Abstract
Background:
Every year, millions of people are exposed to avoidable, life-threatening risks through the trans-fusion of unsafe blood.
Aim:
To determine the survival time of Treponema pallidum in banked donor blood.
Material and Methods:
Two groups of male Wistar rats (group A and B) were inoculated intratesticularly with 0.5ml of artificially infected donor blood (final density of Nichols treponemes: 5×105 /ml) stored at 4°C for various periods of time. In group A, a pair each of the rats was injected every 12 hours, starting at 0 hr, up to a maximal storage time of 96 hr. In group B, the rats were injected after 72, 120, 192 and 336 hours of storage of the treponemes-blood mixture. Group C which is a control group was injected with blood only, while group D rats were injected with heat-killed treponemes suspended in blood every 12 hours. The detection of Treponema pallidum IgG/IgM was based on the principle of double antigen sandwich immunoassay, in which purified recombinant antigens are employed sufficiently to identify antibodies to Syphilis. The outcomes of interest included the proportion of Syphilis positive rats and the maximal survival hours of T. pallidum in banked blood.
Results:
14 rats (77.8%) out of the 18 rats that were involved in group A developed orchitis and positive serology up to 72 hours of storage time, p<0.05. 2 rats (25%) in group B developed orchitis after 72hrs of storage time. All the 18 rats (100%) in the control group C and D showed neither clinical nor serological changes.
Conclusion:
It was concluded that the survival time of T. pallidum in banked donor blood lies between 72-120hrs in this study. Regardless of blood banking temperature, T. pallidum and other transfusion transmissible infections should be screened for prior to allogeneic transfusion.
Keywords: Syphilis, Treponema pallidum, Wistar rat, survival time
Introduction
It is believed in most health facilities as a tradition that T. pallidum spirochaetes are fragile, and cannot withstand blood-bank temperature when subjected to it for long hours. Hence, T. pallidum screening is not carried out on donated units of blood before allogeneic transfusion despite World Health Organization (W.H.O.) recommendations. However, in some extreme emergencies, some donated units of blood that has not been previously screened for syphilis might not be banked at all before transfusion, thereby, putting the recipient at high risk of Syphilis infection.
Every year, millions of people are exposed to avoidable, life-threatening risks through the transfusion of unsafe blood. As per a global database, 6 million of 81 million units of blood collected an-nually in 178 countries are not screened for trans-fusion-transmissible infections[1].
The provision of safe and efficacious blood and blood components for transfusion or manufacturing use involves a number of processes, from the selection of blood donors and the collection, processing and testing of blood donations to the testing of patient samples, the issue of compatible blood and its administration to the patient. There is a risk of error in each process in this “transfusion chain” and a failure at any of these stages can have serious implications for the recipients of blood and blood products. Thus, while blood transfusion can be life-saving, there are associated risks, particularly the transmission of blood-borne infections[2].
The microbial agents of importance to blood transfusion services are those that are transmissible by blood transfusion and can cause morbidity and mortality in recipients. In order to be transmissible by blood, the infectious agent or infection usually has the following characteristics: presence in the blood for long periods; sometimes in high titers, stability in blood stored at 4°C or lower, long incubation period before the appearance of clinical signs, asymptomatic phase or only mild symptoms in the blood donor, hence not identifiable during the blood donor selection process[2].
Donated blood is tested by many methods, but the core tests recommended by the World Health Organization are these four: Hepatitis B Surface Antigen, antibody to Hepatitis C, antibody to HIV; usually subtypes 1 and 2, serologic test for Syphilis. WHO reported in 2006 that 56 out of 124 countries surveyed did not use these basic tests on all blood donations[3].
Syphilis is a sexually transmitted infection (STI) caused by the Treponema pallidum spirochete. The route of transmission of syphilis is almost always by sexual contact, although there may be congenital syphilis via transmission from mother to child in-utero. Syphilis may also be transmitted via blood and blood products, and intravenous drug use[4]. If not treated, syphilis can cause serious effects such as damage to the aorta, brain, eyes, and bones. In some cases these effects may be fatal. More recently, there has been a resurgence of syphilis[4]. Syphilis has also acquired a new potential for morbidity and mortality through association with increased risk for HIV infection[4]. This will make it increasingly difficult to get safe blood because of this blood borne infection.
The aim of this study was to determine the survival time of Treponema pallidum in banked blood with the objective of evaluating the sero-prevalence of Treponema pallidum infection among Wistar rats inoculated with the treponemes-blood mixture.
Materials and Methods
This study was carried out in the Biomedical Sciences Department of the Ladoke Akintola University of Technology, Ogbomoso between the 21st of April and 5th of May, 2010. Laboratory animals were handled applying animal care regulations. The approval for this study was also obtained from the appropriate university committee.
