Abstract
Background:
Mass urinary screening is a useful tool to identify children with asymptomatic progressive renal diseases. A dipstick urinalysis screening was conducted to detect such prevalence and to set up a more effective screening program for children.
Patients and Methods:
A cross sectional study was carried out in seven nurseries and primary schools in different regions of Lebanon (Beirut, North Lebanon, and Valley of Bekaa) between February 2010 and March 2010. Eight hundred seventy asymptomatic children were enrolled in this study. First morning mid steam urine samples were obtained from students and were tested by dipstick method. Children with abnormal findings were re-tested after fifteen days.
Results:
Twenty five (2.9%) children had urinary abnormalities at the first screening; Eighteen (72%) of them still had abnormal results at the second screening. Among all the students, hematuria was the most common abnormality found with a prevalence of 1.5%, followed by nitrituria (0.45%), combined hematuria and nitrituria (0.45%) and proteinuria (0.1%). Urinary abnormalities were more common in females than in males. With respect to age, most positive results were detected at 6 years of age. Hematuria and proteinuria were mainly present in the North of Lebanon.
Conclusion:
Asymptomatic urinary abnormalities might be detected by urine screening program at school age. Further work-up should be offered to define the exact etiology of any abnormal finding and to determine whether early detection of renal disorders in childhood will lead to effective interventions and reduction in the number of individuals who develop end-stage renal disease.
Keywords: Dipstick urine analysis, renal failure in school aged children, urine analysis screening
Introduction
Urine analysis, a simple and inexpensive test, is the cornerstone in the evaluation of the kidney function. Serious renal diseases may be present without any symptoms. Proteinuria as well as hematuria may be the only early signs of renal disease (membranous nephropathy, membranoproliferative glomerulonephritis, post infectious glomerulonephritis, IgA nephropathy and others)[1]. Also, the presence of detectable nitrites in urine has been used to diagnose urinary tract infection. Urinary tract infection is very common in children with severe consequences on the kidney function leading to chronic kidney disease and hypertension if left untreated[2,3].
The basic dipstick method is the most rapid screening procedure that could be helpful in the early detection of renal or urinary tract diseases among apparently healthy or asymptomatic subjects in the hope of preventing and retarding progression to chronic renal failure[4,5].
Worldwide, screening for chronic kidney disease (CKD) is controversial. The primary basis for this controversy is the uncertainty whether early detection of renal disorders in childhood will lead to effective interventions and reduction in the number of individuals who develop end-stage renal disease (ESRD). There appears to be a clear consensus among Japanese, Taiwanese, and Korean investigators that the screening programs currently in place in these countries have led to early detection and effective intervention[6,7]. This opinion is not shared by investigators from North America and Europe, but their estimates of the prevalence of CKD in children predate the emergence of obesity and childhood hypertension. This may well have led to an underestimation of the prevalence of CKD in children[6].
Epidemiological data on the incidence and prevalence of chronic kidney disease in the pediatric population is currently limited, imprecise, and flawed by methodological differences between various data resources. It is estimated to be approximately 18 per 1 million[1].
There are considerable differences in the pattern of renal disease around the world which arise from racial variation in the susceptibility to renal disease compounded with socioeconomic factors[8]. Congenital causes are responsible for the greatest percentage of cases of chronic kidney disease seen in children. Although this is the most common reported etiology from developed countries where chronic kidney disease is diagnosed in its early stages, infectious or acquired causes predominate in developing countries, where patients are referred in the later stages of kidney disease[9].
In Lebanon, which is a developing country, little is known about the prevalence of abnormal urinary findings in asymptomatic children. A dipstick urinalysis screening was conducted to detect such prevalence among school children in different regions of Lebanon and to set up a more effective screening program for children in developing countries.
Patients and Methods
This cross sectional study, approved by the Institutional Review Board Committee of Makassed General Hospital in Beirut, was carried out between February 2010 and April 2010 on 1150 children from seven nurseries and primary schools in different regions of Lebanon.
