Abstract
Background:
Bronchiolitis is a potentially life-threatening respiratory illness commonly affecting children who are less than two years of age. Patients with viral lower respiratory tract infection are at risk for co-bacterial infection.
Aim:
The aim of our study was to evaluate the use of C-reactive protein (CRP) in predicting bacterial co-infection in patients hospitalized for bronchiolitis and to correlate the results with the use of antibiotics.
Patients and Methods:
This is a prospective study that included patients diagnosed with bronchiolitis admitted to Makassed General Hospital in Beirut from October 2008 to April 2009. A tracheal aspirate culture was taken from all patients with bronchiolitis on admission to the hospital. Blood was drawn to test C-reactive protein level, white cell count, transaminases level, and blood sugar level.
Results:
Forty-nine patients were enrolled in the study and were divided into two groups. Group 1 included patients with positive tracheal aspirate culture and Group 2 included those with negative culture. All patients with a CRP level ≥2 mg/dL have had bacterial co-infection. White cell count, transaminases and blood sugar levels were not predictive for bacterial co-infection. The presence of bacterial co-infection increased the length of hospital stay in the first group by 2 days compared to those in the second group.
Conclusion:
Bacterial co-infection is frequent in infants with moderate to severe bronchiolitis and requires admission. Our data showed that a CRP level greater than 1.1 mg/dL raised suspicion for bacterial co-infection. Thus, a tracheal aspirate should be investigated microbiologically in all hospitalized patients in order to avoid unnecessary antimicrobial therapy and to shorten the duration of the hospital stay.
Keywords: Bronchiolitis, Lower respiratory tract infection in children, C-reactive protein, Bacterial co-infection
Introduction
Bronchiolitis is a common respiratory disease in children younger than two years of age. It results in narrowing or obstruction of the lower respiratory tract[1], and occurs in association with viral infection, most commonly the Respiratory Syncytial Virus (RSV), in around 75% of cases. Usually, it is seasonal with peak prevalence in the winter months (from November-March) when such viruses are widespread. Re-infection during a single season is possible[2].
Patients typically present with rhinorrhea, congestion, cough, wheezing, and low-grade fever. In more severe cases, patients may develop respiratory distress that requires prompt hospitalization. Assisted mechanical ventilation is rarely required in those patients[1]. Bacterial co-infection is clinically well documented in viral respiratory disease, but the pathogenic mechanisms are still poorly understood. One possible mechanism is that viral infections facilitate bacterial colonization, adherence and trans-location through the epithelial barrier, paving the way for bacterial disease[3].
Although antimicrobial therapy is not recommended for treatment of patients with bronchiolitis unless there is concern about complications such as secondary bacterial pneumonia, the reported rate of use of antibiotics varies from 28% to 62% across hospitals in America[4]. Multiple studies were carried out in order to identify a predictor of bacterial co-infection in patients with bronchiolitis, but no factor could be identified. The aim of our study was to evaluate C-reactive protein (CRP) as a predictor of such complication in patients hospitalized for bronchiolitis and to correlate the results with the use of antibiotics.
Patients and Methods
All patients under two years of age and admitted to Makassed General Hospital in Beirut with the diagnosis of bronchiolitis between October 2008 and April 2009 were studied prospectively. The diagnosis of bronchiolitis was based on clinical background, defined as the evidence of new onset of lower respiratory tract symptoms of strongly suspected viral infection cause in children less than 2 years of age. Written informed consent was obtained from the parents or a legal guardian of all infants included in the study. The study was approved by the Institutional Review Board and the research and ethics committee at the hospital.
The sex, age, weight and height of the patient as well as duration of hospitalization were recorded. Blood was drawn upon admission and sent for analysis. Automated blood count, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), blood sugar (BS) and C-reactive protein (CRP) were performed. CRP was determined by immune-phelometry. A CRP level> 0.3 mg/dL was considered positive.
