Abstract
Background:
The roles of group C and F streptococci in causing endemic pharyngitis are still controversial, although group C streptococci are implicated in the outbreaks of pharyngitis and associated disorders.
Aim:
The aim of this study was to determine the prevalence and the role of these groups of β-hemolytic streptococci in acute pharyngitis with emphasis on the Streptococcus anginosus group. The antimicrobial susceptibility profile of these bacterial isolates and their ability to produce some virulence factors was also determined.
Materials and Methods:
Throat swab specimens were collected from 177 patients suffering from acute pharyngitis who had been admitted to the Hilla Teaching Hospital, Hilla, Iraq, during October 2009 to January 2010. The necessary biochemical tests were conducted and the organisms identified using standard procedures. Susceptibility of isolates pathogens to several antibiotics was examined using standard susceptibility testing. Virulence factors of these isolates were also determined using standard methods.
Results:
Results revealed that a total of 67 isolates belonged to β-hemolytic streptococci, of which 11(16.4%) isolates belonged to anginosus group streptococci, which possessed Lancefield group C and F antigens. Most of these bacterial isolates have the ability to produce more than one virulence factor such as capsule, hemolysin, CFA III, and lipase enzyme. The bacterial isolates were highly resistant to ampicillin, cefotaxime, and cefepime while they exhibited moderate resistance to tetracycline, ceftriaxone, and ciprofloxacin. On the other hand, they showed a high sensitivity to vancomycin, ofloxacin, and clindamycin.
Conclusion:
This study concluded that groups C and F Streptococci were implicated as a cause of acute pharyngitis in 6.2% of the specimens among other groups of streptococci. Most of these isolates have the ability to produce more than one virulence factor. There was a high rate of resistance among isolates for β-lactam antibiotics; however, they were highly susceptible to vancomycin, ofloxacin, and clindamycin.
Keywords: β-hemolytic streptococci, Group C and F, Streptococcus anginosus
Introduction
The Streptococcus milleri group (SMG) is a highly diverse group which includes three species: Streptococcus anginosus, Streptococcus intermedius, and Streptococcus constellatus. The group also includes hemolytic streptococci belonging to Lancefield groups A, C, F and G as well as non-groupable and non-hemolytic streptococci[1].
The nomenclature, identification and classification of Streptococcus milleri have been confusing. In 1989, it was proposed in the United States to rename this different group under one species name, Streptococcus anginosus. In Great Britain, the designation Strep. milleri was preferred[2].
Group C streptococci are a common cause of infection in animals; however, there is little information about their overall importance as a cause of human infection. Group C and G streptococci were first recognized as human pathogens by Lancefield and Hare in 1935[3].
The role of Lancefield group C beta-hemolytic streptococci in causing endemic pharyngitis is still controversial[4,5], although Lancefield group C streptococci are implicated in the outbreaks of purulent pharyngitis and associated disorders[6,7]. It is well known that group C streptococci are often isolated from clinical specimens. S. anginosus group (SAG) is the most common beta-hemolytic group C streptococcus isolated from the human throat[8,9]. Other group C streptococcal species are generally isolated only from patients with zoonotic infections[7].
Strains belonging to the ‘Streptococcus milleri’ group (SMG) are distinct among the viridans streptococci due to their tendency to cause suppurative infections and bacteremia[10]. The frequent presence of polysaccharide capsule may help these pathogens to escape being phagocytosed before adhering to the site of tissue damage. The production of extracellular enzymes including hyaluronidase, deoxyribonuclease, ribonuclase, gelatinase and collagenase by these organisms may contribute to their pathogenicity by degradation of connective tissues. SMG have also been observed to release extracellular products with immunosuppressive effects, which may allow the organism to survive within an abscess[11]. Strains belonging to the SMG are considered uniformly susceptible to the antibiotics usually employed for streptococcal infections[10]. However, few reports and studies have studied the antimicrobial susceptibility of the SMG and have demonstrated the presence of resistant strains[12,13].
To our knowledge, the prevalence and role of groups C and F in patients with acute pharyngitis have not been documented in our locality. Therefore, this study aims to determine the prevalence and the role of these groups of β-hemolytic streptococci in acute pharyngitis with emphasis on SAG in Hilla City, Iraq. The antimicrobial susceptibility profile of these bacterial isolates and their ability to produce some virulence factors will also be determined.