The parents were instructed how to obtain a clean mid stream urine specimen from their children. Vessels containing urine were brought by parents to the school in the early morning. Out of 1150 children; 251 children did not deliver urine samples, 29 children were excluded for having symptoms of urinary tract infections (dysuria, fever, vomiting, etc.) or taking antibiotics, leaving 870 children for screening (Figure 1).
Fig. 1.
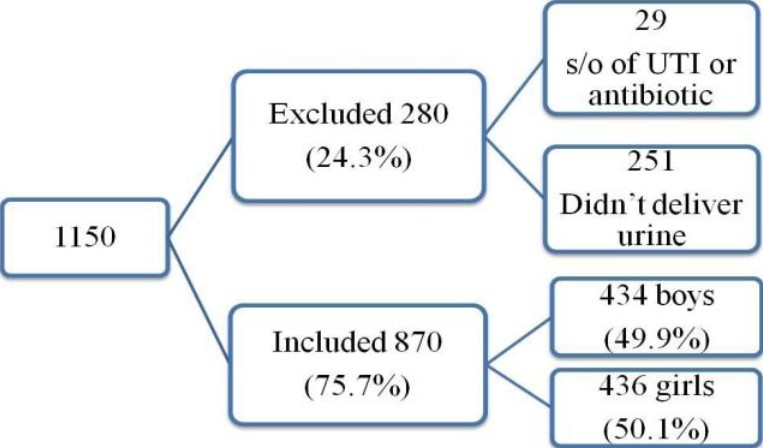
Number of children collected
In this screening program, the dipstick adopted consists of 10 reagents: pH, specific gravity, protein, blood, glucose, leucocytes, nitrites, urobilinogen, bilirubin and ketones (urine quick test; Combur-10-Test™,Roche, Mannheim, Germany).
Dipstick test was performed by a pediatrician. Each reagent strip, impregnated into a chemical, reacts with the substance present in urine and quickly changes color (60-120 seconds). The color of the strip was compared to the color chart present in the bottle label.
Urinalysis was considered abnormal if the following findings were detected:
>5-10 RBC/μl; hematuria (Green dots on yellow test: intact erythrocytes; Uniform green coloration of test: free hemoglobin or hemolysed erythrocytes);
1+ or greater proteinuria (trace, 1+, 2+, 3+, 4+ correspond to 10 mg/dl, 30 mg/dl, 100 mg/dl, 300 mg/dl, 1000 mg/dl respectively);
1+ or greater glycosuria (1+, 2+, 3+ corresponds to 100 mg/dl, 300 mg/dl, 1000 mg/dl respectively);
Positive nitrites; and
>25 WBC/μl; leukocyturia.
All abnormal urinary findings were controlled with the same dipstick brand (used in the first analysis) after 15 days as second screening. This improved the specificity of the test.
Statistical Analysis
Statistical analysis was done by using statistical package of social science SPSS version 16. Qualitative data were expressed in the form of numbers and percentages. Differences between groups were evaluated by chi-square test. Fisher's exact test was used for small samples.
Results
Of the remaining 870 children, 57.6% were from Beirut, 21.4% were from North Lebanon, and 21.0% were from Valley of Bekaa. 434 were boys (49.9%) and 436 (51%) were girls. All boys were circumcised. The ages ranged from 5 to 8 years with mean age of 6.58± 0.89 SD in males and 6.79± 0.98 SD in females (Figure 2).
Fig. 2.
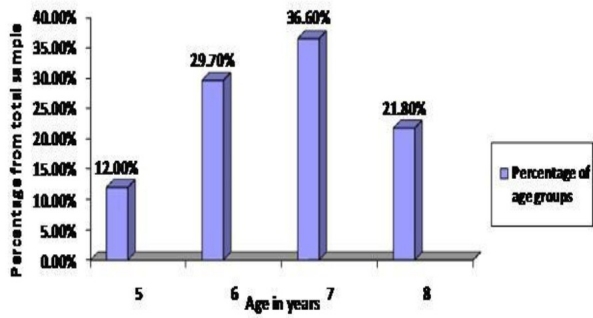
Distribution of children by age
Urinary abnormalities were detected in 2.9% of children at the first screening; 72% of them had urinary abnormalities at the second screening (P=0.016) (Table 1).
