Abstract
Background:
The oxidative stress is considered as major consequence of diabetes mellitus affecting red cell antioxidant enzymes
Aim:
The present study was conducted to assess the impact of oxidative stress (reduced glutathione) on glutathione peroxidase, and glutathione reductse and prevalence of anemia among diabetic patients.
Materials and Methods:
The study involved 100 adult patients attending Buraidah Central Hospital and 30 healthy controls. Blood samples were collected and analyzed for glutathione (GSH) concentration, glutathione peroxidase (GPO), glutathione reductase (GR), fasting blood sugar (RBS), hemoglobin (HGB), red cell count (RBCs) hematocrit (HCT) mean cell volume (MCV) mean cell hemoglobin (MCH) and mean cell hemoglobin concentration (MCHC) and hemoglobin A1c. Blood urea, serum creatinine, and microalbuminuria were measured to exclude diabetes mellitus nephropathy.
Results:
were obtained showed significant correlation between deficiency of glutathione peroxidase, glutathione reductase and deficient of glutathione among diabetics, which has significant correlation between low hemoglobin concentration (females <120 g/L, males <130 g/L), also there is low concentration of red cell count and red cell indices (MCV, MCH and MCHC). The prevalence of anemia was 22% in diabetes patients.
Conclusion:
It can be concluded that there is strong significant effect of oxidative stress (reduced glutathione) on glutathione peroxidase, glutathione reductase level these may reduce hemoglobin concentration in diabetic patients. This means oxidative stress of diabetes mellitus is the possible cause of anemia in diabetics without nephropathy.
Keywords: Oxidative stress, diabetes mellitus, glutathione, glutathione peroxidase, glutathione reductase, anemia
Introduction
Depletion of blood glutathione (GSH)has been documented in many clinical situations such as malnutrition, severe burn injury , human immunodeficiency virus (HIV) infection, and diabetes, both type 1 and type 2. Glutathione depletion may have adverse consequences in diabetic patients, independent of glycemic control, and it may weaken the defense against oxidative stress. This could cause damage to protein, DNA, or membrane lipids and thus potentially lead to cell dysfunction in various tissues. In diabetes, increased oxidative stress is known to play a decisive role in the pathogenesis of vascular complications[1].
The role of glutathione peroxidase (GPO) in cellular defense against oxidant attack has been discussed for many years. The red cell has been a central focus because it is thought to undergo a high endogenous rate of H2O2 production from hemoglobin autoxidation, which can be markedly increased in cells with unstable hemoglobins. In addition, the red cell is probably exposed to H2O2 from stimulated macrophages and, under certain circumstances, from pathogenic bacteria or malarial parasites. It is believed that the glutathione peroxidase enzyme, protects the erythrocyte against peroxides that are generated intracellularly or exogenously. The relative importance of GPO in defending the cell has been debated since the discovery of glutathione peroxidase[2].
Glutathione reductase plays an important role in protecting hemoglobin, red cell enzymes, and biological cell membranes against oxidative damage by increasing the level of reduced glutathione (GSSGR) in the process of aerobic glycolysis. The enzyme deficiency may result in mild to moderately severe hemolytic anemia upon exposure to certain drugs or chemicals. However, hereditary deficiency of the enzyme is extremely rare[3].
Materials and Methods
Ethical approval
Permission from the ethical committee of Qassim university, Buraidah central hospital management were taken and verbal consent from study subjects on explanation of study objectives.
Sampling
Blood samples used in this study were drawn using vacutainer system into heparin and EDTA anticoagulant, also urine specimens were collected from 100 adult diabetic patients attending Buraidah central Hospital, while 30 adult non- diabetic subjects who are apparently healthy, were selected at random as a control.