Patients and Methods
Patients and Specimen Collection
One hundred seventy seven throat swab specimens were collected from patients suffering from acute pharyngitis who were submitted to the Hilla Teaching Hospital, Hilla, Iraq, during a period of three months (from October 2009 to January 2010). The age of the patients ranged from 4-75 years. These specimens were collected with the aid of a physician to avoid any possible contamination. Each swab was inserted into the oropharynx and rotated there then carefully withdrawn avoiding contamination from the mouth. Swabs for culture were placed in tubes containing readymade media to maintain the swab wet until taken to laboratory. Each specimen was inoculated on a blood agar plates. All plates were incubated anaerobically in a Candle Jar at 37°C for 24-48 hrs. Throat swabs were collected aseptically and transported immediately to the laboratory.
Bacterial Isolates
The isolation and identification of SAG bacteria in the patients’ pharynx were performed through colony morphology of bacterial isolates, microscopic gram stain investigation and biochemical tests[14]. Isolates identification was also confirmed using API 20 test system (Biomerieux, France).
Streptex Agglutination Test
Streptex agglutination test (Remel, Lenexa, KS, USA) for Lancefield grouping was used to identify streptococci groups (A, B, C, D, F, and G) according to the manufacturer's instructions.
Detection of Virulence Factors
Virulence factors were detected in bacterial isolates according to standard procedures. Capsule production was detected by Hiss's Method of capsule staining[14]. Hemolytic reaction was detected on blood agar medium by streaking a pure culture of bacterial isolate and incubated at 37°C for 24-48 hrs. The appearance of a clear zone surrounding the colony is an indicator of β-hemolysis, while the greenish zone is an indicator of α-hemolysis[14].
Extracellular protease production was tested and carried out using M9 medium[15]. Colonization Factor antigens (CFA) I and III production test were performed to detect the ability of bacterial isolates to produce colonization factors antigen[16]. Bacteriocin production was performed using cup assay method on Brain Heart Infusion agar (BHI) supplemented with 5% glycerol to enhance their growth and bacteriocin production[17]. Lipase production was carried out in an egg-yolk agar medium[14]. Gliding motility of bacterial isolates was detected by swarm assay method to measure spreading of bacteria over the agar surface[18].
Antimicrobial Susceptibility Testing
The susceptibility of the bacterial isolates to antimicrobial agents was determined using disk diffusion method[19] and interpreted according to Clinical and Laboratory Standards Institute (CLSI) documents[20]. The following antimicrobial agents were obtained as standard reference disks for their known potency in laboratory use: penicillin (P), ampicillin (Am) 10 μg, cefotaxime (CTX), 30 μg, ceftriaxone (CTR) 30 μg, cefepime (FEP) 30 μg, vancomycin (VA) 30 μg, erythromycin (E), 15 μg clarithromycin (AZM) 15 μg, chloramphenicol (C) 30 μg, tetracycline (TE), 30 μg, clindamycin (DA) 2 μg, and ofloxacin (OFX) 5 μg. All of these tests were performed on plates of Mueller- Hinton agar (Oxoid, UK).
A 0.5 MacFarland suspension (Biomιrieux, France) of tested bacterial isolates was applied to the plates, which were dried in an incubator at 35°C for 15 minutes. Antimicrobial disks were placed on the agar with sterile forceps. The agar plates were incubated inverted at 35°C for 18 hours. Results were recorded by measuring the inhibition zone (in millimeters) and interpreted according to Clinical and Laboratory Standards Institute (CLSI) documents[20].
Detection of β-Lactamase Production
This test was performed for all isolates that were resistant to β-lactam antibiotics, using Rapid Iodometric Method[21].
Determination of Minimum Inhibitory Concentration (MIC)
Two methods were used for determination of MICs of isolates against seven different antibiotics. The MICs for cefotaxime and ciprofloxacin were determined by HiComb MIC Test using HiComb strips (Himedia, India).This test was carried out according to the procedure recommended by the manufacturer.