Table 1.
Abnormal urine results by dipstick
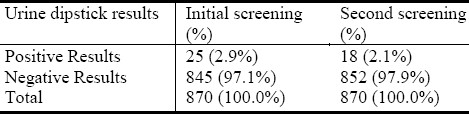
Some subjects had more than one abnormality detected. Microscopic hematuria had the highest prevalence detected in 2.0% of children at the first screening and 1.5% at the second screening; Glycosuria and leucocyturia were not present in any subject.
Hematuria was divided into either hemoglobinuria which was found in 7 children (41.2% of hematuria cases) or intact erythrocytes found in 10 children (58.8% of hematuria cases). Among children with hematuria, 11(1.3%) had isolated hematuria (IH) and 6 (0.7%) had combined hematuria and nitrituria at the first sampling compared to 9 (1.0%) and 4 (0.45%) for isolated hematuria and combined hematuria and nitrituria (CHN) respectively at the second screening (Figure 3).
Fig. 3.
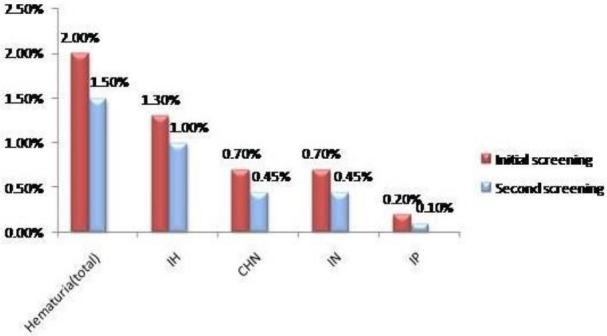
Prevalence of abnormal urinary findings among the school children. IH: Isolated hematuria, IN: Isolated nitrituria, IP: Isolated proteinuria, CHN: Combined hematuria and nitrituria.
The other abnormalities found were isolated nitrituria IN, 6 (0.7%) at the first screening and 4 (0.45%) at the second screening); isolated proteinuria IP, 2 (0.2%) at the first screening and 1 (0.1%) at the second screening (Figure 3).
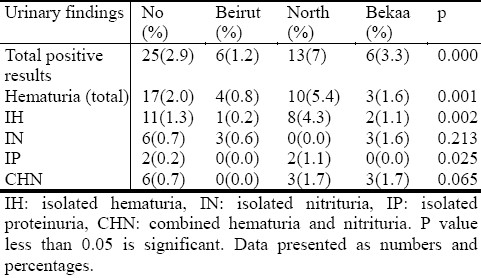
Most abnormal results were found in children from North Lebanon, 13 (7%) (P=0.000) except for nitrituria which had a higher percentage in Valley of Bekaa, although P value was not significant (Table 2). These results were comparable to the results of the second screening.
Table 2.
Comparison of results by region
When stratified by sex, abnormal urine findings were more common among girls than boys (18 girls versus 7 boys) with P value of 0.021; isolated nitrituria was more prevalent in females (5 versus 1) with P value of 0.110 (Figure 4). These results were comparable to the second screening except for proteinuria which was positive only in one boy.
Fig. 4.
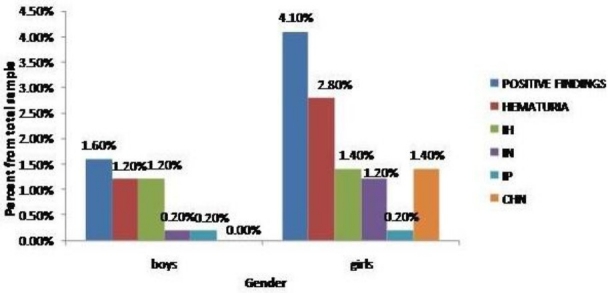
Comparison of results by sex. IH: Isolated hematuria, IN: Isolated nitrituria, IP: Isolated proteinuria, CHN: Combined hematuria and nitrituria.
