intervention in an inner-city ethnically diverse population of children ages 8 to 16 years.[25] However, while successful, this high-intensity (~98 hours of contact) program, which took place at a pediatric obesity clinic, would not be feasible in general primary care settings. Additional studies are needed to determine if a less intense intervention, with less participant burden would be as efficacious and result in lower drop-out rates, given retention is a problem in many large randomized trials that focus on childhood overweight and obesity.[10]
The Positive Lifestyles for Active Youngsters (Team PLAY) trial seeks to address specific gaps in the childhood obesity treatment literature by providing a primary care feasible, moderately intense intervention, directed toward very young children and their parent(s) in a primarily African American urban population. The following presents an overview of the recruitment methods, intervention design, and baseline characteristics of the Team PLAY cohort.
2. Objectives of Team PLAY
The Team PLAY trial was designed to determine if a 6-month moderately intense, family-based behavioral intervention, targeting both child and parent in a primarily African American urban population (that has the potential to be implemented in primary care; e.g. primary care feasible), is superior to standard primary care in promoting healthy weight change in young overweight or obese children ages 4 to 7 years. The Team PLAY intervention focused on behavior change that would facilitate healthy eating patterns and increases in physical activity as a way to slow the rate of weight gain or promote weight maintenance. We hypothesize that lifestyle changes acquired during the Team PLAY intervention will result in a significant decrease in children’s BMI at two years after enrollment, when compared to changes in BMI resulting from the usual care delivered by primary care providers for childhood overweight and obesity. Secondary aims include (a) examining changes in children’s fat-free mass, waist circumference, dietary intake, physical activity, body esteem, child adjustment and parental perception of health provider’s support with respect to the management of their children’s food choices and physical activity behaviors, and (b) evaluating the effect of the intervention on family functioning and parent’s BMI and waist circumference and (c) examining psychosocial measures as potential predictors, mediators, and moderators of change in the BMI, dietary and physical activity behaviors.
3. Study Design
3.1 General Design
Two hundred seventy overweight or obese children between the ages of 4 and 7 years and the child’s parent or a regular caregiver (e.g. grandparent, aunt) were randomly assigned after a baseline assessment to the intervention or standard primary care arm of the study. Participants randomized to the intervention group received intense group-based counseling regarding developmentally appropriate physical activities, strategies for reducing sedentary behaviors, nutrition information, and behavioral counseling that included a self-management program to use at home. Both the intervention and standard primary care groups received usual care from their primary care provider. In an effort to somewhat standardize the usual care of local health care providers, physicians of the enrolled children were provided general written information about the evaluation and treatment of overweight children developed by local experts.[26,27] These included resources for physicians (e.g. evaluation forms, reimbursable ICD-9 codes for obesity, etc.) and patients (e.g., nutrition educational materials) to be used at primary care visits. Study visits consist of a comprehensive physical examination that included a medical history, anthropometric measures, body composition evaluation, nutritional assessment, measures of physical activity, and a behavioral/psychosocial assessment at baseline, 6, 12, 18, and 24 months. Change in child Body Mass Index (BMI) is the primary outcome of the trial.
3.2 Study Population
Recruitment efforts were initially targeted to only local primary care community clinics that serve low-income, minority children. Recruitment strategies were later expanded to include the general population of the urban, racially diverse community in which the trial is being conducted. Given the demographic characteristics of this community we successfully enrolled high minority participation despite the change in recruitment strategies.
3.3 Inclusion Criteria
The study was open to all children 4 to 7 years of age, male or female, of any race who had a BMI ≥ 85% for age and gender.
3.4 Exclusion Criteria
Children were excluded from the study for the any of following: 1) history of diabetes mellitus; 2) history of significant renal, hepatic, cardiovascular, or gastrointestinal disease; 3) receiving drugs known to alter glucose homeostasis; 4) physical disabilities that limit physical activity (i.e. orthopedic, congenital); 5) psychological disabilities that might limit participation; 6) lack of access to a telephone; 7) current participation in another clinical trial (current participation in an observational study is not an exclusion); 8) other medical or behavioral factors that, in the judgment of the Principal Investigator, may interfere with study participation or the ability to follow the intervention protocol; 9) inability to understand and speak English.
3.5 Parent Participation
A parent or legal guardian agreed to random placement into the intervention or standard primary care (control) group. At least one parent or regular caregiver (e.g. grandparent, aunt, etc.) agreed to attend the group sessions on a consistent basis if assigned to the intervention group. Siblings were not allowed to attend the group sessions. Neither childcare nor transportation to intervention sessions were provided.
3.6. Recruitment and Retention
Recruitment of minority and underserved families was a priority in this study since this group has some of the highest rates of childhood obesity [22] and because our community has a concentration of potentially eligible at risk participants.[28,29] Initial recruitment efforts were intentionally targeted only to local community clinics that serve mainly low-income minority children with clinic primary care physicians referring participants to the study (Clinic Recruitment Phase). However, because recruitment goals were not being met using this proactive approach only, recruitment was opened to pediatric private practices and direct mailings were sent to families with children in our target age range. In addition, a study website and local television spots featuring 1996 Olympic gold medalist, Rochelle Stevens, were utilized (Community Recruitment Phase). Throughout recruitment Team PLAY was described as a program to promote healthy growth in children through proper eating and physical activity, rather than as a weight loss program. This description was chosen to convey sensitivity about an often stigmatized condition and minimize the possibility of embarrassment or harm to self-esteem among the participants.[30]
Emphasis was placed on developing and using a sophisticated system for monitoring, scheduling, and tracking participants. Detailed appointment reminder cards and telephone follow-up are being utilized. Enrolled parents are mailed visit reminders and receive phone calls on the day prior to their scheduled assessment visits. All missed appointments are followed up within 24 hours by a call to the participant’s family or to someone on the list of contacts the participant has provided. If the appointment has been missed, another appointment is made promptly. Extensive efforts are made to achieve all appointment windows for outcome data collection. Additionally, at enrollment sufficient time was spent with each child and their family in order to instruct them about the study and what would be expected of them. After enrollment, to increase adherence, clinic personnel has strived to maintain the participants’ interest and enthusiasm. Clinic personnel maintain close contact with participants and their families, make clinic visits pleasant and convenient, and provide clear written instructions to promote attendance at follow-up visits. Clinic staff makes efforts to eliminate barriers for continued follow-up. For example, if a participant has difficulty with transportation to attend their follow-up, clinic personnel would assist in arranging transportation for that individual. Incentives are being used as an aide to adherence for attending study visits. All participants are offered a fixed monetary compensation to offset transportation and childcare costs for each follow-up visit attended (this monetary compensation was not provided for intervention session attendance).
3.7 Screening for Eligibility
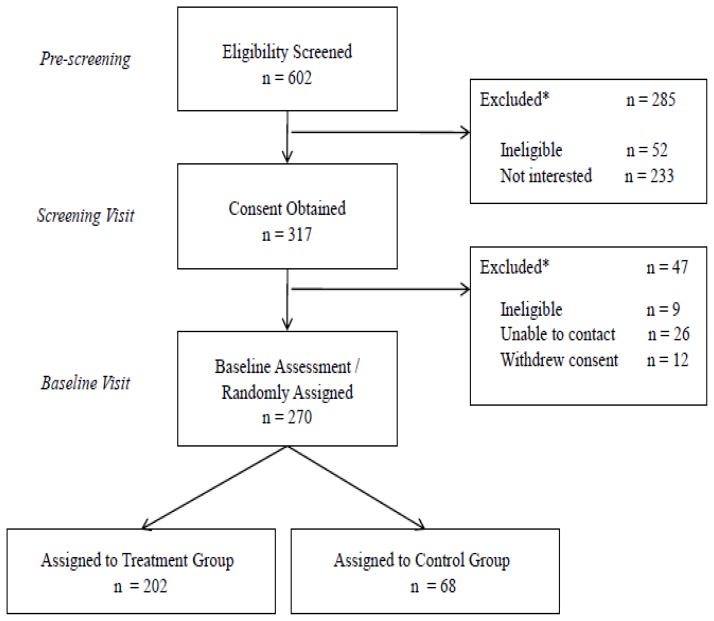
Forty-five percent of families who responded to recruitment efforts were enrolled in the study (Figure 1). Initial contact (pre-screening) with potential participants was conducted by telephone after which a preliminary Screening Visit was scheduled. The purpose of the Screening Visit was to assess eligibility, obtain informed consent, contact information, behavioral information, nutrition and physical activity questionnaires, and medical history. Of 602 respondents, overall 90% were judged eligible on the basis of their age, calculated BMI (from reported weight and height), medical history and ability to read and understand English. Only 3% of respondents were excluded because of a BMI less than the 85th percentile. However, of the eligible respondents, half were unwilling or unable to participate for other reasons (Figure 1, legend). Therefore, consent was obtained and screening performed on only 53% of initial respondents who were then scheduled for a Baseline Visit for completion of study measures and randomization to either standard primary care (control group) or intervention (treatment group). 85% of participants who attended the Screening Visit participated in a Baseline Visit and were randomized into the study.
Figure 1.
