Abstract
Background:
Vaginitis, is an infectious inflammation of the vaginal mucosa, which sometimes involves the vulva. The balance of the vaginal flora is maintained by the Lactobacilli and its protective and probiotic role in treating and preventing vaginal infection by producing antagonizing compounds which are regarded as safe for humans.
Aim:
The aim of this study was to evaluate the protective role of Lactobacilli against common bacterial opportunistic pathogens in vaginitis and study the effects of some antibiotics on Lactobacilli isolates.
Materials and Methods:
In this study (110) vaginal swabs were obtained from women suffering from vaginitis who admitted to Babylon Hospital of Maternity and Paediatrics in Babylon province, Iraq. The study involved the role of intrauterine device among married women with vaginitis and also involved isolation of opportunistic bacterial isolates among pregnant and non pregnant women. This study also involved studying probiotic role of Lactobacilli by production of some defense factors like hydrogen peroxide, bacteriocin, and lactic acid.
Results:
Results revealed that a total of 130 bacterial isolates were obtained. Intrauterine device was a predisposing factor for vaginitis. The most common opportunistic bacterial isolates were Staphylococcus aureus, Escherichia coli, Streptococcus agalactiae, and Klebsiella pneumoniae. All Lactobacilli were hydrogen peroxide producers while some isolates were bacteriocin producers that inhibited some of opportunistic pathogens (S. aureus, E. coli). Lactobacilli were sensitive to erythromycin while 93.3% of them were resistant to ciprofloxacin and (40%, 53.3%) of them were resistant to amoxicillin and gentamycin respectively. Results revealed that there was an inverse relationship between Lactobacilli presence and organisms causing vaginitis. This may be attributed to the production of defense factors by Lactobacilli.
Conclusion:
The types of antibiotics used to treat vaginitis must be very selective in order not to kill the beneficial bacteria (Lactobacilli) that help in preservation of vaginal health and ecosystem as being one of the probiotic bacteria.
Keywords: Probiotic bacteria, lactobacilli, vaginitis
Introduction
Vaginitis, is an infectious or non infectious inflammation of the vaginal mucosa, which sometimes involves the vulva (external genitals). This inflammation often causes itching, burning, irritation, discharge and discomfort. It is one of the most common reasons for women to seek medical care[1]. Vaginitis falls into many forms: irritant, hormonal, foreign body, sexual transmitted diseases and infective. All types can cause great discomfort to the infected woman[2].
Infective vaginitis may be caused by bacteria, fungi, parasites, and viruses. The bacteriological agents associated with vaginitis include a wide variety of bacteria that are dominated by overgrowth and marked by deficiency of hydrogen peroxide- producing Lactobacilli. The most bacterial agents causing vaginitis include Staphylococcus aureus, Echerichia coli, Group B streptococci (GBS), Listeria monocytogenes, Klebsiella pneumoniae, Acinetobacter spp., Neisseria gonorrhoea, and the bacteria that cause bacterial vaginosis in addition to Chlamydia[3].
The most important factor is to maintain the balance of the vaginal flora by the Lactobacilli. The protective and probiotic role of Lactobacilli in treating and preventing vaginal infection is done by their production of antagonizing compounds such as hydrogen peroxide, lactic acid, and bacteriocin[4]. These properties of Lactobacilli was the reason for using of this bacteria as probiotic in addition to being a member of normal flora and are generally regarded as safe for use in humans. For treatment of vaginitis, the normal flora disturbances must be restored by avoiding the use of wrong antibiotic that disturbs the normal vaginal flora[5].
The aim of this study was to evaluate the protective role of Lactobacilli against common bacterial opportunistic pathogens isolated from women suffering from vaginitis in Babylon province, Iraq and study the effects of some antibiotics on the Lactobacilli isolates.