The rats used in this research were male Wistar rats weighing 200g to 230g. Before rats were used, their sera was tested by the venereal disease research laboratory test and found to be nonreactive. Animals were housed individually at a temperature of 16 to 18°C. The virulent Nichols strain of T. pallidum used throughout this work was obtained from a research laboratory. Large pools were maintained frozen and were used to inoculate rats as a source of treponemes for experimental work. Donor blood was obtained from the blood bank department of the Ladoke Akintola University Teaching Hospital, Osogbo.
There were four groups in all (Table 1). Two groups of the rats (group A and B) were inoculated in both testes with 0.5ml of artificially infected donor blood (final density of Nichols treponemes: 5×105 /ml) stored at 4°C for various periods of time. There were nine pairs of rats in each group, except group B which had but four pairs. Group C and D were the control groups.
Table 1.
The experimental protocol

In group A, a pair each of rat was injected every 12 hours, starting at 0 hr, up to a maximal storage time of 96 hr. In group B, each pair of rats was innoculated after 72, 120, 192 and 336 hours of storage of the treponemes-blood mixture. Group C which was a control group was injected with blood only, while group D rats were injected with heat-killed treponemes suspended in blood every 12 hours respectively.
Assay for T. pallidum specific antibodies
Each plasma sample was screened for T. pallidum specific antibodies at room temperature using Syphilis Ultra Rapid Test Strip [ACUMEN® Diagnostics Inc., Livermore, California, USA. Lot No: SYP 8010022]. The test strips were correspondingly labeled prior to the test. The plasma was separated from packed cells before application to the test strip. The syphilis test strip was removed from the pouch, each strip per blood sample (plasma), 2 drops (approximately 50μL) was vertically transferred onto the specimen pad of the test strip with the help of a dropper (each dropper per blood sample), 1 drop of buffer (approximately 40μL) was then added and timer was started immediately, the maximum line on the strip was not exceeded during application. Afterwards, the strip was placed on a non-absorbent surface, and the test strips were observed for red color appearance indicating the presence of T. pallidum specific antibody in the serum. The result was read immediately after 10 minutes. The result was reported as positive, negative, or invalid against the appropriate patient's identification number. Care was taken to ensure the test kits used in this study were not expired.
The Syphilis Ultra Rapid Test Strip (Whole Blood/Serum/Plasma) is a qualitative membrane strip based immunoassay for the detection of T. Pallidum antibodies (IgG and IgM) in whole blood, serum or plasma. In this test procedure, recombinant Syphilis antigen is immobilized in the test line region of the strip. After a specimen is added to the specimen pad it reacts with the Syphilis antigen coated particles that have been applied to the specimen pad. This mixture migrates chromatographically along the length of the test strip and interacts with the immobilized Syphilis antigen. The double antigen test format can detect both IgG and IgM in specimens. If the specimen contains T. pallidum antibodies, a red line will appear in the test line region, indicating a positive result. If the specimen does not contain T. pallidum antibodies, a red line will not appear in this region, indicating a negative result. To serve as a procedural control, a pink line will always appear in the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
Positive test result was recorded as red color which appeared both on the control (C) and test (T) bands indicating the presence of anti T. pallidum antibodies in the serum samples. Invalid test result was recorded as no visible color in the test strip or a red color at (T) without color at (C) bands.
Statistical analysis
The data generated in this study were presented with descriptive statistics. In addition, statistical association between the risk factors and seropositivity was evaluated with Chi Square statistical test at 5% (p<0.05) level of significance.
Results
Table 2 shows the syphilis formation pattern in the treatment groups.
Table 2.
Syphilis serological formation pattern in the treatment groups
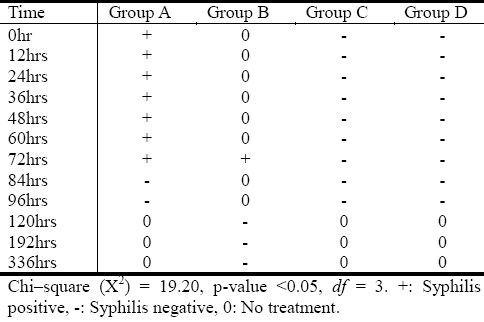
14 rats (77.8%) out of the 18 rats that were involved in group A developed orchitis and positive serology up to 72 hours of storage time, p<0.05. Only both 72hr rats (25%) developed orchitis and a positive syphilis serology in group B. All the 18 rats (100%) in the control group C and D showed neither clinical nor serological changes.