*Specific reason for exclusions: 52 ineligible by criteria (28: age < 4 or > 7 years, 11: BMI < 85th percentile, 10: language barrier, 1: developmental disability, 1: physical disability, 1: diabetic on meds); 77: no contact; 49: missed multiple visits; 56: scheduling conflict; 10: didn’t want to participate in research; 17: participation was too difficult, 16: wrong/bad telephone numbers provided; 8: parent decided that their child didn’t need help. **Specific reasons for exclusions: 9 ineligible by criteria (8: BMI < 85th percentile, 1: developmental disability), 26: missed multiple clinic visits/unable to contact; 12 withdrew consent (3: parent/child was uncomfortable with study related activities; 2: moved out of regional area; 2: family issues; 2: scheduling conflict; 2: didn’t want to participate in research; 1: child was not compliant)
3.8 Randomization
Our randomization strategy differed according to recruitment phase (Clinic versus Community). Initially, we intended to recruit entirely from ten clinics located in areas of severe poverty and other SES stressors (safety-net clinics). These clinics were matched based on race/ethnicity and number of children served, resulting in 5 pairs of clinics. One clinic within each of the five pairs was randomized to the intervention and one was randomized to the standard primary care condition. It was planned after recruiting 10 to 15 participants from each clinic that paired clinics would switch their treatment status. However, we experienced difficulty meeting our recruitment goals using this cross over approach. The low number of participants being recruited during standard primary care assignment led our team to suspect unmasking such that clinics recognized, through patient contact, which treatment status they were currently assigned and preferentially made referrals during intervention enrollment phases (e.g., referral bias). Upon further investigation, Team PLAY health care clinics/providers informed investigators that randomization into the standard primary care arm was a major deterrent to recruitment.
To overcome this barrier, we expanded our recruitment approach to include reactive strategies utilizing the entire community. During the Community Recruitment Phase we included participants from private pediatric primary care offices, utilized direct community mailings, along with use of internet and television advertising. An individual-level randomization scheme of 3 intervention to 1 control participant, stratified by clinic, was used, as this approach allowed us to effectively test our intervention while maintaining sufficient statistical power. By opening recruitment to the entire community and using reactive strategies enrollment no longer depended exclusively on health care provider referrals, which markedly increased the number of clinics from which participants were recruited. Therefore, throughout the Community Recruitment Phase, fewer participants from each clinic were enrolled, many participants were now self-referred, therefore, health care providers were likely less aware of the number and randomization status of their patients enrolled in the Team PLAY study, eliminating the referral bias seen during the earlier Clinic Recruitment Phase.
3.9 Baseline and Follow-up Assessments
A schedule for data collection is presented in Table 1. At the Baseline Visit, informed consent and contact information was reviewed with parents. Anthropometric measures (height, weight, and waist and hip circumference) were obtained on both child and parent. BMI was calculated from weight and height measurements. Although parents completed questionnaires for their children, all other measurements pertained to child participants or their family.
Table 1.
Schedule of clinic activities
| Activity | SV | BV | FU1 | FU2 | FU3 | FU4 |
|---|---|---|---|---|---|---|
| Eligibility assessed | x | x | ||||
| Informed consent obtained | x | |||||
| Contact information | x | x | x | x | x | x |
| Demographics | x | |||||
| Medical history | x | |||||
| Orientation to group assignment | x | |||||
| Physical activity questionnaire | x | x | x | x | x | |
| Diet questionnaire | x | x | x | x | x | |
| Psychosocial questionnaires | x | x | x | x | x | |
| Vital signs | x | x | x | x | x | x |
| Weight/height | x | x | x | x | x | x |
| Waist and hip circumference | x | x | x | x | x | x |
| Physical exam | x | |||||
| Adherence | x | x | x | x | ||
| Interval medical history/adverse events assessed | x | x | x | x | x | |
| DXA scan1 | x | x | x | |||
| Accelerometer data collected | x | x | x | |||
| Parental measuresa | x | x | x |
Note. SV = screening visit. BV = baseline visit. FU = follow-up visits 1–4 1DXA scans included whole body. Females participants who have begun their menstrual cycle were asked to give a urine sample to test for pregnancy prior to scanning.
Parental measures included height, weight, and waist circumference
A physical examination was conducted and children’s blood pressure and resting pulse were obtained. Dual-energy X-ray absorptiometry (DXA) was performed and an accelerometer were placed. Dietary, physical activity and behavioral questionnaires were obtained and participants were randomized to intervention status. At the conclusion of this visit, all parent-child dyads met individually with an interventionist who reviewed and gave feedback and dietary recommendations based on their dietary questionnaire, along with general physical activity recommendations.
Follow-up assessments occur at 6, 12, 18 and 24 months after Baseline Visit. All follow-up visits include: child anthropometric measures, blood pressure, resting heart rate, dietary, physical and behavioral questionnaires as well as documentation of changes to contact information, interval medical history, assessment of any adverse events, and review of adherence. The 12 and 24 month follow-up visits also include collection of body composition by DXA, accelerometry placement, child tanner staging and parent anthropometric data.
3.10 Primary and Secondary Outcome Measures
Baseline measurements are complete. Follow up measurements are underway. Only staff blinded to treatment status perform post-randomization measurements. All staff were trained at the start of the trial using a common standardized protocol and were monitored for drift during follow up.
3.10.1 Socio-Demographic Characteristics
At their Screening Visit, parents provided social and demographic information that included race/ethnicity, number of family members, annual household income, highest educational attainment, and marital status.
3.10.2. Physical Characteristics
General health
To ensure the safety of the children who participated in the study, all children received a complete physical exam at their Baseline Visit by a board-certified pediatrician. This exam assessed all body systems and specifically attempted to identify physical findings that may be associated with overweight and obesity in children such as acanthosis nigricans, abdominal tenderness or hepatosplenomegaly, orthopedic anomalies and abnormal pubertal maturation.
Anthropometric measures
All anthropometric measurers were determined in accordance with the guidelines defined in the NHANES Anthropometric Procedures Manual.[31] Specifically, weight was measured on the Detecto Balance Beam Scale, which was calibrated with fixed known standard weights weekly and certified annually by the local Bureau of Weights and Measures, and measured in kilograms. Height was measured in centimeters as the distance from the soles of the feet to the top of the head with the participant standing erect and looking straight ahead, using a stadiometer attached to the wall. Body mass index (BMI) in kg/m2, the primary outcome, was calculated from the measured weight and height. A standardized BMI score (BMI z-score) was calculated for each child participant following guidelines established by the CDC. Waist circumference, which can provide indirect information about visceral fat, cardiovascular risk, and insulin resistance [32–34], was measured in centimeters using a Grafco tape measure and was assessed at the smallest horizontal circumference in the area between the ribs and the iliac crest.
Body composition
Child body composition was assessed using dual-emission X-ray absorptiometry (DXA). Measurement of total mass, bone free lean mass (LM), fat mass (FM), bone area, bone mineral content (BMC) and bone density (BMD) of the whole body was performed using the Hologic Discovery A (Bedford, MA) software version 8.3 and analyzed using APEX software 2.3. A DXA-certified research assistant performed the DXA measurements using a standardized protocol. Individuals were scanned two times with body replacement after the first scan. The average of the two scans is reported. The coefficient variation was 0.24% for BMC, 0.02% for LM, 0.05% for FM, 0.04% for total mass, 0.03% for % FM and 0.06% for BMD. All DXA scans were reviewed for quality assurance by one of the study co-investigators (F.T.).
Cardiovascular function
Children’s resting blood pressure and radial pulse was measured to determine whether the study intervention has an impact on these important physiologic parameters. Trained personnel utilizing the guidelines of the American Heart Association and the National Heart, Lung, and Blood Institute [35] obtained these measurements. Appropriate arm size was selected and both heart rate and blood pressure measurements were repeated 3 times with the mean of the 3 measurements recorded. At least 30 seconds elapsed between the three readings, and each time the cuff bladder was allowed to fully deflate.
3.10.3. Diet and Physical Activity
Dietary assessment
The Block Kid’s FFQ, a food frequency questionnaire (www.nutritionquest.com) for 2–7 year olds was interviewer administered to the parents of each child participant. All interviewers were certified by a Registered Dietitian and re-certified every 6 months. The Block Kid’s FFQ was developed for commercial use (Nutriquest.com) to assess food and nutrient intake in children across a wide range of nutrients and food items. The food list for this questionnaire was developed from the NHANES III dietary recall data. The nutrient database was developed from the USDA Nutrient Database for Standard Reference. The frequency of 90 food questions was obtained on a daily, weekly or monthly basis reflecting habits over the previous 6 months. While this tool has not specifically been validated for use by parents with children in our age range, other FFQ developed for use with parent report of children ages 1–10 have shown reasonable reliability and validity.[36,37] In children ages 3–5 the Block Kid’s FFQ provide the same median intake as that obtained from a 3 day food record.[38–40]
Physical activity monitoring
The physical activity monitor each child was asked to wear was an ActiGraph GT1M (The ActiGraph, Fort Walton Beach, Florida). For the initial 86 participants the activity monitor was worn on the hip for seven consecutive days (except bathing). After 2 activity monitors were rendered irreparable due to nocturnal enuresis, the protocol was changed and participants were asked to wear the activity monitor only during waking hours. The ActiGraph has a built-in dual axis accelerometer designed to measure and record time varying accelerations ranging in magnitude from approximately 0.05 to 2 Gs. This small (2” × 1.5” × 0.6”) monitor is placed on a belt and secured around the waist of the child and programmed to collect 60 sec epoch (intervals) motion counts. Accelerometry has been shown to provide valid estimates of physical activity among young children [41] and the ActiGraph has been used successfully by other investigators in children.[42]
Correlates of physical activity
The Amherst Health and Activity Survey, previously validated for uses as a proxy measure for reporting physical activity engagement [43], was used to assess correlates of physical activity in children.[44] Of particular interest in the current study were 6 questions that assess aspects of the neighborhood environment shown to promote or hinder physical activity [45], including neighborhood features (i.e., presence of sidewalks, enjoyable scenery, lack of high crime rates), availability of public parks and proximity to parks, and perceptions of neighborhood and park safety. The Amherst neighborhood environment variables have demonstrated acceptable test-retest reliability (ICCs range from .68 to .86).[44]
3.10.4 Child and Family Psychosocial Characteristics
Children and their parents completed a battery of psychosocial measures to evaluate potential predictors, mediators, and moderators of change in the primary study outcomes and specific target behaviors related to the intervention. These are described below.