Materials and Methods
Patients and Specimen Collection
One hundred and ten vaginal swabs taken from women suffering from vaginitis, who admitted to Babylon hospital for maternity and pediatrics in Babylon province, Iraq during the period from November 2008 to April 2009. Vaginal swabs were also taken from private clinics. One hundred swabs were taken from married women, of these, 20 swabs from pregnant and 80 were from non-pregnant women. In addition, 70 of married women were with intrauterine device (IUD) and 30 of them were without. The study also included ten swabs from unmarried women.
Swabs for culture were placed in tubes containing normal saline to maintain the swabs moist until being taken to the laboratory. Each specimen, except specimen for isolation of Lactobacilli, was immediately inoculated on blood agar plates, nutrients agar, chocolate agar and MacConkey's, plates. All plates were incubated aerobically at 37 °C for 24-48 hours. For isolation of Lactobacilli, specimen was inoculated on deMan Rogosa Sharpe (MRS) broth and agar and incubated anaerobically using Gas Pack jar.
Isolation and Identification of Bacterial Isolates
The isolation and identification of lactobacilli and bacterial opportunistic pathogens in patient's vaginal swabs were performed through colony morphology of bacterial isolates, microscopic gram stain investigation, and biochemical tests[6]. Isolates identification was also confirmed using API 20 test system (Biomerieux/France).
Detection of Defense Factors of Lactobacilli
Hydrogen peroxide (H2O2) production test: Lactobacilli were streaked onto a 20 ml MRS agar plate containing 5 mg of tetrmethyl-benzidine (TMB) and (0.20) mg of horse radish peroxidase. Peroxidase generates O2 from any H2O2 produced by the Lactobacilli and the TMB stains the colonies blue in the presence of O2 after 48 hours of anaerobic incubation. Thus colonies that produce H2O2 on MRS agar appear dark blue, while non-producers are colorless[7]. The media were used within 48 hours after preparation.
Bacteriocin production test: All Lactobacilli isolates were examined for their ability to produce bacteriocin by cup assay method[8]. Clinical isolates of S. aureus and E. coli (obtained from Microbiology Lab, College of medicine, Kufa University, Iraq) were used as indicator isolates against the bacteriocin-producing isolates of Lactobacilli. Brain heart infusion medium supplemented with 5% glycerol was used for this purpose.
Lactic Acid Production Test: pH of the buffer (0.1 m of Tris-HCl) was measured and adjusted to 7. A loopfull of Lactobacilli isolates were inoculated and incubated for 24-48 hours anaerobically. After 24 hours, pH was measured. Any decrease in pH reading was attributed to production of lactic acid. After 48 hours, pH was also measured and the increase in pH was attributed to an entrance of the organism into another metabolic pathways[9].
Antimicrobial Susceptibility Testing
The susceptibility of bacterial isolates of Lactobacilli to antimicrobial agents was determined using disk diffusion method[10] and interpreted according to Clinical and Laboratory Standards Institute (CLSI) documents[11]. The following antimicrobial agents were obtained (from Oxoid, U.K.) as standard reference disks as known potency for laboratory use: Amoxicillin (Amx) 10 μg, Vancomycin (VA) 30 μg, Gentamycin (GN) 10 μg, Erythromycin (ER ), 15 μg, and Ciprofloxacin (CIP) 5 μg.
All these tests were performed on plates of Muller-Hinton agar (Oxoid, UK). A 0.5 MacFarland suspension (provided by Biomérieux, France) of tested bacterial isolates was applied to the plates, which were dried in an incubator at 35 °C for 15 minutes. Antimicrobial disks were placed on the agar with sterile forceps. The agar plates were incubated inverted at 35 °C for 18 hours. Results were recorded by measuring the inhibition zone (in millimeters) and interpreted according to CLSI documents[11].
In this method, 50 microliters of the pellet of an overnight culture was diluted in MRS broth to obtain 107 CFU/ml. Muller-Hinton agar plates containing 5% sheep blood were flooded with this suspension in order to give confluent colonies, air dried for 15 min, and the discs impregnated with antibiotics were positioned on the plates. After 36 hours. of anaerobic incubation at 37 °C, the diameters of the bacteria–free zones were measured.