The 77.8% positive serology recorded in group A was thought to be as a result of the sero-conversion that has taken place in the experimental rats, regardless of the incubation time and the temperature (4°C) the treponemes-blood mixture was subjected to, before inoculation.
The 75% negative serology observed in group B was thought to be as a result of longer incubation period (≥72hrs) the treponemes-blood mixture was subjected to, before inoculation time, though, 25% of the rats presented with orchitis and a positive syphilis serology.
100% negative serology was observed in groups C and D. Initially, 50% of group D animals lost weight, hostile and aggressive but they later regained weight after ad-libitum feeding.
Discussion
While blood transfusion can be life-saving, there are associated risks, particularly the transmission of blood-borne infections[2]. This study was therefore aimed at determining the survival time of T. pallidum in banked blood. The literature also notes that Syphilis can occur in blood donors[5–7].
From this study, Treponema pallidum survival in blood bank temperature is of alarming rate i.e. (≥72hrs), p<0.05. The survival time of T. pallidum in this study was similar to that suggested by W.H.O.[2] in 2009. The ≥72hrs T. pallidum survival time found in this study calls for a major review of the practice of screening donor blood in Nigeria. Based on the results of this study, the present policy in which prospective donors are screened clinically and for only HIV and HBV infections therefore require urgent revision.
On the other hand, it is a well-known fact that HIV, HBV and HCV are global infectious pathogens contributing to mortality and morbidity in all ages[8,9]. Hence, there should be a consensus among transfusion scientists in Nigerian medical institutions on the need for routine donor screening of transfusion transmissible infections (TTIs) like HIV 1&2, HBV, HCV, and Syphilis. There are commercially available rapid test kits to determine the serum antibodies to the aforementioned TTIs.
The implication of T. pallidum survival in donor blood is the risk of transmission of this pathogen to recipients of blood and blood products. This can contribute to the ever-widening pool of infection in the wider population. Syphilis has also acquired a new potential for morbidity and mortality through association with increased risk for HIV infection[4] thus making safe blood more difficult to get.
Conclusion
It was concluded that the survival time of T. pallidum in banked donor blood lies between 72-120hrs in this study. Comparison of our results with literature findings suggests that the experimentally found survival time could depend on the number of treponemes initially present in the donor blood. However, it is fully unclear whether infectivity in rat’ testes can be paralleled with infectivity in the human system. In addition, progression of animal versus human disease may be quite different.
It is therefore recommended that regardless of blood banking temperature, it is required in all Nigerian hospitals to screen for T. pallidum and other transfusion transmissible infections prior to allogeneic transfusion, which may help in avoiding transfusion related Syphilis and its probable long-term effects. Syphilis positive pints of blood should be discarded and the affected donor treated appropriately.
Acknowledgement
The authors appreciate the management of Ladoke Akintola University of Technology, for permission to carry out this work.
References
- 1.Global data base on blood safety—report 2001-02. Geneva: World Health Organization; 2004. World Health Organization; pp. 5–17. [Google Scholar]
- 2.Geneva: WHO; 2009. World Health Organization. Screening Donated Blood for Transfusion Transmissible Infections-Recommendations; p. 30. [PubMed] [Google Scholar]
- 3.World Blood Donor Day. World Health Organization. 2006. [Accessed April 21, 2010]. at http://www.who.int/mediacentre/news/releases/2006/pr33/en/index.html .
- 4.Olokoba A, Olokoba L, Salawu F, et al. Res J Med Sci. 2008;2(5):217–219. [Google Scholar]
- 5.Chikwem J, Mohammed I, Okara G, Ukwandu N, Ola T. Prevalence of transmissible blood infections among blood donors at the University of Maiduguri Teaching Hospital, Maiduguri, Nigeria. East Afr Med J. 1997;74(4):213–216. [PubMed] [Google Scholar]
- 6.Ejele O, Erhabor O, Nwauche C. The risk of transfusion-transmissible viral infections in the Niger-Delta area of Nigeria. Sahel Med J. 2005;8(1):16–19. [Google Scholar]
- 7.Fiekumo I, Musa A, Jeremiah Z. Sero-epidemiology of transfusion-transmissible infectious diseases among blood donors in Osogbo, South-west, Nigeria. Blood Transfusion. 2009;1:1–10. doi: 10.2450/2009.0071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanki PJ, Adeyi O. “AIDS in Nigeria: A nation on the threshold.” Introduction. Harvard Center for Population and Development Studies. 2006:23–45. [Google Scholar]
- 9.Mustapha SK, Jibrin YB. The Prevalence of Hepatitis B Surface Antigen in Patients with Human Immunodeficiency Virus Infection in Gombe, Nigeria. Ann Afr Med. 2004;3(1):10–12. [Google Scholar]