The MICs of the other antibiotics (ampicillin, ceftriaxone, vancomycin, erythromycin, and tetracycline) were determined by the two-fold agar dilution susceptibility method[22]. The MIC values were based on break points recommended by Clinical and Laboratory Standards Institute (CLSI) documents[20] for estimation of the response. The break point represents the optimum concentration of the drug that can reach the serum and provide a high level of therapy. The microorganism was considered sensitive if the estimated MICs were less than the break point. Standard strain (E. coli ATCC 25922) was used as a negative control.
Results
Morphological and biochemical characterization revealed that 137 isolates belonged to the genus Streptococcus, of which 67 (37.8%) were β-hemolytic isolates. The results showed that out of 67 β-hemolytic streptococcal isolates, 11(16.4%) isolates belonged to Strep. anginosus group. This represented 6.2% of all the positive culture throat swabs collected in this study.
In this study, there was a difference in the isolation rates of BHS between males and females. Of the 11 isolates which belonged to the anginosus group, 4 isolates (36.3%) were recovered from males and 7 isolates (63.6%) from females.
Results found that the API 20 strep system used for identification of SAG was not sufficient for identification of this group of β-hemolytic streptococci, so the following tests were used in addition to tests using the API 20 strep system: Colony characteristics and type of hemolysis (on blood agar), Catalase production, and sugar fermentation (Mannitol, Salicin, Raffinose). In addition to these morphological and biochemical tests, Lancefield group reaction was also used.
Figure 1 showed that the isolates in our study possessed Lancefield group antigens C, 2 (18.1%) and F, 8 (72.7%) with one isolate possessing both C and F antigens that were found in β-hemolytic streptococci.
Fig. 1.
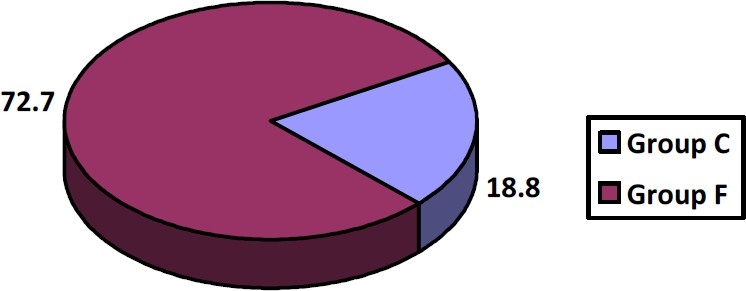
Percentages of group C and F antigens in the β-hemolytic streptococci recovered in this study.
Virulence Factors of the Bacterial Isolates
The virulence factors of SAG isolates in the present study were detected and the results are shown in Table 1.
Table 1.
Virulence factors detected in isolates of anginosus group streptococci
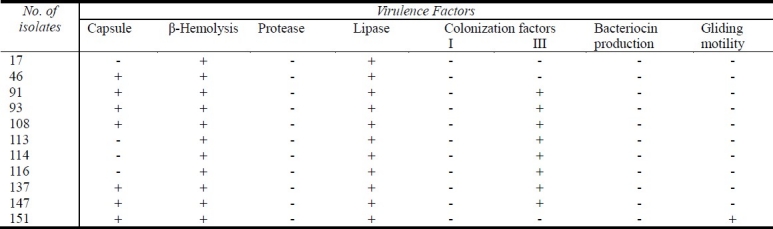
All isolates in the present study showed β-hemolysis, but no isolate was able to produce the enzyme protease when tested on M9 medium containing gelatin (Table 1). Conversely, all isolates produced the enzyme lipase after incubation for 48 hrs on egg yolk agar medium; most of them (72.7%) possessed the colonization factor antigens III (Table 1).
No isolate in the present study produced bacteriocin when tested with sensitive Gram positive indicator isolates and only one isolate showed a positive result regarding gliding motility test (Table 1).
Antibiotic Susceptibility of the Isolates
The results of the disk diffusion method revealed that most isolates were found to be resistant to at least 4 antibiotics tested (Table 2). The results also revealed that there was a highly bacterial resistance to β-lactam antibiotics ampicillin (90%), third generation cephalosporins (cefotaxime and ceftrixone) (100%), and fourth generation cephalosporin (cefepime) (81.8%). The result of a beta-lactamase detection test in this study revealed that there was no detectable beta-lactamase activity in all isolates of this group.