Tracheal aspirate was directly collected at admission by an inhalation therapist in the presence of a doctor and nurse. Specimens were obtained by passage of a 10F suction catheter into the trachea, while avoiding oropharyngeal contamination. The suction catheter was attached to a sterile mucus trap. Although routine radiological study is not recommended by the American Academy of Pediatrics in the diagnosis of bronchiolitis, a plain chest radiograph was performed for all patients to exclude a concomitant bacterial pneumonia. The chest radiograph was checked by the radiologist who was blinded to the study. Antibiotics, steroids, bronchodilators and oxygen supplement were added according to the clinical results of the patient and tracheal aspirate culture.
Patients were divided into two groups according to the results of the tracheal aspirate culture: group 1 included patients with positive tracheal aspirate culture and group 2 included those with negative culture. Exclusion criteria were: 1)Evidence of pneumonia on chest radiograph upon admission, 2)Patients with evidence of other associated infections (Acute otitis media, acute gastroenteritis), 3)Patients with underlying chronic lung disease, 4)Prior use of antibiotics 2 weeks before admission, 5)Patients with systemic disease, 6) Previous exposure to mechanical ventilation, 7)Malnutrition, 8)Immunocompromised patients, and 9)Presence of two morphotypes from tracheal aspirate culture.
Statistical Analysis
Statistical analysis was performed with the use of Sigma stat software (SPSS Inc) version 13.0. For comparison of categorical data, chi-square and Fisher exact tests were used. Results were analyzed by repeated measures Analysis of Variance (repeated measures ANOVA). A p value <0.05 was considered significant.
Results
A total of 69 patients were hospitalized with the diagnosis of bronchiolitis during the study period. Twenty patients were excluded: twelve have had pneumonia on chest radiograph upon admission, tracheal aspirate was not taken in five patients due to family refusal, and three patients were mechanically ventilated at birth.
Therefore, a total of 49 patients were enrolled in the study: 29 males and 20 females. 29 out of 49 patients have had positive tracheal aspirate culture (Group 1), while 20 out of 49 patients have had negative culture (Group 2). The mean age of the patients was similar in both groups (107±80 days in group 1 versus 128±114 days in group 2) as well as the growth parameters. The most common organisms cultured were Haemophilus influenzae (n=9), Moraxella catarrhalis (n=5), Escherichia coli n=5, Staphylococcus aureus (n=3), Streptococcus pneumoniae (n=3), Klebsiella pneumonia (n=2), and Pseudomonas aeruginosa (n=2).
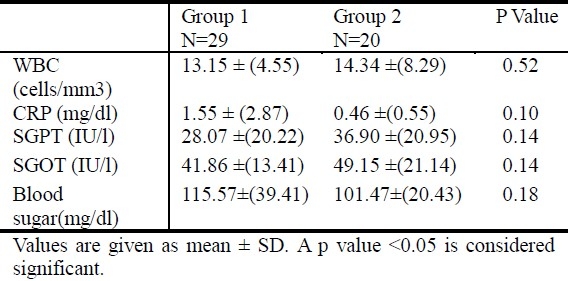
The mean CRP level was 1.55± 2.87 mg/dL in group 1 versus 0.46± 0.55 mg/dL in group 2, but the difference was not significant. The tracheal aspirate culture was positive in 83.3% of patients in group 1 compared to 18% in group 2 when the CRP level was ≥1.1 mg/dL (p<0.05). Five patients have had bacterial co-infection when the CRP level was ≥ 2 mg/dL (Table 1). Table 2 summarizes the results of laboratory studies in both groups.
Table 1.
Results of tracheal aspirate culture in correlation with the CRP level
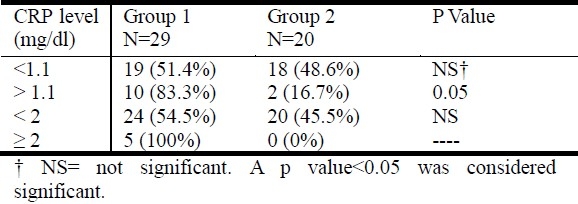
Table 2.