Body esteem
The Revised Body-Esteem Scale was administered to assess children’s attitudes and feelings about their body and physical appearance.[46] A 3-point response scale (1= no, 2= sometimes, and 3 = yes) was used to rate agreement with items rather than a yes/no response format in order to increase variability, as was done in previous studies with young children.[47] The total score on this 20-item questionnaire is calculated as the sum of individual items, and ranges from 20 to 60 with a higher score indicating better body esteem. Examination of this measure indicated that at baseline our 4-year-old participants were not able to provide reliable self-report data on body esteem, evidenced by a much lower internal consistency estimate when including these children (α = .60 in the full sample versus α = .83 after excluding 4-year-olds).
Child adjustment
The MacArthur Health and Behavior Questionnaire (HBQ) [48], a comprehensive caregiver-report questionnaire measuring caregiver perceptions of children’s functioning across multiple domains, was used to assess aspects of children’s mental health and social functioning. The HBQ mental health scales examined in the Team PLAY study contained 75 items scored on a 3-point Likert scale (0 = “rarely applies”, 1 = “applies somewhat”, 3 = “certainly applies”). These items provided three scales scores: Internalizing Symptoms, Externalizing Symptoms, and Attention-Deficit/Hyperactivity Disorder (ADHD) Symptoms. The social functioning scales contained 40 items scored using either a 3-point Likert scale (0 = “rarely applies”, 1 = “applies somewhat”, 3 = “certainly applies”) or a 4-point Likert scale (1 = “not at all like”, 1 = “very little like”, 3 = “somewhat like”, 4 = “very much like”). These items provided three scales scores: Peer Relations, Social Withdrawal, and Prosocial Behavior.
Evaluation of the HBQ’s overall psychometric qualities revealed that this instrument has high test-retest reliability and cross-informant agreement, as well as strong predictive and discriminate validity.[48,49] Internal consistency estimates in the current sample for the three mental health symptom scales were acceptable (Internalizing α = .77, Externalizing α = .79, and ADHD symptoms α = .83), as were alphas for the three social functioning scales (Peer Relations α = .80, Social Withdrawal α = .74, and Prosocial Behavior α = 90).
Family functioning
Family functioning was measured using the Family Adaptability and Cohesion Evaluation Scales (FACES-IV) [50], a self-report instrument designed to assess dimensions of cohesion and flexibility as outlined by the Circumplex Model of Marital and Family Systems.[51] Respondents are asked to rate the extent of their agreement with 42 statements using a 5-point Likert scale (1 = strongly disagree to 5 = strongly agree). Responses are used to create two balanced scales (Cohesion and Flexibility- higher scores indicate better functioning) and four unbalanced scales tapping the extremes of cohesion and flexibility (Disengaged, Enmeshed, Rigid, and Chaotic- higher scores on these scales reflect greater family dysfunction.). Scores on these six scales are calculated as the sum of responses on the 7 items comprising each scale. The FACES-IV also includes 20 additional items that can be used to assess respondents’ perceptions of communication and satisfaction within the family. The Family Communication and Family Satisfaction scales are each comprised of ten items scored using a 5-point Likert scale (1 = strongly disagree to 5 = strongly agree).
Previous versions of the FACES have demonstrated acceptable reliability and validity, and available psychometric information for the FACES-IV suggests that this newer version has similar psychometric qualities.[52] In the present sample, internal consistency estimates for the six family functioning scales were as follows: Cohesion α = .76, Flexibility α = .66, Disengaged α = .80, Enmeshed α = .71, Chaotic α = .81, and Rigid α = .70). Coefficient alphas for Family Communication and Family Satisfaction were also acceptable (αs = .89 and .92, respectively).
Health care provider autonomy support
An adapted version of the Health Care Climate Questionnaire (HCCQ) [53] was used to assess parents’ perceptions of the degree to which their child’s health care providers are supportive with respect to the management of their children’s food choices and physical activity behaviors. Specifically, this measure focused on parents’ feelings of autonomy related to health care providers’ encouragement of their questions, the provision of choices, level of understanding of the caregivers’ perspective, and whether they convey confidence in caregivers’ ability to manage their children’s diet and physical activity habits (e.g., “My child’s health care providers try to understand how I see my child’s diet before suggesting any changes”; “My child’s health care providers convey confidence in my ability to make changes regarding my child’s physical activity”).
Parents rated their level of agreement with 12 statements using a 7-point scale that ranged from 1=not at all true to 7=very true. A total score ranging from 12–84 was obtained by summing responses across all 12 items. The HCCQ demonstrated excellent internal consistency in the current sample (α = .96), similar to what has been reported in other studies using the original HCCQ.[54]
4. Intervention
4.1 Delivery of the Intervention
The dietary, physical activity and behavioral components of the intervention were delivered via 14 one-hour group sessions over a six-month period. Groups of 10 to 15 parent-child dyads met at community sites that were chosen for location and convenience (e.g. safe, accessible, and free parking), once a week for the initial 8 weeks, biweekly for 8 weeks, and thereafter monthly for 2 months. Parents and their children were instructed separately for the majority of the session time. Parent group sessions, instructed by a trained interventionist, co-occurred while the children were playing at a moderate to vigorous intensity and learning about healthy foods. Given the young age of the children, the overall intervention mainly targeted the parent as the agent of change, however the children were also instructed in intervention content as described below. Each group session integrated dietary, physical activity, and behavioral modification instruction (Table 2). Parents were introduced to healthy eating using an adaption of the “We Can” moderately restrictive diet (described below), and were taught how to model age-appropriate physical activities and received instruction on utilizing common household items to engage their children in physical activity at home. Parents were supplied with an intervention resource guide, the Team PLAYbook, that contained all presented session materials, healthy habit monitoring worksheets for use by adults and children, healthy choice food substitution suggestions as well as recipes, serving size and physical activity guidelines.
Table 2.
Team PLAY Parent/Child* Intervention Content
| Session 1 | Monitoring Eating and Activity Behaviors
|
| Session 2 | Setting Energy Balance Goals
|
| Session 3 | Cues for Healthful Eating and Activity
|
| Session 4 | Road Map for Change
|
| Session 5 | Rewarding Activities
|
| Session 6 | Family Round Table
|
| Session 7 | Recipes for Success
|
| Session 8 | The Choice is Yours
|
| Session 9 | Going Healthy Places
|
| Session 10 | Support for Change
|
| Session 11 | Barrier Busting
|
| Session 12 | Creating Confidence with Healthy Habits
|
| Session 13 | The Caution Zone
|
| Session 14 | Ways to Stay Motivated
|
Cognitive factors in the Social Cognitive Theory model were addressed with parents only
While parents participated in adult group sessions, children were simultaneously led by study staff with a teaching or physical activity background, in age appropriate versions of the nutrition, physical activity and parts of the behavior modification content of the adult sessions. The physical activities were designed to engage them at a moderate to vigorous level for at least 50% of the session. The goal being to increase the children’s MVPA minutes per week, as well as to improve motor skill development, both of which have been inversely linked to childhood obesity.[55] Specifically, the physical activity component of all intervention sessions taught children and caregivers how to efficiently perform individual motor skills (such as jumping, hoping, kicking or throwing), while utilizing various physical activity implements (such as ropes, hula hoops, bean bags or ribbon wands). Children’s books and music, that contained healthy nutrition and physical activity themes, were provided to the families throughout the intervention to help reinforce the behavior change messages at home.
During the last 15 minutes of each group session, parents and children joined together and parents modeled for their children locomotor and object control skills, MVPA engagement and healthy choice behaviors taught in the sessions (e.g. throwing, jumping, hoping, identification of skim milk and water as healthy beverages choices and soda as an unhealthy choice). The unique design of this intervention model allows both parents and children to benefit personally from physical activity engagement, while building their dietary and physical activity skills. Moreover, self-regulatory skills delivered to parents during the adult sessions were integrated into combined sessions in order to promote understanding and to encourage application of dietary and physical activity behaviors within their home environments. Session content was delivered in an interactive format that encouraged discussion among participants and incorporated messages set to music for experiential learning and modeling of healthful eating and activity behaviors. Most session resources, including the PLAYbook, physical activity props and the music played during all group sessions, were sent home for playtime use. These resources served to reinforce key behavioral, nutrition, and physical activity messages practiced during the intervention sessions.
Since special efforts were made to recruit minority participants, we endeavored to make the interventions address the needs of this group. For example, the dietary component included cooking with foods readily available and used by African Americans and making healthier fast food choices. The music provided varied and was appropriate for young children of any racial background. The lyrics promoted and complemented the healthy behaviors being taught at the sessions. Healthy lifestyle changes that were modeled included dancing, singing and reading with parents (culturally- and topic-appropriate books were provided). There was a mix of ethnic and racial backgrounds among the interventionists.
Following the intensive intervention phase, a 6 month intervention follow-up is conducted. Each month families are mailed a newsletter (“Go Notes”) containing general nutritional and physical activity information (that reinforces intervention session content) and are contacted by telephone to discuss problems or answer questions that may arise.