Table 2.
Antibiotic resistance of anginosus group streptococci
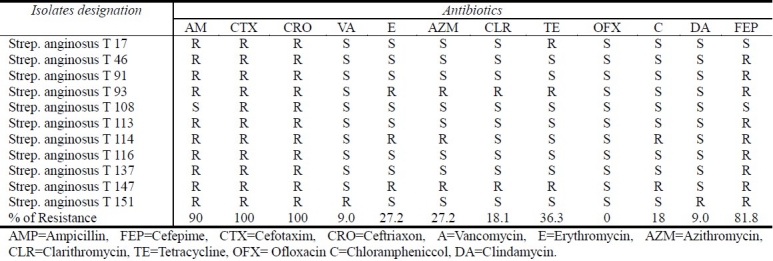
Bacterial susceptibility to the macrolide group (erythromycin, azithromycin, and clarithromycin) has also been investigated (Table 2). It was found that most isolates were sensitive to these antibiotics with a sensitivity rate of 72.7% for erythromycin, 72.7% for azithromycin, and 81.8% for clarithromycin.
Most isolates were sensitive to tetracycline (Table 2) with a resistance rate of 36.3%.
Results also showed that most isolates (10 of 11) were highly sensitive to vancomycin, clindamycin and chloramphenicol with a very low rate of resistance (9.0%), (18%), (9.0%), respectively. Table 2 showed that one isolate of SAG was resistant to vancomycin.
The results also showed that all isolates (100%) were sensitive to fluoroquinolone (ofloxacin). The results of β-Lactamase production test were negative for all tested isolates in this study.
MICs Determination of Anginosus Group Isolates
All 11 anginosus group isolates were highly resistant to ampicillin with a concentrations of one fold of the break point (≥128μg/ml). Most of them were highly to moderately resistant to ceftriaxone (7 isolates) and cefotaxime (8 isolates) with MIC values ranging from 0.25 to 8 μg/ml and from 1 to 30 μg/ml, respectively (Table 3).
Table 3.
MICs of a number of antibiotics against Strep. anginosus group isolates
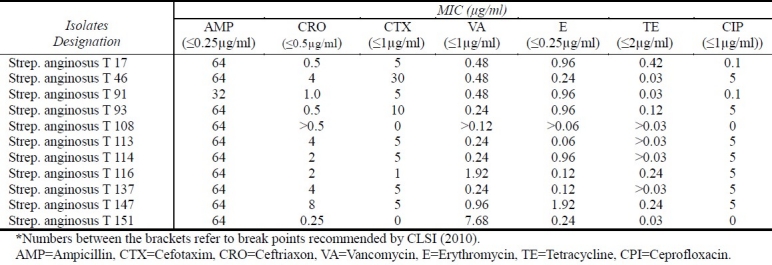
All isolates in the present study were highly susceptible to vancomycin and tetracycline (Table 3). However, one isolate was found to be less susceptible to vancomycin with MIC reached to (7.68 μg/ml). Results also showed a resistance rate of 45% to erythromycin. For ciprofloxacin, seven anginosus group isolates (Table 3) were able to grow in concentrations one fold of the break point (≥4μg/ml).
Discussion
The percentage of positive isolates (6.2%) belonged to the SAG among all β-hemolytic streptococci collected from throat swabs in the present study was higher than that recorded by other studies[23] that reported that 4.4% of throat swabs were positive for group C streptococci. This could be attributed to many factors such as cultural, ecological, and others (e.g., using antibiotics without medical roles). The high incidence of infections among females by BHS group of bacteria was inconsistent with other investigators who found a higher incidence of infections among males by this group of bacteria[24].
The use of biochemical profiling tests in the API 20 strep system test was not sufficient for final identification of species of this group of streptococci. This result was in accordance with other researchers who reported that identification may be difficult, and many laboratories reported the identification of these bacteria to the group level but not the species level[2].
The percentages of Lancefield group antigens C (18.1%) and F (72.7%) in SAG isolates in our study was close to results of[25] who found that Strep. milleri isolated in their clinical laboratory, represented 56% of group C, and 100% of group F, whereas the incidence of Strep. milleri among group A and G streptococci was estimated to be low.