Results of laboratory studies in both groups
A chest radiograph was obtained for all patients who are admitted with the diagnosis of bronchiolitis. Antimicrobial therapy was initiated in 86.2% of patients in group 1 compared to 50% in group 2 (p<0.006). The use of bronchodilators and steroids was similar in both groups. The duration of hospitalization of patients was 7.60±2.38 days in group 1 compared to 4.50±0.71 days in group 2 and it was not significant.
Discussion
Bronchiolitis is a disorder most commonly caused in infants by viral lower respiratory tract infection[5]. Respiratory Syncytial Virus (RSV) is the most important cause during infancy worldwide[6,7]. It is assumed that nearly all children become infected with RSV within the first 2 years of life[8,9]. Clinicians should diagnose bronchiolitis and assess disease severity on the basis of history and physical examination and should not routinely order laboratory or radiological studies for diagnosis[5]. In our study, the diagnosis of bronchiolitis was based on clinical background and the chest radiograph was performed in all patients to exclude the presence of pneumonia.
Although bacterial co-infection in viral respiratory disease is clinically well documented, pathogenic mechanisms are still poorly understood[3] .The reported prevalence of such complication in the literature varies between 33% and 44% according to several centers[10–12]. Thorburn et al. reported that up to 40% of children with RSV bronchiolitis admitted to PICU were infected with bacteria in their lower airways and were at increased risk of bacterial pneumonia[13]. Kneyber et al. found that 33% of children (9 of 24) in whom admission endotracheal aspirates were performed had a positive bacterial culture[10,11]. Among our population, we found a higher percentage of positive cultures (60%) most commonly Hemophilus influenza, Moraxella catarrhalis, and Escherichia coli. Sanford et al. in 1978 was the first to introduce an adherence assay to verify increased susceptibility of mammalian cells to bacterial adherence as a result of viral infection[14] .One possible mechanism is that viral infections facilitate bacterial colonization, adherence and trans-location through the epithelial barrier, paving the way for bacterial disease[3]. Studies have focused on changes in colonization and adherence capacity of bacteria during viral infection[3]. Duttweiler et al. studied retrospectively 127 infants admitted to the ICU for RSV bronchiolitis and found that 44% of those ventilated and endotracheally sampled had “concomitant bacterial pneumonia”[15].
Since bacterial co-infection in patients with bronchiolitis may complicate the natural course of the disease and thus may increase the morbidity and duration of hospitalization stay, multiple studies were carried out to identify a predictor for such complication. The highest CRP value was found to have significant correlation with indirect markers of bronchiolitis severity[16]. In a study analysis of 48 patients hospitalized for bronchiolitis, analyzed CRP value, along with Procalcitonin, Interleukin-6 and leukocyte counts in infants with bronchiolitis, concluded that bronchiolitis severity did not have a significant influence on any of the values[17]. Serum Procalcitonin, CRP and IL-6 have little value in the differentiation of bacterial and viral pneumonia in children[18]. However, in some patients with very high serum PCT, CRP or IL-6 values, bacterial pneumonia is probable[18]. In our study, we found that the mean CRP value was not different between patients with positive or negative tracheal aspirate culture. However, we noticed a high probability of bacterial co-infection when the CRP level was greater than 1.1mg/dL, compared to 100% as the CRP level exceeds 2 mg/dL. To our knowledge, this is the first prospective study that evaluates CRP as a predictor of bacterial co-infection in patients with bronchiolitis.
Bronchiolitis is more common in males, in those who have not been breastfed, and in those who live in crowded conditions[19]. Nagayama et al. speculated that gender-specific responses to RSV infection might account for male susceptibility[20]. The analysis of the demographic data of our patient did not show any difference between the two groups concerning age, sex, weight and height. We did note that all the males with a CRP level of more than 1.1 mg/dL showed a positive growth from the tracheal aspirate.