4.2. Dietary Intervention
The goal of the dietary component of the intervention was to promote healthful eating behaviors for healthy growth. It was modeled after the Traffic Light Diet [56] and modified for delivery to parents based on “Ways to Enhance Children’s Activity and Nutrition at home (We Can)” as presented on the National Institute of Health (NIH) website (Website: http://www.nhlbi.nih.gov/health/public/heart/obesity/wecan/). We Can is a collaborative effort of the National Institutes of Heart, Blood and Lung; Diabetes and Digestive and Kidney Diseases; Child Health and Human Development and The National Cancer Institute. The We Can program is a basis for community and wide scale interventions. The materials are geared to parents of 8–13 year olds and were based on the results of the Child and Adolescent Trial for Cardiovascular Health (CATCH).[57] The intervention adopted the “Go, Slow, Whoa food system” as a way to encourage healthier foods choices, reduce portion sizes and decrease excess energy intake from high sugar/high fat food and beverages. The Go foods are lowest in fat and sugar, relatively low in calories, have higher nutrient density and are great to eat anytime. Examples of Go foods are fruits and vegetables, low fat dairy products, whole grains, lean meat, poultry and fish, beans, eggs and nuts. Slow foods are relatively higher in calories, fat and sugar are to be eaten sometimes/less often than Go foods. Examples of Slow foods are canned fruit in syrup, high fat cheeses, and fruit juices. Whoa foods are highest in fat and calories and are to be consumed only once in a while on special occasions in small portions. Examples of Whoa foods include sweetened beverages, french fries, fruit pies, cakes, doughnuts and high fat meats or those fried in extra fat or breading. The Team PLAY dietary intervention modified the We Can materials to focus on ways to improve the eating habits and cooking practices of families residing in our predominantly African American, urban, Mid-South community. As part of the intervention, parents were provided with dietary goals to meet the energy and nutrient requirements for healthy growth based on their child’s age. Recommendations for daily servings of each food groups were based on the USDA’s MyPyramid guide for 4–8 year olds (Website: http://www.mypyramid.gov). Children’s books and music, that contained healthy nutrition themes, were provided to the families throughout the intervention to help reinforce the nutrition messages at home.
4.3 Physical Activity Intervention
The physical activity component, adhering to the recommendations of the U.S. Surgeon General [58] and Healthy People 2010 and 2020 [59,60], was designed to instruct parents in ways to increase the total amount of time their children spend being physically active (e.g. counting daily steps with a pedometer) and increase the percentages of those total minutes the children spend engaged in MVPA. While parents were taught how to model the age-appropriate physical activities and received instruction on utilizing common household items to engage their children in physical activity, the children were led in age-appropriate physical activities at a moderate to vigorous level for at least 50% of the lesson time. During the last 15 minutes of each group session, parents modeled the home activities with their children and families were given simple physical activity implements, such as ropes, bean bags or ribbon wands, to enhance movement skills and the play experience at home.
4.4 Behavioral Intervention
Based on Social Cognitive Theory (SCT), parents and children were instructed in proven health behavior change techniques (e.g. stimulus control, contingency management, and reinforcement) that were integrated into each session in order to help children successfully achieve the dietary and physical activity goals above. For instance, during all child sessions, in an effort to increase MVPA, each child wore a pedometer that the instructor referred to as a “happy feet counter.” These were regularly checked and the child rewarded with a “Team PLAY ticket” when they achieved the desired counts. Physical activity (hoola hoops, balls, bean bags) and food (fruit/vegetable cutouts, serving containers) implements were used as cues to help prompt the children to learn about MVPA and Slow, Go, Whoa food choices. Additionally, session instructors gave frequent verbal feedback to encourage and reinforce the desired healthy behaviors. In order to encourage continued practice at home, each child/parent dyad was given a session play pack (implements) that reinforced the skills performed during the session. Cognitive factors in the SCT model (e.g. self-efficacy and the self-regulatory skills; monitoring, goal-setting, and problem-solving), which are necessary for implementation of this intervention, were also addressed with parents but not children given their young age and age-related cognitive differences among youth.
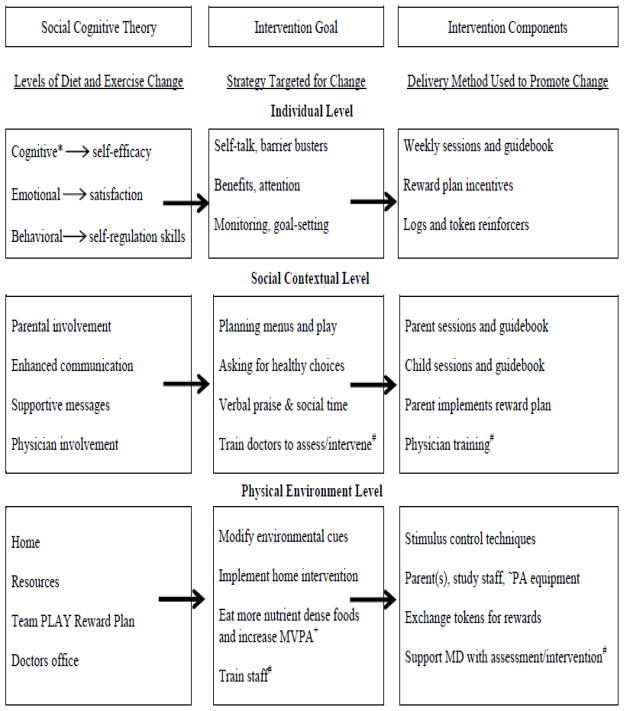
The target behavioral goals for children were to consume nutrient dense foods as often as possible throughout the day by asking parents for these foods, and to increase play time that involves moderate to vigorous levels of physical activity for 60 minutes per day. Moving from SCT theory to intervention delivery was based on methods described by Bauman et al. [61] (Figure 2).
Figure 2.
*Targets change in parents only; #Not part of efficacy trial; − Translational phase only; and + Moderate to vigorous physical activity (modified Coday et al., 2002 [62]).
4.5 Standard Care (Control) Condition
Families assigned to the standard care condition will complete assessments at baseline, 6, 12, 18 and 24 months (Table 1). As previously noted, the child’s primary care provider was sent general written information about the evaluation and treatment of overweight children developed by local experts.[26,27] These included resources for physicians (e.g. evaluation forms, reimbursable ICD-9 codes for obesity, etc.) and patients (nutrition educational materials) to be used at primary care visits. However, no intervention was offered to this group but rather they received routine care and follow-up that is typically prescribed by their primary care provider.
4.6 Interventionist Training
Intervention sessions were conducted by research study staff with a background in teaching, physical activity and/or nutrition and were trained by a study investigator (P.R.). Training included 4–6 hours of observation of sessions (led by P.R. or a trained intervention staff) and 3–4 hours of “hands on” training of specific session activities. Prior to leading a session, all intervention staff were required to demonstrate competency in the delivery of the adult and/or child session components. An elaborate interventionist instruction manual was developed that contained all the essential components of the intervention sessions and detailed scripts for training and delivery of the intervention. This manual will allow replication and translation of the intervention in the future. In order to maintain fidelity to the intervention, sessions were periodically videotaped and feedback was provided to the interventionists.
5. Statistical Approach
5.1 Sample Size
Power estimation assumed four clinics in both the intervention and standard primary and fifteen subjects within each clinic (Clinic Recruitment Phase). We expected at most a 20% dropout over the two years resulting in at least 12 subjects in each of the clinics per cycle. To estimate power associated with the original study design assumptions were made concerning intraclass correlation for the same subject over time (ICCS) of 0.20 (an estimate for the points furthest apart −2 years), an intraclass correlation for a common group of subjects at a given time (ICCG1) of 0.10, and an intraclass correlation for a common group of subjects at different times (ICCG2) of 0.04. A difference of 2 kg/m2 between the intervention and standard primary care groups was judged to be meaningful and achievable over a two year period. We estimated the standard deviation within clinic at a given time to be 6.7 kg/m2, and targeted detecting a moderate interaction effect size of f =.15 given two-tailed alpha = .05.[63] Under these assumptions 240 subjects were required to produce power for detecting a significant interaction of intervention (yes or no) by time (five time points) of 86%.
5.2 Data Analysis
Treatment effects on BMI will be analyzed, based on intention to treat, via mixed effect covariance pattern models. Sex, race, and baseline age will be assessed as potential confounding or effect modifier variables. Since the original study design was modified in phase two of recruitment to no longer require clinics to be completely nested in treatment levels, a fixed effect capturing the effect of recruitment phase as well as a random effect for clinic will be incorporated into all analyses. The exponential covariance pattern will be adopted to reflect variation in covariance effects over time (measured continuously as elapsed time since baseline visit) with visits that are closer together expected to have stronger BMI covariation. This pattern is more reasonable than the conventional assumption of compound symmetry common to classic repeated measures models and is expected to be more realistic for this study.
The test of intervention effectiveness will be assessed by evaluating the treatment (intervention versus standard primary care) by visit (baseline and four follow-ups) interaction effect. If treatment is effective, we expect an initial decrease in BMI followed by tapering further decrease (or even a flat line) in intervention subjects but a relatively flat line over time in BMI for subjects in the standard primary care condition.
The analytic process will incorporate initial examination of covariate slopes for heterogeneity of regression. If warranted, separate covariate slopes by treatment level will be retained for modeling purposes. When appropriate, slopes will be pooled for study covariates. Modeling will be accomplished using the SAS Proc Mixed procedure with omnibus hypothesis tests conducted at alpha of .05. Alpha levels for testing contrasts estimated to explain main effects for visit or interaction effects for visit X treatment will be adjusted for the number of contrasts to contain alpha inflation.