In the present study, the virulence factors of SAG isolates were detected and results found that all isolates showed β-hemolysis (Table 1). This result is similar to that in a study that reported that strains of Strep. constellatus and Strep. anginosus (13 of 15 isolates), which belonged to Lancefield serological group F, were beta-hemolytic[26]. However, this result is not matched with the results of other studies, which reported that most strains of Strep. milleri were non-hemolytic. Only 25% were beta-hemolytic and may possess Lancefield group A, C, F, or G antigens[27].
The inability of our isolates to produce the enzyme protease (gelatinase) was also confirmed by another study, which reported that all Strep. milleri isolates tested were unable to show gelatinase activity[28]. The ability of all isolates to produce the enzyme lipase on egg yolk agar medium was inconsistent with a study that found that all isolates examined gave a negative reaction in a test for lipase production[29]. Another study reported that lipase has been observed in some strains of SMG[28].
The possession of colonization factor antigens by most isolates in the present study (72.7%) was consistent with other investigators who have demonstrated fine fimbrial structures on the surface of oral streptococci, including Strep. Milleri. This may be important in its adherence to surfaces and in inter-species interactions that are common in the mixed flora of mucosal surfaces, dental plaque, and purulent lesions[30].
No isolate in the present study produced bacteriocin (Table 1). This result disagreed with a study that showed that three of 30 human strains of group C Streptococci produced inhibitors that had bacteriocin-like properties[31].
A gliding type of motility by only one isolate in this study was consistent with a report that described isolates belonging to the SMG that appeared to exhibit a gliding type of motility. This is expressed as spreading growth on certain types of chocolate agar medium[32].
Results of disk diffusion method revealed that most isolates were found to be highly resistant to beta-lactam antibiotics tested. These results were comparable with the data reviewed which reported that resistance to penicillin G was associated with resistance to the other beta-lactam antibiotics[33].
The erythromycin resistance rate seen in this study was significantly higher than that of SAG isolates from the Netherlands (2.6%) and Spain (17.1%)[12,35]. Due to the high frequency of genetic exchange between different streptococcal species, it is likely that the increased macrolide resistance among viridans streptococci has contributed to an increased macrolide resistance among SAG isolates[13].
Although most isolates were sensitive to tetracycline (Table 2), a resistance rate of 36.3% detected in the present study was comparable with a study that found that the resistance rate for tetracycline of 70 clinically significant isolates of SMG was 37.1%[36] ..
Although one isolate of SAG showed reduced susceptibility (resistance) to vancomycin disk diffusion test (Table 2), CLSI documents[20] reported that the disk diffusion procedure cannot differentiate isolates with reduced susceptibility to vancomycin from susceptible isolates (MIC range ≤1 μg/mL) even when incubated for 24 hours. Since all isolates of SAG (100%) were sensitive to ofloxacin, this drug might serve as the preferred drug for the treatment of infections caused by this group of bacteria.
The results of β-lactamase production test were negative for all tested isolates in this study. The inability of all isolates to produce the beta-lactamase enzyme may be attributed to the fact that the mechanism of beta-lactam resistance in this group of bacteria is due to altered target (penicillin binding proteins, PBPs) rather than beta-lactamase production.
This result is in agreement with other researchers who mentioned that the intrinsic penicillin resistance mechanism in viridans group streptococci involves alterations of the target enzymes for β-lactams, essential penicillin-binding proteins, with a decreased affinity of all β-lactam antibiotics[37]. This mechanism, together with selective antibiotic pressure, may play a role in resistance of viridans group streptococci to β-lactam antibiotics. Since Penicillin-resistance of viridans streptococci is not due to beta-lactamase production, no benefit from using agents such as ampicillin-sulbactam as bacterial therapy[34].
Results of MICs determination of anginosus group isolates showed that all 11 anginosus group isolates ranged from highly to moderately resistant to beta-lactam antibiotics (Table 3). These results are not agreed with results of[38] who reported that the β-lactam group provides the antibiotic of choice in infections caused by streptococci, and with[37] who mentioned that many other β-lactam antibiotics have in vitro activity similar to that of penicillin against the SMG, but the susceptibilities to different cephalosporins are quite variable.