Rasmussen et al. reported that abnormal WBC count and differential cell count determined during the first 24 hours after admission of an unselected group of acutely ill children with infections could not be used as predictions of bacterial etiology[21]. Most children with documented viral infections had low WBC and CRP levels[22]. High WBC, CRP levels, or both were documented in children with respiratory adenoviral infections[22,23].
There were no significant differences in the WBC count between the two groups in our study. Whether evaluation of WBC pattern at a later stage of the infection might be used as prediction of bacterial etiology was not investigated in this study.
Hepatitis is commonly associated with RSV-positive bronchiolitis with reported prevalence of 49%[24]. RSV disease in ventilated children was more severe if transaminases levels were elevated[25]. In addition, Nadal et al. in 1990 reported a case where RSV was isolated from liver tissue and extrahepatic biliary atresia material[26]. We speculate that hepatitis could occur in patients with bronchiolitis regardless of its etiology. This is the reason for which we determine the level of transaminases in all infants included in our study. Eight patients (16%) had elevated SGOT and SGPT level, but the percentage of its occurrence did not differ between the 2 groups.
In RSV infection, the association between severity of illness and counter-regulatory response could explain the association of hyperglycemia with bronchiolitis[27]. Hyperglycemia is frequent in children with bronchiolitis requiring mechanical ventilation. Patients with higher sustained blood glucose level were more likely to require high-frequency oscillatory ventilation and higher peak flow[28]. In our study, hyperglycemia was not frequent in patients with bronchiolitis or those with bacterial co-infection. We must consider the limitation of this result since the timing of blood glucose analysis was not standardized.
The current management of bronchiolitis is primarily supportive, concentrating on the major effects of the condition, namely inadequate feeding, respiratory distress and apneas[29]. There are a few studies that have shown the benefit of using steroids[29]. Bronchodilators can be administered orally or by inhalation and they have been shown to provide a short period of symptomatic relief in some patients[30]. However, most of the studies have shown no change in hospitalization rates, oxygen requirement, mechanical ventilation, the duration of hospital stay, or the duration of the illness with the use of bronchodilators and steroids[30–32]. In addition, the American Academy of Pediatrics (AAP) recommends that both bronchodilators and steroids should not be used routinely in the management of bronchiolitis[1]. In our study, we did not apply special criteria for management and did not follow the guidelines.
Antibacterial medications should only be used in children with bronchiolitis who have specific indications of the coexistence of a bacterial infection[20]. When present, bacterial infection should be treated in the same manner as in the absence of bronchiolitis[20]. Children with bronchiolitis frequently receive antibacterial therapy because of fever, young age, or the concern for secondary bacterial infection. However, our data revealed that almost half of the patients with negative tracheal aspirate culture initially were unnecessarily treated with antibiotics and the majority of those with positive culture were adequately treated. Since the probability of bacterial co-infection was higher in patients with high CRP level, the level of CRP would help the clinician treat patients for whom antimicrobial therapy is necessary.
According to Christakis et al, the mean length of stay varied considerably across hospitals in America (range: 2.40 –3.90 days)[4]. In our study, the presence of bacterial co-infection increased the length of hospital stay in the first group by 2 days compared with those in the second group. This difference could be attributed to the time spent to obtain the result of tracheal aspirate culture.
Conclusion
In infants with bronchiolitis who are moderate to severe enough to require admission, a bacterial co-infection is frequent. Our data showed that a CRP level greater than 1.1 mg/dL raises the suspicion of bacterial co-infection. Thus, a tracheal aspirate should be investigated microbiologically in all hospitalized patients in order to avoid unnecessary antimicrobial therapy and to shorten the duration of the hospital stay.