Inclusion criteria
In this study, test samples were collected from diabetic patients with fasting blood glucose level >140 mg/dL and hemoglobin A1c (Hb A1c) percentage >8%. It is important to state that adequate measures were taken in making sure that the study controls and diabetic patients were not suffering from nephropathy. However study subjects with blood urea <45 mg/dL, serum creatinine below 1 mg/dL, and microalbuminuria level below 30 mcg/mg were used for this study.
Sample preparation
The heparinized, and EDTA samples were labeled by serial number and the date of collections were noted.
Samples were mixed well and tested within one hour for Hb A1c estimation, GPO, GR measurement, and reticulocyte count. After which the samples were tested using coulter machine (Sysmex company, Japan) to determine the hemoglobin (HGB), red cell (RBCs) count, hematocrit (HCT), mean cell volume (MCV), mean cell hemoglobin(MCH) and mean cell hemoglobin concentration (MCHC). The heparinized samples were centrifuged at 1000 × g for 10 min and plasma was harvested to be tested for blood glucose level, blood urea, plasma creatinine and blood total and direct bilirubin.
All reagents were obtained from Randox laboratories LTD, Ardmore, Diamond Road, Crumlin, Co. Antrim, United Kingdom BT29 4QY. HbA1c was assayed using the hemolysate preparation, where the labile fraction is eliminated, hemoglobins are retained by a cationic exchange resin. Hemoglobin A1c (HbA1c) is specifically eluted after washing away the hemoglobin A1a+b fraction (HbA1a+b), and is quantified by direct photometric reading at 415nm.
GSH was estimated by reducing (GSSG) in the presence of NADPH, which is oxidized to NADP+. The absorbance at 340 is measured spectrophotometerically. Reagents for GSH, and HbA1c measurement were obtained from Biosystems S.A. Costa Brava 30, Barcelona. Spain. Reticulocyte cells were counted using flowcytometer as described by Davis and Bigelow[4]. Microalbumiuria was measured as the ratio of albumin to creatinine in urine samples using commercial kits from Randox laboratories LTD, Ardmore, Diamond Road, Crumlin, Co. Antrim, United Kingdom BT29 4QY.
Statistical analysis
Data were analyzed statistically using student t-test to see if there is any significant difference between the mean of patients and the mean controls results and also to correlate between hematology parameters and GSH, GPO, and GR using Pearson correlation with significance less or equal to 0.05 level.
Results
As shown in Table 1 the level of GSH, GPO, GR, fasting blood sugar, total bilirubin in diabetic patients are highly significant (p ≤0.001) in comparison with healthy controls, while the direct bilirubin shows a significant increase (p ≤0.05).
Table 1.
Total glutathione (GSH), glutathione peroxidas (GPO), glutathione reductase (GR), fasting blood sugar (FBS), total bilirubin, direct bilirubin, blood urea, serum creatinine, microalbuminuria (means ± S.E.M) in Diabetic patients (n=100) and healthy controls (n=30).
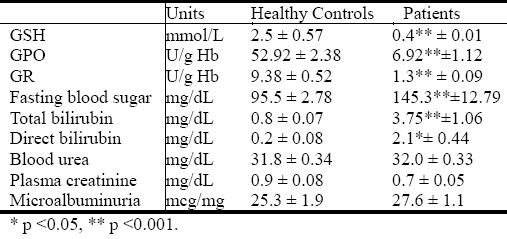
Hematological changes observed are presented in Table 2. There is a significant decrease (p ≤0.001) in HGB concentration and MCH value (p ≤0.05) in all diabetic patients in comparison with controls also the table shows highly significant increase in Hb A1c, and reticulocyte count.
Table 2.
Comparative hematological values (means ± S.E.M) in diabetic patients (n=100), and control (n=30).