Although all isolates in the present study were highly susceptible to vancomycin and tetracycline (Table 3). The percentage of tetracycline resistance (100%) among isolates of this group was higher than that recorded in a study that found that more variable results were obtained with tetracyclines. Rates of resistance in the range of 0-37% have been described[36].
However, one isolate was found to be less susceptible to vancomycin with MIC reaching 7.68 μg/ml, which is seven times more than the break point recommended by the Clinical and Laboratory Standards Institute (≤1 μg/ml)[20] .The results of the single isolate that was less susceptible to vancomycin may be considered as vancomycin tolerant. No vancomycin resistance had been recorded previously locally or worldwide in this group of bacteria, but this resistance was found in Staphylococcus aureus and Streptococcus pneumoniae. To our knowledge, this is the first resistant isolate recorded in Iraq. However, this group may be tolerant to vancomycin rather than being resistant.
Vancomycin tolerance in this group matches reported tolerance to vancomycin among pharyngeal isolates of non-group A β-hemolytic streptococci (mostly GCS and GGS) from children[39], and with a study that reported that a few clinical isolates exhibited tolerance to vancomycin[40]. The less susceptibility (or resistance) of this isolate to vancomycin may be recorded only when the resistance genes (VanA, VanB, VanC, etc could be detected by genetic methods (polymerase chain reaction [PCR]). This was not determined in the present study.
Table 3 showed a resistance rate of 45% to erythromycin. This result is much higher than that previously reported that mentioned that resistance to erythromycin-lincomycin-clindamycin group, although currently rare, is transferable among streptococci, including the SMG, and can be as high as 12-14%[41].
The ability of seven anginosus group isolates (out of 11) (Table 3) to grow in ciprofloxacin in concentrations one fold of the break point (≥4μg/ml) was in accordance with previous data that showed that even in ciprofloxacin-susceptible isolate, the ciprofloxacin MICs were very close to the resistance break points and suggested that ciprofloxacin is inadequate for treatment of SAG infections[13]. In a study of the antimicrobial susceptibility of the SMG, a steady increase in the susceptibility to ciprofloxacin was observed over the study period[35]. While some authors reported that ciprofloxacin was the less active agent than the other agents tested, it was the least active quinolones, especially against all species of streptococci[42].
Conclusion
This study concluded that group C and F Streptococci were implicated as a cause of acute pharyngitis in 6.2% of the clinical specimens among other groups of β-hemolytic streptococci in a hospital in Hilla, Iraq. Most of these isolates have the ability to produce more than one virulence factor. There was a high rate of resistance among these isolates for β-lactam antibiotics, but they were highly susceptible to vancomycin, ofloxacin, and clindamycin.
References
- 1.Whiley RA, Beighton D. Emended description and recognition of Streptococcus constellatus, Streptococcus intermedius, and Streptococcus anginosus as distinct species. Int J Syst Bacteriol. 1991;41:1–5. doi: 10.1099/00207713-41-1-1. [DOI] [PubMed] [Google Scholar]
- 2.Belko J, Goldman DA, Marcone A, Zaidi AK. Clinically significant infections with organisms of the Streptococcus milleri group. Pediatr Infect Dis J. 2002;21:715–723. doi: 10.1097/00006454-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 3.ReiBmann S, Friedrichs C, Rajkumari R, et al. Contribution of Streptococcus anginosus to Infections Caused by Groups C and G Streptococci, Southern India. Emerg Infect Dis. 2010;16:656–663. doi: 10.3201/eid1604.090448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier FA, Centor RM, Graham L, et al. Clinical and microbiological evidence for endemic pharyngitis among adults due to group C streptococci. Arch Intern Med. 1990;150:825–829. [PubMed] [Google Scholar]
- 5.Turner JC, Hayden GF, Kiselica D, et al. Association of group C beta-hemolytic streptococci with endemic pharyngitis among college students. JAMA. 1990;264:2644–2647. [PubMed] [Google Scholar]