5.3 Safety Monitoring
Adverse events, including illnesses and injuries, and serious adverse events, including conditions that required hospitalization, were queried at baseline and at all subsequent follow-up visits. The child’s caregiver was notified by the study pediatrician and study staff of any abnormal physical finding (e.g. a heart murmur, wheeze) or blood pressure measurement obtained outside norms for age, sex and height that were detected on baseline physical examination. An external data safety monitoring committee was also established prior to enrollment of participants in the study.
6. Results
A total of 270 (45% of those potentially eligible) children and one parent were assigned to either the active intervention group (n = 202) or the standard primary care group (n = 68). Baseline comparisons used independent samples t-tests for continuous variables and chi-square tests for categorical variables. Although no differences between the intervention and standard primary care groups were found with respect to child age (M = 6.3 years, SD = 1.1) or race/ethnicity (79.9% minorities), there were significant group differences in the mean values and distributions for several of the other socio-demographic and psychosocial variables collected (see Table 3). Specifically, families in the intervention group included parents that had more education, reported higher total family income, more were more likely to be married and scored higher on the FACES-IV family functioning enmeshed and disengaged scales. However, results of subgroup analyses that tested for differences between the intervention and standard primary care participants recruited initially (Clinic Recruitment Phase, n = 98) as compared to those recruited subsequent to changing our recruitment approach which was implemented to eliminate referral bias (Community Recruitment Phase, n = 172) revealed that most group differences at baseline were among participants recruited initially during the clinic level approach. These analyses were performed in order to better understand the significant group differences described above. Randomization was successful following the changes in recruitment strategies, with all the differences between groups, with the exception of sweetened beverages, eliminated in the Community Recruitment Phase (see Table 4).
Table 3.
Baseline characteristics of Team PLAY participants (mean [SD] or n [%]
| Variable | Intervention | Standard Care | t or χ2 | All participants |
|---|---|---|---|---|
| N | 202 | 68 | 270 | |
| Socio-demographic measures | ||||
| Child’s age in years | 6.2 (1.1) | 6.5 (1.1) | −1.35 | 6.3 (1.1) |
| Number children living in household | 2.3 (1.2) | 2.4 (1.3) | −0.77 | 2.3 (1.2) |
| Child sex, n (%) | 5.65* | |||
| Male | 83 (41.1) | 17 (25.0) | 100 (37.0) | |
| Female | 119 (58.9) | 51 (75.0) | 170 (63.0) | |
| Child race/ethnicity, n (%) | 1.47 | |||
| Caucasian, non-Hispanic | 44 (21.8) | 10 (14.9) | 54 (20.1) | |
| Racial/ethnic minoritya | 158 (78.2) | 57 (85.1) | 215 (79.9) | |
| Parent partnered status | 7.66* | |||
| Married or Living with partner | 116 (57.4) | 26 (38.2) | 142 (52.6) | |
| Single | 63 (31.2) | 32 (47.1) | 95 (35.2) | |
| Separated, Divorced, or Widowed | 23 (11.4) | 10 (14.7) | 33 (12.2) | |
| Parent educational attainment, n (%) | 8.13* | |||
| High school diploma or less | 44 (21.8) | 26 (38.2) | 70 (25.9) | |
| Associate’s degree or Some college | 87 (43.1) | 25 (36.8) | 112 (41.5) | |
| College graduate | 37 (18.3) | 11 (16.2) | 48 (17.8) | |
| Post-college education | 34 (16.8) | 6 (8.8) | 40 (14.8) | |
| Annual household income, n (%) | 10.67* | |||
| < $10,000 | 41 (20.3) | 23 (33.8) | 64 (23.7) | |
| $10,000–29,999 | 53 (26.2) | 21 (30.9) | 74 (27.4) | |
| $30,000–49,999 | 40 (19.8) | 14 (20.6) | 54 (20.0) | |
| ≥ $50,000 | 68 (33.7) | 10 (14.7) | 78 (28.9) | |
| Physical measures | ||||
| % Body fat (DXA)b | 39.1 (5.5) | 39.9 (5.1) | −0.98 | 39.3 (5.4) |
| Waist circumference, cm | 70.9 (9.0) | 71.1 (8.3) | −0.14 | 71.0 (8.8) |
| Resting heart rate | 84.3 (12.4) | 84.1 (11.5) | 0.12 | 84.3 (12.2) |
| Acanthosis nigricans, n (% yes)b | 112 (56.7) | 36 (53.7) | 0.16 | 149 (56.0) |
| Systolic blood pressure, n (%) | 0.23 | |||
| Normal | 136 (68.0) | 45 (66.2) | 181 (67.5) | |
| Prehypertensive | 28 (14.0) | 9 (13.2) | 37 (12.7) | |
| Hypertensive | 36 (18.0) | 14 (20.6) | 50 (25.4) | |
| Diastolic blood pressure, n (%) | 0.40 | |||
| Normal | 126 (63.0) | 40 (58.8) | 166 (61.9) | |
| Prehypertensive | 25 (12.5) | 9 (13.2) | 34 (12.7) | |
| Hypertensive | 49 (24.5) | 19 (27.9) | 68 (25.4) | |
| Child BMI (z-score) | 2.6 (0.6) | 2.5 (0.5) | 0.91 | 2.6 (0.6) |
| Child body mass index, kg/m2 | 24.3 (4.0) | 25.1 (4.4) | −1.35 | 24.5 (4.1) |
| Child BMI percentile, n (%) | 1.69 | |||
| BMI 85th to 95th percentile | 22 (10.9) | 8 (11.8) | 30 (11.11) | |
| BMI >95th percentile | 180 (89.1) | 60 (88.2) | 240 (88.9) | |
| Parent body mass index, kg/m2 | 36.9 (9.1) | 38.6 (9.8) | −1.24 | 37.3 (9.3) |
| Dietary intake/day | ||||
| Energy, kcal | 1797.3 (580.3) | 2018.4 (735.5) | −2.19* | 1852.8 (628.8) |
| Fruits, servings | 1.7 (0.9) | 1.6 (0.9) | 0.05 | 1.7 (0.9) |
| Vegetables, servings | 1.2 (0.5) | 1.1 (0.6) | 0.15 | 1.1 (0.5) |
| Dairy, servings | 1.8 (0.9) | 2.0 (1.0) | −1.67 | 1.8 (0.9) |
| Grains, servings | 4.8 (2.0) | 4.5 (1.9) | 0.83 | 4.7 (2.0) |
| Meat, servings | 1.8 (0.8) | 1.9 (0.8) | −0.82 | 1.8 (0.8) |
| Sweetened beverages (frequency/day) | 0.6 (0.4) | 0.8 (0.5) | −3.38*** | 0.7 (0.5) |
| Sweetened beverages, including juice (frequency/day) | 1.3 (0.7) | 1.6 (0.8) | −2.33* | 1.4 (0.8) |
| Physical activity | ||||
| Daily moderate-to-vigorous activity, min | 18.6 (12.3) | 17.8 (8.9) | −0.47 | 18.37 (11.51) |
| Amherst neighborhood environment | ||||
| Neighborhood characteristics | 5.8 (1.5) | 5.4 (1.5) | 1.72 | 5.7 (1.5) |
| Neighborhood safety | 2.6 (1.4) | 2.4 (1.4) | 0.78 | 2.5 (1.4) |
| Access to facilities | 3.4 (1.5) | 3.2 (1.7) | 0.94 | 3.3 (1.6) |
| Park distance, miles | 3.5 (3.2) | 3.8 (4.2) | −0.53 | 3.5 (3.5) |
| Park safety | 3.8 (1.2) | 3.6 (1.2) | 1.15 | 3.7 (1.2) |
| Park frequency | 1.6 (1.0) | 1.6 (1.0) | −0.03 | 1.6 (1.0) |
| Psychosocial characteristics | ||||
| Body Esteemc | 44.9 (8.9) | 45.2 (6.9) | −0.29 | 45.0 (8.4) |
| Health Care Climated | 56.3 (20.3) | 61.8 (21.8) | −1.72 | 57.5 (20.7) |
| HBQ child psychosocial adjustment | ||||
| Internalizing | 0.36 (0.25) | 0.40 (0.23) | −1.21 | 0.37 (0.24) |
| Externalizing | 0.29 (0.21) | 0.33 (0.26) | −1.24 | 0.30 (0.23) |
| ADHD symptoms | 0.71 (0.38) | 0.73 (0.37) | −0.47 | 0.71 (0.38) |
| Peer relations | 2.70 (0.34) | 2.70 (0.27) | −0.03 | 2.70 (0.32) |
| Social withdrawal | 0.51 (0.30) | 0.59 (0.32) | −1.86 | 0.53 (0.33) |
| Prosocial behavior | 1.31 (0.37) | 1.33 (0.38) | −0.44 | 1.32 (0.38) |
| FACES-IV family functioning | ||||
| Cohesion | 29.5 (4.4) | 28.6 (4.2) | 1.39 | 29.3 (4.3) |
| Flexibility | 21.0 (5.0) | 21.0 (4.4) | −0.03 | 21.0 (4.8) |
| Chaotic | 13.4 (5.6) | 14.1 (5.4) | −0.94 | 13.5 (5.6) |
| Enmeshed | 12.0 (4.7) | 14.1 (5.3) | −3.02** | 12.5 (5.0) |
| Disengaged | 11.3 (5.1) | 13.3 (4.8) | −2.85** | 11.8 (5.1) |
| Rigid | 17.8 (4.9) | 18.6 (5.8) | −1.16 | 17.9 (5.1) |
| Family communication | 39.1 (7.4) | 39.7 (5.9) | −0.62 | 39.3 (7.0) |
| Family satisfaction | 37.0 (7.9) | 37.0 (6.5) | −0.02 | 37.0 (7.5) |
Note.
p <.05,
p <.01,
p <.001.