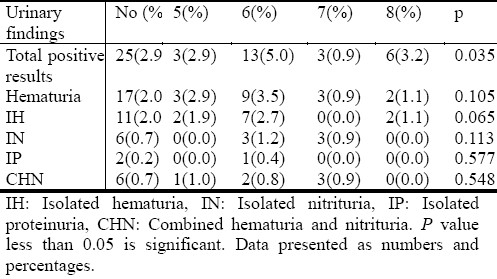
When compared by age, approximately most abnormalities were detected at 6 years of age (Table 3).
Table 3.
Comparison of results by age
Discussion
Many renal and urinary tract disorders may be asymptomatic for a long period of time. Routine urine screening programs are recommended as a basic fundamental step in early identification of renal damage. This has proved to be extremely important in reducing the growing burden of chronic kidney disease (CKD) in both developed and developing countries. To our knowledge, this is the first report on dipstick urine analysis screening among asymptomatic children in Lebanon.
Our findings of hematuria, proteinuria and nitrituria in school children were compared to other studies. The finding of urinary abnormalities was in nearly 2.1% of the studied group which was similar to the 2.5% and the 2.3% reported in Northern Iran and Malaysia respectively[10,11]; higher than the 0.6% and the 0.72% reported in Tokyo and Egypt respectively[12,13]; lower than the 7.2% and the 9.6% reported in Bolivian and Nigerian studies respectively[14,15].
Hematuria was the most common abnormality found in our studied group. This was contrasted to other studies as in Egypt and Nigeria where proteinuria was the most common positive finding[13,15]. The development of asymptomatic microscopic hematuria is relatively common in children. Its prevalence in school aged children has been estimated at 0.5% to 2.0% depending on the population screened. This was comparable to our results that showed a prevalence of 1.5% at the second screening (1.0% for isolated hematuria IH; 0.45% for combined hematuria and nitrituria CHN). Nigerian and Xiamen cities reported a prevalence of 1.5% and 1.21% respectively[15,16]. On the other hand, hematuria had a lower prevalence in Malaysia, Egypt and Shanghai (0.21%, 0.36% and 0.46% respectively)[11,13,17]. The finding of CHN was not reported previously.
The clinical significance of asymptomatic hematuria remains unclear and the merit of such an evaluation has not been confirmed. The child with persistent asymptomatic isolated microscopic hematuria of longer than 2 week duration poses a dilemma in regard to the degree of further diagnostic testing that should be performed. Based on medical recommendations, a child with persistent microscopic hematuria should be followed closely every 3 months and for one year[1].
Bergstein et al evaluated 342 children referred to their nephrology clinic for asymptomatic isolated microscopic hematuria. Among these patients, they found no diagnosis in 274; hypercalciuria was the most common finding (16%); followed by membrano-proliferative glomerulonephritis (MPGN) and post-streptococcal glomerulonephritis (PSGN)[18]. Other authors like Vehaskari et al performed biopsy samples from 22 children with microscopic hematuria having no family history of kidney disease and a negative evaluation for causation. Two children had IgA nephropathy and one child had Alport syndrome. The remaining biopsies were normal and showed non-specific focal tubular changes[19]. In a screening done in Japan, they found 6 cases of IgA nephropathy and 7 cases of minor glomerular abnormalities among 220 children with asymptomatic hematuria[20].
These studies showed that microscopic asymptomatic hematuria might be benign but it can also be an important sign of underlying disease. However, limitations of these studies were the absence of long term follow-up and thus, the frequency of development of complications and occult kidney disease was not known. Furthermore, it seems that in patients with microscopic hematuria due to occult glomerular disorders, progression to clinically significant disease will be accompanied by the development of hypertension with or without proteinuria or gross hematuria. Thus, long term follow-up in children with microscopic hematuria is mandatory[21].