- 6.Benjamin J, Perriello VA. Pharyngitis due to group C hemolytic streptococci in children. J Pediatr. 1976;89:254–255. doi: 10.1016/s0022-3476(76)80459-3. [DOI] [PubMed] [Google Scholar]
- 7.Cimolai N, Elford RW, Bryan L, et al. Do non-group A strep cause endemic pharyngitis? Rev Infect Dis. 1988;10:587–601. doi: 10.1093/clinids/10.3.587. [DOI] [PubMed] [Google Scholar]
- 8.Lebrun L, Gulbert M, Wallet P, et al. Human Fc (gamma) receptors for differentiation in throat cultures of group C “Streptococcus equisimilis” and group C “Streptococcus milleri.”. J Clin Microbiol. 1986;24:705–707. doi: 10.1128/jcm.24.5.705-707.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox K, Turner J, Fox A. Role of Beta-Hemolytic Group C Streptococci in Pharyngitis: Incidence and Biochemical Characteristics of Streptococcus equisimilis and Streptococcus anginosus in Patients and Healthy Controls. J Clin Microbiol. 1993;31(4):804–807. doi: 10.1128/jcm.31.4.804-807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988;10:257–285. doi: 10.1093/clinids/10.2.257. [DOI] [PubMed] [Google Scholar]
- 11.Arala-Chaves MP, Porto MT, Arnaud P. Fractionation and characterization of the immunosuppressive substance in crude extracellular products released by Streptococcus intermedius. J Clin Invest. 1981;68(1):294–302. doi: 10.1172/JCI110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs JA, Stobberingh EE. In-vitro antimicrobial Susceptibility of the “Streptococcus milleri” group (Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius) J Antimicrob Chemother. 1996;37:371–375. doi: 10.1093/jac/37.2.371. [DOI] [PubMed] [Google Scholar]
- 13.Asmah N, Eberspächer B, Regnath T, et al. Prevalence of erythromycin and clindamycin resistance among clinical isolates of the Streptococcus anginosus group in Germany. J Med Microbiol. 2009;58:222–227. doi: 10.1099/jmm.0.001560-0. [DOI] [PubMed] [Google Scholar]
- 14.Forbes BA, Daniel FS, Alice SW. Baily and Scot's Diagnostic microbiology? 12th ed. USA: Mosby Elsevier Company; 2007. [Google Scholar]
- 15.Piret J, Millet J, Demain A. Production of intracellular protease during sporulation of Bacillus brrevis. Eur J Appl Microbiol Biotechnol. 1983;17:227–230. [Google Scholar]
- 16.Sambrook J, Rusell DW. Cold Spring Harbor. Third ed. NY: Cold Spring Harbor Laboratory Press; 2001. Molecular cloning. A laboratory manual. [Google Scholar]
- 17.A1-Qassab AO, Al-Khafaji ZM. Effect of different conditions on inhibition activity of enteric lactobacilli against diarrhea-causing enteric bacteria. J Agric Sci. 1992;3(1):18–26. [Google Scholar]
- 18.Wolfe AJ, Berg HG. Migration of bacteria in semisolid agar. Proc. Natl. Acad Sci. 1989;86:6873–6977. doi: 10.1073/pnas.86.18.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards (NCCLS) Approved standard M2-A8. 3rd ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2003. Performance standards for disc susceptibility tests. [Google Scholar]
- 20.Clinical and Laboratory Standards Institute (CLSI) Approved standard M100-S20. 1. Vol. 30. Wayne, PA. USA: National Committee for Clinical Laboratory Standards; 2010. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 21.WHO. Techniques for the detection of β- lactamase producing strains of Neisseria gonorrhoeae. 1978;616:137–143. [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards (NCCLS) Approved standard M7-A6. 6th ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. [Google Scholar]
- 23.Lewis RFM, Balfour AE. Group C streptococci isolated from throat swabs: a laboratory and clinical study. J Clin Pathol. 1999;52:264–266. doi: 10.1136/jcp.52.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs JA, Pietersen HG, Stobberingh EE, et al. Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius. Clinical relevance, hemolytic and serologic characteristics. Am Int Clin Pathol. 1995;104:547–553. doi: 10.1093/ajcp/104.5.547. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence J, Yajko DM, Hadley WK. Incidence and characterization of beta-hemolytic Streptococcus milleri and differentiation from Strep. payogenes (group A), S. equisimilis (group C), and large colony group G streptococci. J Clin Microbiol. 