73.7% African American (199/270), 3.3% Hispanic (9/270), and 2.9% other (8/270).
Missing data for 7 participants.
Does not include 4-year-old participants.
This measure was added after enrollment had begun, resulting in missing data for 36 participants.
Table 4.
Baseline characteristics of Team PLAY participants (mean [SD]) or n [%], by recruitment site
| Variable | Clinic (n = 98) | Community (n = 172) | ||||
|---|---|---|---|---|---|---|
| Intervention (n = 61) | Standard Care (n = 37) | t or χ2 | Intervention (n = 141) | Standard Care (n = 31) | t or χ2 | |
| Socio-demographic measures | ||||||
| Child’s age in years | 5.9 (1.1) | 6.6 (1.0) | −2.94** | 6.4 (1.1) | 6.3 (1.1) | 0.41 |
| Child sex, n (%) | 3.87 | 1.57 | ||||
| Male | 25 (40.1) | 8 (21.6) | 58 (41.1) | 9 (29.0) | ||
| Female | 36 (59.0) | 29 (78.4) | 83 (58.9) | 22 (71.0) | ||
| Parent partnered status | 4.56 | 0.69 | ||||
| Married or Living with partner | 28 (45.9) | 9 (24.3) | 88 (62.4) | 17 (54.8) | ||
| Single | 27 (44.3) | 23 (62.2) | 36 (25.5) | 9 (29.0) | ||
| Separated, Divorced, or Widowed | 6 (9.8) | 5 (13.5) | 17 (12.1) | 5 (16.1) | ||
| Parent education level, n (%) | 10.91* | 1.16 | ||||
| High school diploma or less | 18 (29.5) | 22 (59.5) | 26 (18.4) | 4 (12.9) | ||
| Associate’s degree/Some college | 26 (42.6) | 12 (32.4) | 61 (43.3) | 13 (41.9) | ||
| College graduate | 11 (18.0) | 3 (8.1) | 26 (18.4) | 8 (25.8) | ||
| Post-college education | 6 (9.8) | 0 (0.0) | 28 (19.9) | 6 (19.4) | ||
| Annual household income, n (%) | 11.03* | 3.55 | ||||
| < $10,000 | 16 (26.2) | 20 (54.1) | 25 (17.7) | 3 (9.7) | ||
| $10,000–29,999 | 22 (36.1) | 12 (32.4) | 31 (22.0) | 9 (29.0) | ||
| $30,000–49,999 | 10 (16.4) | 4 (10.8) | 30 (21.3) | 10 (32.3) | ||
| ≥ $50,000 | 13 (21.3) | 1 (2.7) | 55 (39.0) | 9 (29.0) | ||
| Physical measures | ||||||
| BMI z-score | 2.8 (0.7) | 2.5 (0.5) | 2.65* | 2.5 (0.6) | 2.5 (0.6) | −0.44 |
| Dietary measures | ||||||
| Energy, kcal | 1981.0 (612.9) | 2151.9 (822.9) | −1.13 | 1719.1 (549.9) | 1867.2 (600.0) | −1.31 |
| Sweetened beverages (frequency/day) | 0.7 (0.4) | 0.9 (0.5) | −2.18* | 0.6 (0.5) | 0.8 (0.5) | −1.94† |
| Sweetened beverages, including juice (frequency/day) | 1.4 (0.7) | 1.7 (0.8) | −1.80† | 1.3 (0.7) | 1.4 (0.8) | −0.91 |
| Psychosocial measures | ||||||
| FACES-IV enmeshed scale | 12.9 (5.1) | 15.7 (5.5) | −2.63* | 11.6 (4.6) | 12.1 (4.3) | −0.51 |
| FACES-IV disengaged scale | 12.7 (6.1) | 15.4 (4.7) | −2.25* | 10.6 (4.5) | 10.8 (3.7) | −0.19 |
Note.
p <.10,
p <.05,
p <.01.
Children in the intervention and standard primary care groups did not differ with respect to physical measures (see Table 3). Most children recruited for this study were extremely overweight as demonstrated by the overall mean BMI z-score of 2.6. While children with BMIs > 85th percentile were eligible to participate, only 11% of participants had BMIs between the 85th and 95th percentiles, with the majority (88%) of participants having BMIs over the 97th percentile. Twenty-five percent of children had blood pressures in the hypertensive range based on sex, age and height, mean waist circumference was greater than the 90th percentile for 7 year olds [64], and 56% had acanthosis nigricans on physical exam. The average child BMI was 24.5 kg/m2 (SD = 4.1) and most children (88.9%) had a BMI percentile > 95th percentile. The average percent body fat, measured by DXA scan, was 39.3 (SD = 5.4).
Regarding dietary intake, we found no group differences with respect to children’s daily consumption of fruits (M = 1.7 servings/day), vegetables (M = 1.1 servings/day), grains (M = 4.7 servings/day) or meats (M = 1.8 servings/day). Compared with children receiving standard primary care, children in the intervention group consumed significantly fewer daily calories (1797.3 kcal versus 2018.4 kcal), which may be attributed to a significantly lower number of servings of sweetened beverages (1.0 versus 1.7 servings/day). Both groups exceeded the daily caloric recommendations of the American Heart Association for this age group.[65]
No differences between the groups in minutes of MVPA were found. All children were extremely physically inactive, showing only 18.6 and 17.8 minutes of moderate to vigorous physical activity (MVPA) per day for intervention and standard primary care groups respectively, as compared to the national recommendations of 60 minutes per day.
7. Discussion
The Team PLAY trial was designed to test the efficacy of a 6-month moderately intense, family-based behavioral intervention, targeting the child-parent dyad, in a majority African American population. This study examines the feasibility of recruiting/enrolling high-risk participants, baseline characteristics of the sample, and the gap this intervention potentially fills.
Recruitment of underserved participants for this trial was more difficult than anticipated. Although effort was placed into recruitment advertisements that promoted healthy lifestyles and healthy growth as opposed to weight loss, there was still a lack of public interest in participating in this type of a program among families with younger (ages 4 to 7 years) and minority children. Baseline results reveal that while overweight children were eligible to participate; almost 90% of the participants were obese with many of those in the severely obese category. It may be that parents of overweight children did not feel their child needed or would benefit from a weight intervention. Indeed, a study by West and colleagues reported parental perceptions of overweight, particularly in young and African American children, is low.[66] Additionally, among clinicians it is well documented that the use of BMI to assess childhood overweight is low compared to use in the diagnosis of obesity.[7,67] The high rate of those not interested, unable to reach, or who withdrew consent (45%) during screening (Figure 1) speaks to the challenges in treating obesity in at risk families.
Along with overcoming inherent difficulties in recruiting hard to reach populations [68–71], another unique challenge we faced was the initial recruitment approach that relied exclusively on “proactive” methods in off-site locations (Clinic Recruitment Phase).[69,72] Specifically, primary care providers located in participating clinics made referrals of their young obese patients during a regularly scheduled check-up. Perceived desire for obesity treatment at these regular doctor visits may have been lower than anticipated, as seen by the high rate of disinterest or inability to participate. Additionally, as described earlier, the initial group randomization scheme used in the Clinic Recruitment Phase appeared to lead to an allocation bias based on referrals whereby, despite efforts to maintain blinding, providers preferentially made referrals when their clinic was enrolling into the treatment condition (Intervention Arm). However, differences noted in the baseline characteristics between intervention versus standard primary care using a proactive recruitment virtually disappeared with adoption of the Community Recruitment Phase which used both proactive and reactive strategies.
There are several key strengths to this prospective behavioral trial. First, this study has a very young school age population, which represents an understudied subset of children at high risk for developing weight-related health problems. Specifically, we recruited a sample of underserved minority youngsters between the ages of 4 and 7 years, a population in which few intervention studies on healthy growth have been done.[15,16] Treatment to promote healthy growth in overweight and obese children at an early age is critical, as it may improve individuals’ health across the life course by preventing obesity. Second, by including parents and family function in the assessment of outcomes, in addition to between-group comparisons of child weight change, we will be able to conduct within-group correlational analyses that may elucidate the relationship between family functioning and treatment outcomes.[14,18]
Another key strength is that most children receive their health care in a primary care setting. Thus, primary care providers are uniquely poised to intervene with efficacious interventions to prevent and treat childhood overweight and obesity. Team PLAY was designed for translation to the primary care setting. The parent and child group sessions could be performed in most healthcare offices. The group design allows more time for education and discussion than an individual office visit and is likely to be more cost-effective. This is supported by several studies that suggest that group well-child care is at least as good as traditional well-child care.[73–75] As previously described, an intervention manual with detailed instructions, including sample scripts, has been developed along with participant materials. While our interventionists included registered dieticians and research assistants with physical activity and teaching backgrounds, professional staff from a primary care setting would likely have the ability to conduct this type of program without extensive additional training.
Lastly, Team PLAY is a moderately intense intervention with a two year follow-up period. As mentioned previously, moderate to high intensity interventions have been found to produce significant improvements in weight in overweight/obese children.[15] The Bright Bodies trial [25] experience, however, suggests the participation burden of a high intensity program may lead to a high drop out rate. Findings from Team PLAY will provide additional information regarding the intensity required in pediatric overweight and obesity treatment and long-term follow-up will add to the limited information about treatment sustainability in obese minority youngsters.