Another positive finding in our study was isolated nitrituria (IN) that accounted for 0.45% of the studied group at the second screening compared to the 1.5% in the Nigerian screening[15]. Isolated nitrituria was not reported in other studies. Both the findings of IN and CHN could rise the suspicion of urinary tract infections although subjects were asymptomatic. Urinary nitrites are produced by bacterial breakdown of dietary nitrates. The reliability of the nitrite test for urinary tract infections has been investigated by several workers, most of whom concluded that false positive results were rare and that the test had a higher specificity for urinary tract infections[22]. False positive results in general are explained by vaginal contamination or exposure of the dipstick to air for 1-2 weeks. Specificity of urinary nitrite test is about 98% (Rushton and long 2002). The sensitivity of the test has been reported to be low (21-59%) by some authors[23] and high (80-93%) by others[25]. One report in 1987 showed that urinary nitrite test detected 83% of asymptomatic urinary tract infections[24].
Proteinuria can be a major cause of underlying kidney disease or a transient finding in normal children. In our study, first morning urine sample helped in excluding orthostatic proteinuria as a cause of isolated proteinuria in children The dipstick is sensitive to albumin mainly whereas quantitative methods detect all kidney proteins. Proteinuria is a strong independent and risk factor of end stage renal disease ESRD. Therefore, asymptomatic proteinuria warrants further work up to detect and even prevent ESRD[25].
In this study, the proportion of students with proteinuria was 0.1% at the second screening. In Northern Iran and Nigeria, they reported that the prevalence of proteinuria was 1.6% and 3.5% respectively[10,15]. These reports were higher than those of Tokyo and Egypt with a prevalence 0.08% and 0.12% respectively[12,13].
In a screening done in Korea, renal biopsy was performed in 63.1% of children with IH, 10.5% of IP and 69.9% of combined hematuria and proteinuria. IgA nephropathy was the most common finding, followed by mesangial proliferative glomerulonephritis, Henoch-Shönlein nephritis, membranoproliferative glomerulonephritis and then lupus nephritis[26].
Most positive findings were more common in girls than in boys in our screening.This was similar to the results of the Nigerian study[15] but contrasted with the results of the Egyptian study which showed that age and sex had no impact in the results of the screening done[13]. The finding of higher nitrites among females could be a reflection of higher rates of asymptomatic bacteriuria in the girls of the studied group,since nitrites are generated in urine by bacteria. Studies among school-age children have shown that bacteriuria is 30 times the prevalence among boys, attributed to the fact that girls have short urethra which predisposes them to ascending bacterial infection[15,27].
Although the number of children and the number of females at age of 6 years were not higher compared to the other groups, we reported most positive results at this age group. Most abnormal urinary findings were found in North of Lebanon especially in the regions with poor socioeconomic status. This rises the question whether a correlation between chronic renal diseases and low social class could exist. In our screening, we did not perform other investigations as done by other groups. Most common causes of urinary abnormalities and the prevalence of kidney disease in Lebanon are still not yet known. If these causes are similar to those of previous studies done worldwide, this implies that early detection of these abnormalities could be of importance in improving the health of children.
A number of recommendations regarding urinary screening as part of well child care have been published by the American Academy of Pediatrics (AAP) over the past 20 year. In 1977 and 1992, the AAP recommended a screening urinalysis at 4 periods during a child's life. In 2000, the pediatric health care guidelines were revised to recommend a screening urinalysis at 5 years of age and during adolescence. In 2007, the screening urinalysis was removed altogether[28]. However, urinalysis screening is still mandatory in many countries of Asia as in Japan, Taiwan and Korea. Therefore, it's clear that there's no global consensus as to whether screening for CKD should be undertaken in children. In Japan, the number of children and adolescents reaching ESRD decreased from 166 in 1984 to 86 in 2002. Also, Murakami et al compared the situation in Japan and the United States. The number of children starting ESRD therapy is 6 per million in Japan compared with 30 per million in the United States[6]. It is particularly important that the prevalence data for CKD in children worldwide should be updated and additional evidence should be obtained on whether effective interventions will reduce the number of children with ESRD.