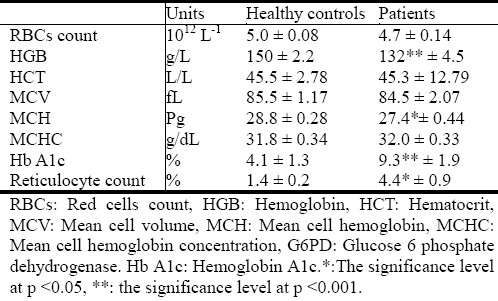
The data in Table 3 shows significant correlation between low HGB concentration, GSH,GPO and GR deficiency at level 0.01 (two tailed). Also the data indicates that HGB concentration have high significant correlation with RBCs count, MCV, MCH, and MCHC at level (0.01), while the prevalence of anemia constitutes 22% among diabetic patients as shown in Figure 1.
Table 3.
Correlation between hematology values, total glutathione (GSHa) glutathione peroxidase (GPOa), and glutathione reducrase (GRa)
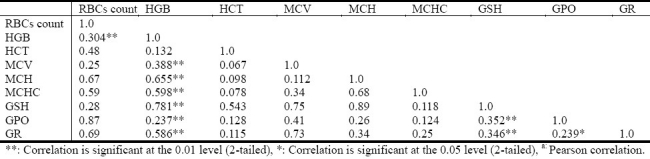
Fig. 1.
The prevalence of anemia (HGB for females <120g/L, <130 g/L for males) among diabetic patients.
Discussion
Glutathione can be used to “detoxify” reactive oxygen species such as hydrogen peroxide H2O2, a process in which glutathione is oxidized to the dimer glutathione disulfide in a reaction catalyzed by glutathione peroxidase. Reduced glutathione in turn is regenerated from glutathione disulfide by glutathione reductase in a reaction requiring NADPH as a cofactor[5].
Free radicals are constantly produced as a consequence of the endogenous reactions. High level of exogenous or endogenous free radicals could lead to destruction of major components of cellular structure, including nucleic acids, proteins, amino acids, lipids, and carbohydrates and would affect various cell functions such as membrane function, metabolism and even gene expression, and they can cause a number of other pathological conditions[6] As such, they are an important antioxidant defense in nearly all cells exposed to oxygen.
The red cell has been a central focus of this research, because it is thought to undergo a high endogenous rate of production H2O2 from hemoglobin autoxidation, which can be markedly increased in cells with unstable hemoglobins. In addition, the red cell is exposed to H2O2, superoxide, and NO in under certain conditions like diabetes[2].
The etiology and pathogenesis of anemia in diabetes mellitus is multifactorial. Chronic hyperglycemia might result in abnormal red blood cells, oxidative stress, and sympathetic denervation of the kidney related to autonomic neuropathy. These factors promote a hypoxic environment in the renal interstitium, which leads to impaired production of erythropoietin by the peritubular fibroblasts. Inappropriately low erythropoietin level is an important cause of early anemia in patients with diabetes mellitus[7]. In this study the erythropoietin deficiency factor has been excluded, because all patients were selected have no nephropathy or any renal problem
The present study demonstrates that red cell GSH, GPO and GR are significantly reduced in uncontrolled diabetic patients without nephropathy, compared with age and sex matched group of healthy volunteers. The reduction of these enzymes is due to a decrease in GSH concentration which leads to an increase in oxidative stress. However, it is important to note that elevation of blood glucose level generates oxidative stress1, which could contribute to increase in glutathione utilization[8].
The deficiency of most potent antioxidant GSH, and GPO, GR enzymes leads to increase free radical such H2O2, superoxide, and NO which are major oxidant in diabetic oxidative stress.
In Figure 1, 22% of diabetic patients are suffering from anemia due decrease in the HGB, RBCs count and HCT as shown in Table 1. This is as result of reduced antioxidants GSH, GPO and GTR. The deficiency of antioxidants lead to increase free radical such H2 O2, superoxide, and NO which are major oxidant in diabetes oxidative stress which could further lead to oxidation of red cell components, such as HGB and the cell becomes functionless and unable to carry oxygen. These red cells will be taken by reticuloendothelial system, which decreases the number of circulating RBCs and HGB concentration, which results to hemolytic anemia.