Limitations
Despite the numerous strengths of Team PLAY, there are also several limitations. Although there is growing evidence for the effectiveness and cost benefit of programs that intervene only on the parent [76,77], this evidence was limited at the conception of Team PLAY and we elected to have complimentary child sessions during the parent sessions. We hypothesized that the fun and interactive nature of the child sessions would appeal to the children who in turn would encourage their parents to attend the sessions and also provide a setting where the child/parent dyad could interact without the distraction of other siblings or family members. Furthermore, by including the children we were able to promote their participation in MVPA at the sessions and observe and instruct the parents as they modeled the physical activities with their children. However, the child sessions added significant cost to the intervention and determining if they contribute significantly to the outcomes (positive or negative) will be difficult. This may have important implications for future translation of Team PLAY.
Furthermore, although the intervention is an efficacy trial designed for translation to primary care, it is not being conducted in a primary care setting and therefore is not measuring feasibility and acceptability in that setting. It requires staffing, intervention resources and space that could limit its translatability to primary care and other settings. Moreover, some of the intervention’s strengths could arguably be limitations. Children in the study are young (4–7 years) and the cohort is predominantly African American so the generalizability of the findings to other populations may be limited. The majority of participants are obese, limiting information on the treatment of overweight children. Finally, the intervention had a moderate degree of participant burden and as described in detail above, the difficulties encountered in recruiting minority, underserved, and very young overweight or obese children could make Team PLAY’s approach of offering face-to-face family based lifestyle change unfeasible for retention to treatment in some of the most at risk population subgroups.
In summary, evidence is lacking regarding efficacious weight reduction and obesity prevention interventions aimed at young minority children. Data from this trial will provide information regarding factors important to successful implementation of such an intervention. If proven efficacious, Team PLAY would offer a treatment model for early intervention for obese children that might be translated to other settings such as medical, community, school, or faith-based locations.
Acknowledgments
This work was supported by a grant from the National Institute of Child Health and Human Development (R01HD050895-03) awarded to Grant Somes, Ph.D. and Marion Hare, M.D., M.S. The authors would like to dedicate this publication to their friend and colleague Grant W. Somes, Ph.D., who died in 2010. He provided invaluable support and contribution to this study and publication, and is greatly missed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daniels SR, Arnett DK, Eckel RH, et al. Overweight in children and adolescents: Pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111(15):1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 2.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: The Bogalusa heart study. Pediatrics. 1999;103(6 Pt 1):1175–1182. doi: 10.1542/peds.103.6.1175. [DOI] [PubMed] [Google Scholar]
- 3.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disord. 1999;23 (Suppl 2):S2–11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 4.Holt N, Schetzina KE, Dalton WT, 3rd, Tudiver F, Fulton-Robinson H, Wu T. Primary care practice addressing child overweight and obesity: A survey of primary care physicians at four clinics in southern Appalachia. South Med J. 2011;104(1):14–19. doi: 10.1097/SMJ.0b013e3181fc968a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein JD, Sesselberg TS, Johnson MS, et al. Adoption of body mass index guidelines for screening and counseling in pediatric practice. Pediatrics. 2010;125(2):265–272. doi: 10.1542/peds.2008-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.August GP, Caprio S, Fennoy I, et al. Prevention and treatment of pediatric obesity: An endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2008;93(12):4576–4599. doi: 10.1210/jc.2007-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlow SE Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120 (Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 8.Bernard-Bonnin AC, Stachenko S, Bonin D, Charette C, Rousseau E. Self-management teaching programs and morbidity of pediatric asthma: A meta-analysis. J Allergy Clin Immunol. 1995;95(1 Pt 1):34–41. doi: 10.1016/s0091-6749(95)70150-8. [DOI] [PubMed] [Google Scholar]
- 9.McLean N, Griffin S, Toney K, Hardeman W. Family involvement in weight control, weight maintenance and weight-loss interventions: A systematic review of randomised trials. Int J Obes Relat Metab Disord. 2003;27(9):987–1005. doi: 10.1038/sj.ijo.0802383. [DOI] [PubMed] [Google Scholar]
- 10.Oude Luttikhuis H, Baur L, Jansen H, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;1(1):CD001872. doi: 10.1002/14651858.CD001872.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Cohen DM, Lumley MA, Naar-King S, Partridge T, Cakan N. Child behavior problems and family functioning as predictors of adherence and glycemic control in economically disadvantaged children with type 1 diabetes: A prospective study. J Pediatr Psychol. 2004;29(3):171–184. doi: 10.1093/jpepsy/jsh019. [DOI] [PubMed] [Google Scholar]
- 12.Duke DC, Geffken GR, Lewin AB, Williams LB, Storch EA, Silverstein JH. Glycemic control in youth with type 1 diabetes: Family predictors and mediators. J Pediatr Psychol. 2008;33(7):719–727. doi: 10.1093/jpepsy/jsn012. [DOI] [PubMed] [Google Scholar]
- 13.Wysocki T, Harris MA, Buckloh LM, et al. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. J Pediatr Psychol. 2006;31(9):928–938. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- 14.Williams NA, Coday M, Somes G, Tylavsky FA, Richey PA, Hare M. Risk factors for poor attendance in a family-based pediatric obesity intervention program for young children. J Dev Behav Pediatr. 2010;31(9):705–712. doi: 10.1097/DBP.0b013e3181f17b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: A targeted systematic review for the USPSTF. Pediatrics. 2010;125(2):e396–418. doi: 10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- 16.Kuhl ES, Clifford LM, Stark LJ. Obesity in preschoolers: Behavioral correlates and directions for treatment. Obesity. 2011 doi: 10.1038/oby.2011.201. [DOI] [PubMed] [Google Scholar]
- 17.Barton M US Preventive Services Task Force. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2010;125(2):361–367. doi: 10.1542/peds.2009-2037. [DOI] [PubMed] [Google Scholar]
- 18.Kitzmann KM, Beech BM. Family-based interventions for pediatric obesity: Methodological and conceptual challenges from family psychology. J Fam Psychol. 2006;20(2):175–189. doi: 10.1037/0893-3200.20.2.175. [DOI] [PubMed] [Google Scholar]
- 19.Epstein LH, Wing RR, Woodall K, Penner BC, Kress MJ, Koeske R. Effects of family-based behavioral treatment on obese 5–8 year-old children. Behavior Therapy. 1985;16(2):205–212. [Google Scholar]
- 20.Epstein LH, Valoski A, Koeske R, Wing RR. Family-based behavioral weight control in obese young children. J Am Diet Assoc. 1986;86(4):481–484. [PubMed] [Google Scholar]
- 21.McKee MD, Maher S, Deen D, Blank AE. Counseling to prevent obesity among preschool children: Acceptability of a pilot urban primary care intervention. Ann Fam Med. 2010;8(3):249–255. doi: 10.1370/afm.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 23.Seo DC, Sa J. A meta-analysis of obesity interventions among U.S. minority children. J Adolesc Health. 2010;46(4):309–323. doi: 10.1016/j.jadohealth.2009.11.202. [DOI] [PubMed] [Google Scholar]
- 24.Kitzmann KA, Dalton WT, Stanley CM, et al. Lifestyle interventions for youth who are overweight: A meta-analytic review. Health Psychology. 2010;29(1):91–101. doi: 10.1037/a0017437. [DOI] [PubMed] [Google Scholar]
- 25.Savoye M, Nowicka P, Shaw M, et al. Long-term results of an obesity program in an ethnically diverse pediatric population. Pediatrics. 2011;127(3):402–410. doi: 10.1542/peds.2010-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burghen GA. Prevention and treatment of obesity, metabolic syndrome, and type 2 diabetes in children and adolescents. Diabetes Spectrum. 2005;18(4):210–212. [Google Scholar]
- 27.Stender SRS, Burghen GA, Mallare JT. The role of health care providers in the prevention of overweight and type 2 diabetes in children and adolescents. Diabetes Spectrum. 2005;18(4):240–248. [Google Scholar]
- 28.Bailey J, Gibson D, Tang J, Hare M. Greater Memphis area progress report. A collaborative report by the University of Tennessee, University of Memphis, Healthy Memphis Common Table. Nov, 2005. Reversing the epidemic of obesity and diabetes. [Google Scholar]
- 29.Klesges RC, Obarzanek E, Kumanyika S, et al. The Memphis Girls’ health Enrichment Multi-site Studies (GEMS): An evaluation of the efficacy of a 2-year obesity prevention program in African American girls. Arch Pediatr Adolesc Med. 2010;164(11):1007–1014. doi: 10.1001/archpediatrics.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barlow SE, Bobra SR, Elliott MB, Brownson RC, Haire-Joshu D. Recognition of childhood overweight during health supervision visits: Does BMI help pediatricians? Obesity. 2007;15(1):225–232. doi: 10.1038/oby.2007.535. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES) Anthropometry Procedures Manual. 2007. [Google Scholar]