From the above statement, it is evident that there's difference in data between Asia and United States. Lebanon is one of the developing countries of Asia with poor socioeconomic status and poor education in its periphery where routine visits to pediatricians are infrequent. This reinforces the necessity of screening children at school entry by dipstick urine analysis.
Conclusion
This study helped to assess the prevalence of urinary abnormalities in school-aged children of Lebanon, for the first time. Hematuria was found to be the most prevalent abnormality.
The controversies over the value of urine screening in young children raise the question of whether renal diseases are more prevalent in Asian children and therefore it is of great importance to be identified early in the course of disease, and whether the last AAP guideline would be modified in the near future such as screening will be limited to a selected number of children.
By the time we have answers to these questions, we suggest that routine urinalysis should be part of screening of children at the school entry in Lebanon, and that further follow-up should be offered to determine the exact etiology of any abnormal finding.
Acknowledgment
The authors would like to thank Dr. Nahida Rifai, Fouad Ziade, PhD., and Mme Dima Abou Daher for their valuable contribution to this work.
References
- 1.Philadelphia: Saunders; 2004. Kliegman: Nelson Textbook of Pediatrics. [Google Scholar]
- 2.Kumar P, Clark M. Urinary tract infection. In: Kumar P, Clark M, editors. Clinical medicine. 4th edition. London: Harcourt Brace and Company; 1998. p. 548. [Google Scholar]
- 3.Gorelick M, Shaw KN. Screening tests for urinary tract infection in Children: A meta-analysis. Pediatrics. 1999;104:54. doi: 10.1542/peds.104.5.e54. [DOI] [PubMed] [Google Scholar]
- 4.Devillé W, Yzermans J, Van Duijn N, Bezemer PD, Van der Windt D, Bouter LM. The urine dipstick test useful to rule out infections. A meta-analysis of the accuracy. BMC Urology. 2004;4:4. doi: 10.1186/1471-2490-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shumann GB, Nancy FG. Usefulness of macroscopic urinalysis as a screening procedure – a preliminary report. Am J Clin Path. 1979;71:452–456. doi: 10.1093/ajcp/71.4.452. [DOI] [PubMed] [Google Scholar]
- 6.Hogg RJ. Screening for CKD in children: A Global Controversy. Clin J Am Soc Nephrol. 2009;4:509–515. doi: 10.2215/CJN.01210308. [DOI] [PubMed] [Google Scholar]
- 7.Kitagawa T. Lessons learned from the Japanese nephritis screening study. Pediatr Nephrol. 1988;2:256–263. doi: 10.1007/BF00862602. [DOI] [PubMed] [Google Scholar]
- 8.Avner E, Harmon W, Niaudet P. Wilkins, Philadelphia: Lippincott, Williams & Amp; 2004. Textbook of Pediatric Nephrology. [Google Scholar]
- 9.Bradley A, Chadha W, Chadha V. Chronic kidney disease in children: the global perspective. Pediatr Nephrol. 2007;22(12):1999–2009. doi: 10.1007/s00467-006-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badeli H, Heidarzadeh A, Ahmadian M. Prevalence of Hematuria and Proteinuria in Healthy 4 to 6 Year Old Children in Daycare Centers of Rasht (Northern Iran) Iran J Pediatr. 2009;19(2):169–172. [Google Scholar]
- 11.Zainal D, Baba A, Mustaffa BE. Screening proteinuria and hematuria in Malaysian children. Southeast Asian J Trop Med Public Health. 1995;26(4):785–788. [PubMed] [Google Scholar]