References
- 1.Valois Asser EA, Asser AS, Mintz ML. Current Clinical Practice: Disorders of the Respiratory Tract: Common Challenges in Primary Care. Springer: 2006. Bronchiolitis; pp. 249–259. [Google Scholar]
- 2.Scottish Intercollegiate Guidelines Network, Bronchiolitis in children, National clinical guidelines. 2006 Nov [Google Scholar]
- 3.Hament JM, Kimpen JL, Fleer A, Wolfs TF. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol Med Microbiol. 1999;26:189–195. doi: 10.1111/j.1574-695X.1999.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 4.Christakis DA, Cowan CA, Garrison MM, Molteni R, Marcuse E, Zerr DM. Variation in inpatient diagnostic testing and management of bronchiolitis. Pediatrics. 2005;115:878–884. doi: 10.1542/peds.2004-1299. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics, Subcommittee on Management of Bronchiolitis, Pediatrics. 2006;118(4) [Google Scholar]
- 6.Bont L, Kimpen JL. Immunological mechanisms of severe respiratory syncytial virus bronchiolitis. Intensive Care Med. 2002;28:616–621. doi: 10.1007/s00134-002-1256-z. [DOI] [PubMed] [Google Scholar]
- 7.Collins PL, McIntosh K, Chanock RM. Field Virology. New York: Raven Press; 1996. Respiratory syncytial virus; pp. 1313–1351. [Google Scholar]
- 8.Weigl JA, Puppe W, Schmitt HJ. Can respiratory syncytial virus etiology be diagnosed clinically? A hospital-based case-control study in children under two years of age. European J Epidemiol. 2003;18:431–439. doi: 10.1023/a:1024213400297. [DOI] [PubMed] [Google Scholar]
- 9.Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussig LM, Lebowitz MD. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991;133:1135–1151. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- 10.Thorburn K, Van Saene H. Pulmonary bacterial co-infection in children ventilated for severe respiratory syncytial virus bronchiolitis is common. IIntensive Care Med. 2007;33:565. doi: 10.1007/s00134-006-0486-x. [DOI] [PubMed] [Google Scholar]
- 11.Duttweiler L, Nadal D, Frey B. Pulmonary and systemic bacterial coinfections in severe RSV bronchiolitis. Arch Dis Child. 2004;89:1155–1157. doi: 10.1136/adc.2004.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kneyber MCJ, Blusse van Oud-Albas H, van Vliet M, Uiterwaal CSPM, Kimpen JLL, van Vught AJ. Concurrent bacterial infection and prolonged mechanical ventilation in infants with respiratory syncytial virus lower respiratory tract infection. Intensive Care Med. 2005;131:680–685. doi: 10.1007/s00134-005-2614-4. [DOI] [PubMed] [Google Scholar]
- 13.Thorburn K, Harigopal S, Reddy V, Taylor N, Van Saene HK. High incidence of pulmonary bacterial co-infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax. 2006;61(7):611–615. doi: 10.1136/thx.2005.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanford B, Shelokov A, Ramsey M. Bacterial adherence to virus-infected cells: a cell culture model of bacterial superinfection. J Infect Dis. 1978;137:176–181. doi: 10.1093/infdis/137.2.176. [DOI] [PubMed] [Google Scholar]
- 15.Duttweiler L, Nadal D, Frey B. Pulmonary and systemic bacterial co-infections in severe RSV bronchiolitis. Arch Dis Child. 2004;89:1155–1157. doi: 10.1136/adc.2004.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa S, Rocha R, Tavares M, Bonito-Vitor A, Guedes-Vaz L. C-Reactive protein and disease severity in bronchiolitis. Rev Port Pneumol. 2009;15(1):55–65. [PubMed] [Google Scholar]
- 17.Resch B, Gusenleiner W, Muller W. Procalcitonin, Interleukine-6, C-reactive protein and leukocyte counts in infants with bronchiolitis. Pediatr Infect Dis J. 2003;22(5):475–476. doi: 10.1097/01.inf.0000066196.23839.b0. [DOI] [PubMed] [Google Scholar]