- 32.Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatr. 2006;148(2):188–194. doi: 10.1016/j.jpeds.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Maffeis C, Pietrobelli A, Grezzani A, Provera S, Tato L. Waist circumference and cardiovascular risk factors in prepubertal children. Obes Res. 2001;9(3):179–187. doi: 10.1038/oby.2001.19. [DOI] [PubMed] [Google Scholar]
- 34.Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Relat Metab Disord. 2000;24(11):1453–1458. doi: 10.1038/sj.ijo.0801401. [DOI] [PubMed] [Google Scholar]
- 35.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 36.Klohe DM, Clarke KK, George GC, Milani TJ, Hanss-Nuss H, Freeland-Graves J. Relative validity and reliability of a food frequency questionnaire for a triethnic population of 1-year-old to 3-year-old children from low-income families. J Am Diet Assoc. 2005;105(5):727–734. doi: 10.1016/j.jada.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi T, Kamimura M, Imai S, et al. Reproducibility and validity of the Food Frequency Questionnaire for estimating habitual dietary intake in children and adolescents. Nutr J. 2011;10:27. doi: 10.1186/1475-2891-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall TA, Eichenberger Gilmore JM, Broffitt B, Stumbo PJ, Levy SM. Relative validity of the Iowa Fluoride Study targeted nutrient semi-quantitative questionnaire and the Block Kids’ Food Questionnaire for estimating beverage, calcium, and vitamin D intakes by children. J Am Diet Assoc. 2008;108(3):465–472. doi: 10.1016/j.jada.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Byers T, Trieber F, Gunter E, et al. The accuracy of parental reports of their children’s intake of fruits and vegetables: Validation of a food frequency questionnaire with serum levels of carotenoids and vitamins C, A, and E. Epidemiology. 1993;4(4):350–355. [PubMed] [Google Scholar]
- 40.Parrish LA, Marshall JA, Krebs NF, Rewers M, Norris JM. Validation of a food frequency questionnaire in preschool children. Epidemiology. 2003;14(2):213–217. doi: 10.1097/01.EDE.0000041256.12192.23. [DOI] [PubMed] [Google Scholar]
- 41.Sirard JS, Trost SG, Dowda M, Pate RR. Calibration of the computer science and applications, inc. physical activity monitor in preschool children. Medicine & Science in Sports & Exercise. 2001;33(5):S144. [Google Scholar]
- 42.Reilly JJ, Coyle J, Kelly L, Burke G, Grant S, Paton JY. An objective method for measurement of sedentary behavior in 3- to 4-year olds. Obes Res. 2003;11(10):1155–1158. doi: 10.1038/oby.2003.158. [DOI] [PubMed] [Google Scholar]
- 43.Dowda M, Pate RR, Sallis JF, et al. Agreement between student-reported and proxy-reported physical activity questionnaires. Pediatr Exerc Sci. 2007;19(3):310–318. doi: 10.1123/pes.19.3.310. [DOI] [PubMed] [Google Scholar]
- 44.Sallis JF, Taylor WC, Dowda M, Freedson PS, Pate RR. Correlates of vigorous physical activity for children in grades 1 through 12: Comparing parent-reported and objectively measured physical activity. Pediatric Exercise Science. 2002;14:30–44. [Google Scholar]
- 45.Sallis JF, Johnson MF, Calfas KJ, Caparosa S, Nichols JF. Assessing perceived physical environmental variables that may influence physical activity. Res Q Exerc Sport. 1997;68(4):345–351. doi: 10.1080/02701367.1997.10608015. [DOI] [PubMed] [Google Scholar]
- 46.Mendelson BK, White DR. Development of self-body-esteem in overweight youngsters. Dev Psychol. 1985;21(1):90–96. [Google Scholar]
- 47.Davison KK, Birch LL. Processes linking weight status and self-concept among girls from ages 5 to 7 years. Dev Psychol. 2002;38(5):735–748. doi: 10.1037//0012-1649.38.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Essex MJ, Boyce WT, Goldstein LH, et al. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the MacArthur Health and Behavior Questionnaire. J Am Acad Child Adolesc Psychiatry. 2002;41(5):588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 49.Luby JL, Heffelfinger A, Measelle JR, et al. Differential performance of the MacArthur HBQ and DISC-IV in identifying DSM-IV internalizing psychopathology in young children. J Am Acad Child Adolesc Psychiatry. 2002;41(4):458–466. doi: 10.1097/00004583-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Olson DH. FACES IV manual. Minneapolis: Life Innovations; 2008. [Google Scholar]
- 51.Olson DH. Circumplex model of family systems. Journal of Family Therapy. 2000;22(2):144–167. [Google Scholar]
- 52.Olson D. FACES IV and the circumplex model: Validation study. J Marital Fam Ther. 2011;37(1):64–80. doi: 10.1111/j.1752-0606.2009.00175.x. [DOI] [PubMed] [Google Scholar]
- 53.Williams GC, Deci EL. Internalization of biopsychosocial values by medical students: A test of self-determination theory. J Pers Soc Psychol. 1996;70(4):767–779. doi: 10.1037//0022-3514.70.4.767. [DOI] [PubMed] [Google Scholar]
- 54.Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. J Pers Soc Psychol. 1996;70(1):115–126. doi: 10.1037//0022-3514.70.1.115. [DOI] [PubMed] [Google Scholar]
- 55.Nervik D, Martin K, Rundquist P, Cleland J. The relationship between body mass index and gross motor development in children aged 3 to 5 years. Pediatr Phys Ther. 2011;23(2):144–148. doi: 10.1097/PEP.0b013e318218d356. [DOI] [PubMed] [Google Scholar]
- 56.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol. 1994;13(5):373–383. doi: 10.1037//0278-6133.13.5.373. [DOI] [PubMed] [Google Scholar]
- 57.Luepker RV, Perry CL, McKinlay SM, et al. Outcomes of a field trial to improve children’s dietary patterns and physical activity. The child and adolescent trial for cardiovascular health. CATCH collaborative group. JAMA. 1996;275(10):768–776. doi: 10.1001/jama.1996.03530340032026. [DOI] [PubMed] [Google Scholar]
- 58.Office of the Surgeon General (US), Office of Disease Prevention and Health Promotion (US), Centers for Disease Control and Prevention (US), National Institutes of Health (US) 2001 [Google Scholar]
- 59.U.S. Department of Health and Human Services. Proposed Healthy People 2020 objectives. 2009. [Google Scholar]
- 60.U.S. Department of Health and Human Services. Healthy People 2010. 2000. [Google Scholar]
- 61.Bauman L, Stein R, Ireys H. A framework for conceptualizing interventions. Sociological Practice Review. 1991;2:241–251. [Google Scholar]
- 62.Coday M, Klesges LM, Garrison RJ, Johnson KC, O’Toole M, Morris GS. Health opportunities with physical exercise (HOPE): Social contextual interventions to reduce sedentary behavior in urban settings. Health Educ Res. 2002;17(5):637–647. doi: 10.1093/her/17.5.637. [DOI] [PubMed] [Google Scholar]
- 63.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 64.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145(4):439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 65.Gidding SS, Dennison BA, Birch LL, et al. Dietary recommendations for children and adolescents: A guide for practitioners: Consensus statement from the American Heart Association. Circulation. 2005;112(13):2061–2075. doi: 10.1161/CIRCULATIONAHA.105.169251. [DOI] [PubMed] [Google Scholar]
- 66.West DS, Raczynski JM, Phillips MM, Bursac Z, Heath Gauss C, Montgomery BE. Parental recognition of overweight in school-age children. Obesity. 2008;16(3):630–636. doi: 10.1038/oby.2007.108. [DOI] [PubMed] [Google Scholar]
- 67.Barlow SE, Dietz WH, Klish WJ, Trowbridge FL. Medical evaluation of overweight children and adolescents: Reports from pediatricians, pediatric nurse practitioners, and registered dietitians. Pediatrics. 2002;110(1 Pt 2):222–228. [PubMed] [Google Scholar]
- 68.Brown DR, Fouad MN, Basen-Engquist K, Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Ann Epidemiol. 2000;10(8 Suppl):S13–21. doi: 10.1016/s1047-2797(00)00197-6. [DOI] [PubMed] [Google Scholar]
- 69.Harris KJ, Ahluwalia JS, Catley D, Okuyemi KS, Mayo MS, Resnicow K. Successful recruitment of minorities into clinical trials: The Kick it at Swope project. Nicotine Tob Res. 2003;5(4):575–584. doi: 10.1080/1462220031000118540. [DOI] [PubMed] [Google Scholar]
- 70.Winkleby MA, Robinson TN, Sundquist J, Kraemer HC. Ethnic variation in cardiovascular disease risk factors among children and young adults: Findings from the third National Health and Nutrition Examination Survey, 1988–1994. JAMA. 1999;281(11):1006–1013. doi: 10.1001/jama.281.11.1006. [DOI] [PubMed] [Google Scholar]
- 71.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the national heart, lung, and blood institute. N Engl J Med. 2000;343(7):475–480. doi: 10.1056/NEJM200008173430706. [DOI] [PubMed] [Google Scholar]
- 72.Webb MS, Seigers D, Wood EA. Recruiting African American smokers into intervention research: Relationships between recruitment strategies and participant characteristics. Res Nurs Health. 2009;32(1):86–95. doi: 10.1002/nur.20299. [DOI] [PubMed] [Google Scholar]
- 73.Dodds M, Nicholson L, Muse B, 3rd, Osborn LM. Group health supervision visits more effective than individual visits in delivering health care information. Pediatrics. 1993;91(3):668–670. [PubMed] [Google Scholar]
- 74.Osborn LM, Woolley FR. Use of groups in well child care. Pediatrics. 1981;67(5):701–706. [PubMed] [Google Scholar]
- 75.Taylor JA, Davis RL, Kemper KJ. A randomized controlled trial of group versus individual well child care for high-risk children: Maternal-child interaction and developmental outcomes. Pediatrics. 1997;99(6):E9. doi: 10.1542/peds.99.6.e9. [DOI] [PubMed] [Google Scholar]
- 76.Janicke DM, Sallinen BJ, Perri MG, et al. Comparison of parent-only vs family-based interventions for overweight children in underserved rural settings: Outcomes from project STORY. Arch Pediatr Adolesc Med. 2008;162(12):1119–1125. doi: 10.1001/archpedi.162.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Silverstein JH, Brumback B. Comparison of program costs for parent-only and family-based interventions for pediatric obesity in medically underserved rural settings. J Rural Health. 2009;25(3):326–330. doi: 10.1111/j.1748-0361.2009.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]