- 12.Murakami M, Yamamoto H, Ueda Y, Murakami K, Yamauchi K. Urinary screening of elementary and junior high-school children over a 13 year period in Tokyo. Pediatr Nephrol. 1991;5:50–53. doi: 10.1007/BF00852844. [DOI] [PubMed] [Google Scholar]
- 13.Bakr A, Sarhan A, Hammad A, et al. Asymptomatic urinary abnormalities among primary school children in Egypt. World J Pediatr. 2007;3(3):214–217. [Google Scholar]
- 14.Plata R, Silva C, Yahuita J, Perez L, Shieppati A, Remuzzi G. The first clinical and epidemiological programme on renal disease in Bolivia: A model for prevention and early diagnosis of renal diseases in the developing countries. Nephrol Dial Transplant. 1998;13(12):3034–3036. doi: 10.1093/ndt/13.12.3034. [DOI] [PubMed] [Google Scholar]
- 15.Akor F, Okolo S, Agaba E, Okolo A. Urine examination findings in apparently healthy new school entrants in Jos, Nigeria. SA J Child Health. 2009;3(2):60–63. [Google Scholar]
- 16.Tong S, Gui-lan P, Hui-fen Z, et al. Urine routine screening of 34455 school children in Xiamen city. J Child Health Care. 2009;1 [Google Scholar]
- 17.Rao J, Zhou L, Shen Q, et al. School urinalysis screening in Shanghai. World J Pediatr. 2006;3:195–198. [Google Scholar]
- 18.Stapleton F. Asymptomatic microscopic hematuria. Arch Pediatr Adolesc Med. 2005;159:398–399. doi: 10.1001/archpedi.159.4.398. [DOI] [PubMed] [Google Scholar]
- 19.Vehaskari VM, Rapola J, Koskimies O, Savilahti E, Vilska J, Hallman N. Microscopic hematuria in schoolchildren: epidemiology and clinicopathologic evaluation. J Pediatr. 1979;95:676–684. doi: 10.1016/s0022-3476(79)80710-6. [DOI] [PubMed] [Google Scholar]
- 20.Murakami M, Hayakawa M, Yanagihara T, Hukunaga Y. Proteinuria screening for children. Kidney Int. 2005;68:S23–27. doi: 10.1111/j.1523-1755.2005.09406.x. [DOI] [PubMed] [Google Scholar]
- 21.Bergstein J, Leiser J, Andreoli S. The Clinical Significance of Asymptomatic Gross and Microscopic Hematuria in Children. Arch Pediatr Adolesc Med. 2005;159:353–355. doi: 10.1001/archpedi.159.4.353. [DOI] [PubMed] [Google Scholar]
- 22.Powell H, McCredie D, Artchie M. Urinary nitrite in symptomatic and asymptomatic urinary infection. Arch Dis Child. 1987;62:138–140. doi: 10.1136/adc.62.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skelton IJ, Hogan M, Stokes B, Hurst JA. Urinary tract infections in childhood. The place of nitrite test. Med J Aust. 1977;1:882–886. doi: 10.5694/j.1326-5377.1977.tb131211.x. [DOI] [PubMed] [Google Scholar]
- 24.Sheifele DW, Smith AL. Home-testing for recurrent bacteriuria using nitrite strips. Am J Dis Child. 1978;132:46–48. doi: 10.1001/archpedi.1978.02120260048013. [DOI] [PubMed] [Google Scholar]
- 25.Hanif R, Ally S, Jalal-ud-din , Khan K. Effectiveness of routine urinalysis of patients attending rural health care centers in abbottabad. J Ayub Med Coll Abbottabad. 2006;18(3) [PubMed] [Google Scholar]
- 26.Cho BS, Kim SD. School urinalysis in Korea. Pediatr Nephrol. 2007;12(Suppl 3):S3–S7. doi: 10.1111/j.1440-1797.2007.00873.x. [DOI] [PubMed] [Google Scholar]
- 27.Travis LB, Brouhard BH. nfections of the urinary tract. In: Rudolph AM, editor. Rudolph's Paediatrics. 12th ed. Stamford: Appelton and Lange; 1996. pp. 1388–1392. [Google Scholar]
- 28.Sekhar D, Wang L, Hollenbeak C, Widome M, Paul I. A cost-effectiveness Analysis of Screening Urine Dipsticks in Well-Child Care. Pediatrics. 2010;125:660–663. doi: 10.1542/peds.2009-1980. [DOI] [PubMed] [Google Scholar]