The lifespan of red blood cells might be decreased in patients with diabetes mellitus[9], so red blood cells are affected by various disturbances in the hematopoietic milieu, such as chronic hyperglycemia and hyperosmolarity[10], and these disturbances lead to elevated internal viscosity and increased membrane rigidity in these blood cells[11], so that the number of red blood cell is significantly reduced.
In comparison with previous cross-sectional study done by Thomas et al[12] shows that approximately 25% of patients who attended a diabetes clinic were anemic, but in this present study only 22% of the diabetic patients are anemic.
Conclusion
It can be concluded that there is strong significant impact of oxidative stress (low glutathione) on glutathione peroxidase, glutathione reductase level could reduce hemoglobin concentration in diabetic patients, that means oxidative stress of diabetes mellitus is the possible cause of anemia in diabetic patients without nephropathy.
Acknowledgement
This study was supported by a grant from the Research council, college of Medical Applied Science. Qassim University.
The author states that there are no conflicts of interest that may have influenced this work.
References
- 1.Dominique D, Shiela DS, Shawn S, et al. Evidence for accelerated rates of glutathione utilization and glutathione depletion in adolescents with poorly controlled type 1 diabetes. Diabetes. 2005;54(1):190–196. doi: 10.2337/diabetes.54.1.190. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RM, Goyette G, Ravindranath Y, Ho Y-S. Red cells from glutathione peroxidase-1-deficient mice have nearly normal defenses against exogenous peroxides. Blood. 2000;96(5):1985, 1988. [PubMed] [Google Scholar]
- 3.Chang JC, van der Hoeven LH, Haddox CH. Glutathione reductase in the red blood cells. Annals of Clinical and Laboratory Science. 1978;8(1):23–29. [PubMed] [Google Scholar]
- 4.Davis BH, Bigelow NC. Reticulocyte analysis and reticulocute maturity index. In: Darzynkiewicz Z, Crissman HA, editors. Flow cytometry. Methods in Cell Biology. 3rd ed. San Diego Academic Press; 1994. pp. 74–263. [DOI] [PubMed] [Google Scholar]
- 5.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defense against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 6.Young IS, Woodside JV. Anti-oxidants in health and disease. J Clin Pathol. 2001;54:176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosman DR, Winkler AS, Marsden JT, Macdougall IC, Watkins PJ. Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. J Diabetes Care. 2001;24:495–499. doi: 10.2337/diacare.24.3.495. [DOI] [PubMed] [Google Scholar]
- 8.De Mattia G, Laurenti O, Bravi C, Ghiselli A, Iuliano L, Balsano F. Effect of aldose reductase inhibition on glutathione redox status in erythrocytes of diabetic patients. Metabolism. 1994;43:965–968. doi: 10.1016/0026-0495(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 9.Virtue MA, Furne JK, Nuttall FQ, Levitt MD. Relationship between GHb concentration and erythrocyte survival determined from breath carbon-monoxide concentration. J Diabetes Care. 2004;27:931–935. doi: 10.2337/diacare.27.4.931. [DOI] [PubMed] [Google Scholar]
- 10.Schmid-Schonbein H, Volger E. Red-cell aggregation and red-cell deformability in diabetes. J Diabetes. 1976;25:897–902. [PubMed] [Google Scholar]
- 11.McMillan D E, Utterback NG, La PJ. Reduced erythrocyte deformability in diabetes. J Diabetes. 1978;27:895–901. doi: 10.2337/diab.27.9.895. [DOI] [PubMed] [Google Scholar]
- 12.Thomas MC, Cooper ME, Rossing K, Parving H. Anaemia in diabetes: is there a rationale to TREAT. Diabetologia. 2006;49:1151–1157. doi: 10.1007/s00125-006-0215-6. [DOI] [PubMed] [Google Scholar]