- 18.Toika P, Irjala K, Juven T, et al. Serum procalcitonin, C-reactive protein and interleukine-6 for distinguishing bacterial and viral pneumonia in children. Pediatr Infect Dis J. 2000;19(7):598–602. doi: 10.1097/00006454-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kliegman RM, Behrman RE, Jenson HB, Stanton BF. 18th ed. Saunders: 2000. Nelson Textbook of Pediatrics. [Google Scholar]
- 20.LaVia W, Marks MI, Stutman HR. Respiratory syncytial virus puzzle: clinical features, pathophysiology, treatment, and prevention. J Pediatr. 1992;121:503–510. doi: 10.1016/s0022-3476(05)81135-7. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen NH, Rasmussen LN. Predictive value of white blood cell count and differential cell count to bacterial infections in Children. Acta Pcediatr Scand. 1982;71:775–778. doi: 10.1111/j.1651-2227.1982.tb09518.x. [DOI] [PubMed] [Google Scholar]
- 22.Peltola V, Mmertsola J, Ruuskanen O. Comparison of total white blood cell count and serum C-reactive protein levels in confirmed bacterial and viral infections. J Pediatr. 2006;149:721–724. doi: 10.1016/j.jpeds.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 23.Ruuskanen O, Putto A, Sarkkinen H, Meurman O, Irjala K. C-reactive protein in respiratory virus infections. J Pediatr. 1985;107:97–100. doi: 10.1016/s0022-3476(85)80624-7. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhut M, Thorburn K. Hepatitis Associated with Severe Respiratory Syncytial Virus-positive Lower Respiratory Tract Infection. Scand J Infect Dis. 2002:931–934. doi: 10.1080/00365540110077191. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhut M, Thorburn K, Ahmed T. Transaminase levels in ventilated children with respiratory syncytial virus bronchiolitis. Intensive Care Med. 2004;30:931–934. doi: 10.1007/s00134-004-2236-2. [DOI] [PubMed] [Google Scholar]
- 26.Nadal D, Wunderli W, Merumann O, Briner J, Hirsig J. Isolation of respiratory syncytial virus from liver tissue and extrahepatic biliary atresia material. Scand J Infect Dis. 1990;22:91–93. doi: 10.3109/00365549009023125. [DOI] [PubMed] [Google Scholar]
- 27.Tasker RC, Roe MF, Bloxham DM, White DK, Ross-Russell RI, O’Donnell R. The neuroendocrine stress response and severity of acute respiratory syncytial virus bronchiolitis in infancy. Intensive Care Med. 2004;30:2257–2262. doi: 10.1007/s00134-004-2470-7. [DOI] [PubMed] [Google Scholar]
- 28.Branco RG, Tasker RC. Glycemic level in mechanically ventilated children with bronchiolitis. Pediatr Crit Care Med. 2007;8:546–550. doi: 10.1097/01.PCC.0000288712.67749.45. [DOI] [PubMed] [Google Scholar]
- 29.Garrison MM, Christakis DA, Harvey E, Cummings P, Davis RL. Systemic corticosteroids in infant bronchiolitis: a meta-analysis. Pediatrics. 2000;105(4):E44. doi: 10.1542/peds.105.4.e44. [DOI] [PubMed] [Google Scholar]
- 30.Patel H, Platt RW, Pekeles GS, et al. A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr. 2002;141:818–824. doi: 10.1067/mpd.2002.129844. [DOI] [PubMed] [Google Scholar]
- 31.Patel H, Platt R, Lozano JM, Wang EL. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2004;3:CD004878. doi: 10.1002/14651858.CD004878. [DOI] [PubMed] [Google Scholar]
- 32.Cade A, Brownlee KG, Conway SP, et al. Randomised placebo controlled trial of nebulised corticosteroids in acute respiratory syncytial viral bronchiolitis. Arch Dis Child. 2000;82:126–130. doi: 10.1136/adc.82.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]