1985;22:772–777. doi: 10.1128/jcm.22.5.772-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiley RA, Hardie JM, Beighton D. Phenotypic differentiation of Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosue “Streptococcus milleri group”. J Clin Microbiol. 1990;28:1497–1501. doi: 10.1128/jcm.28.7.1497-1501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ball LC, Parker MT. The cultural and biochemical characters of Streptococcus milleri strains isolated from human sources. J Hyg. 1979;82:63–78. doi: 10.1017/s002217240002547x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bridge PD, Sneath PH. Numerical taxonomy of Streptococcus. J Gen Microbiol. 1983;129:565–597. doi: 10.1099/00221287-129-3-565. [DOI] [PubMed] [Google Scholar]
- 29.Ruoff KL, Ferraro MJ. Hydrolytic enzymes of Streptococcus milleri. J Clin Microbiol. 1987;25(9):1645–1647. doi: 10.1128/jcm.25.9.1645-1647.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handley PS, Carter PL, Wyatt JE, et al. Surface structures (peritrichous fibrils and tufts of fibrils) found on Streptococcus sangguis strains may be related to their ability to coaggregate with other oral genera. Infect Immun. 1985;47:217–227. doi: 10.1128/iai.47.1.217-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schofield CR, Tagg JR. Bacteriocin-like activity of group B and group C streptococci of human and of animal origin. J Hyg Camb. 1983;90:7–18. doi: 10.1017/s0022172400063774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergman S, Selig M, Collins JA, et al. “Streptococcus milleri” Strains displaying a gliding type of motility. Int J Syst Bacteriol. 1995;45:235–239. doi: 10.1099/00207713-45-2-235. [DOI] [PubMed] [Google Scholar]
- 33.Clermont D, Horaud T. Identification of chromosomal antibiotic resistance genes in Streptococcus anginosus (“Strep. milleri”) Antimicrob Agents Chemother. 1990;34:1685–1690. doi: 10.1128/aac.34.9.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melia M, Auwaerter P. Streptococcus species. Infect. Dis. Society of America. 2009:394–434. [Google Scholar]
- 35.Limia A, Jimenez ML, Alarcón T, et al .Five-year analysis of antimicrobial susceptibility of the Streptococcus milleri group. Eur J Microbiol Infect Dis. 1999;18:440–444. doi: 10.1007/s100960050315. [DOI] [PubMed] [Google Scholar]
- 36.Gómez-Garcés JL, Alos JI, Cogollos R. Bacteriologic characteristics and antimicrobial susceptibility of 70 clinically significant isolates of Streptococcus milleri group. Diagn Microbiol Infect Dis. 1994;19:69–73. doi: 10.1016/0732-8893(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 37.Alcaide F, Linares J, Pallares R, et al. In vitro activities of 22 β-lactam antibiotics against penicillin-resistant and penicillin-susceptible viridians group streptococci isolated from blood. Antimicrob Chemother. 1995;39:2243–2247. doi: 10.1128/aac.39.10.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruoff KL. Streptococcus anginosus (Streptococcus milleri): The unrecognized pathogen. Clin Microbiol Rev. 1988;1:102–108. doi: 10.1128/cmr.1.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaoutis T, Schnieder B, Moore LS, et al. Antibiotic susceptibilities of group C and group G streptococci isolated from patients with invasive infections: evidence of vancomycin tolerance among group G serotypes. J Clin Microbiol. 1999;37:3380–3383. doi: 10.1128/jcm.37.10.3380-3383.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noble JT, Tyburski MB, Berman M, et al. Antimicrobial tolerance in group G streptococcus. Lancet. 1980;ii:982. doi: 10.1016/s0140-6736(80)92143-1. [DOI] [PubMed] [Google Scholar]
- 41.Horodniceanu T, Bouguelert L, Bieth G. Conjugative transfer of multiple-antibiotic resistance markers in β-hemolytic group A, B, F and G streptococci in the absence of extrachromosomal deoxyribonucleic acid. Plasmids. 1981;5:127–137. doi: 10.1016/0147-619x(81)90014-7. [DOI] [PubMed] [Google Scholar]
- 42.Citron DM, Goldstein EJC, Merriam CV, et al. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J Clin Microbiol. 2007;45:2819–2828. doi: 10.1128/JCM.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