Statistics Study
Mean value and percentages were used for prevalence of lactobacilli distributions and for determination of the isolation rates of opportunistic bacterial isolates from pregnant and non-pregnant women and in women with and without IUD.
Results
Role of Intrauterine Device as Predisposing Factor in Vaginitis
Results found that the incidence of vaginitis was higher among married women (92.3%) than unmarried women (7.7%) and the incidence of vaginitis among married women that used IUD (71%) was higher than married women without IUD (29%).
Out of 110 vaginal swabs (obtained from women suffering from vaginitis), 105 samples gave positive bacterial culture whereas five samples showed no bacterial growth. Out of the positive cultures, a total of 130 bacterial isolates were obtained,10 from unmarried women and 120 from married women. Of these 120 bacterial isolates, 15 isolates were belonged to Lactobacilli and 105 isolates were of opportunistic pathogens (Table 1).
Table 1.
Numbers of bacterial isolates among pregnant and non pregnant women with vaginitis

From the results, it was shown that gram positive bacteria constituted (55.2%) of total bacterial isolates compared to gram negative bacteria which constituted (44.8%).
Opportunistic Bacterial Isolates in Women with Vaginitis
Thirty nine cases of vaginitis were caused by single bacterial species in non pregnant and sixteen in pregnant women, as shown in Table 2 and 3, respectively. S. aureus constituted (8/39), E. coli (5/39), followed by L. monocytogenes (7/39), group B streptococci- GBS (5/39), Pseudomonas aeruginosa (4/39), K. pneumoniae (2/39) while other bacterial isolates constituted (5/39). For pregnant women, different percentages of bacterial isolates were recorded, as shown in Table 3.
Table 2.
Types of opportunistic bacterial isolates from non-pregnant women with vaginitis
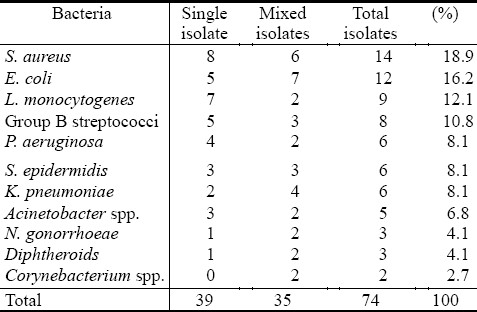
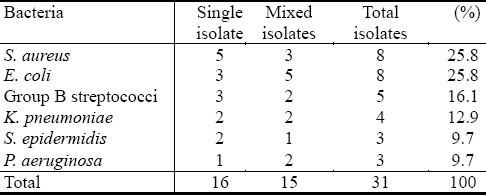
Table 3.
Types of opportunistic bacterial isolates from pregnant women with vaginitis
Isolation of Lactobacilli
Results found that fifteen isolates of Lactobacilli were isolated anaerobically from all patients with vaginitis, 11 isolates (73.3%) from non pregnant and four (26.7%) from pregnant women.
Defense Factors of Lactobacilli as Probiotic Bacteria
The ability of Lactobacilli to produce hydrogen peroxide (H2O2) was investigated and the result showed that these isolates did not have the ability to produce H2O2. Bacteriocin production activity of Lactobacilli was also studied and it was found that two isolates (13.3%) of lactobacilli inhibited the growth of E. coli while two other isolates of lactobacilli (13.3%) inhibited the growth of S. aureus. It was found that the largest zone of inhibitions were 21 mm and 12 mm against E. coli and a S. aureus respectively (Table 4).
Table 4.
Bacteriocin production by lactobacilli isolates

Regarding lactic acid production by Lactobacilli, Results of Figure 1, showed that changes in pH measurement of the media containing lactobacilli (after 24 hours of incubation) were attributed to lactic acid production. The results revealed that the pH of the broth was decreased after 24 hr of anaerobic incubation of Lactobacilli and then neutral pH (7) was returned after 48 hours. of incubation. This low pH is inhibitory for many potentially pathogenic organisms that colonized the vagina.
Fig. 1.
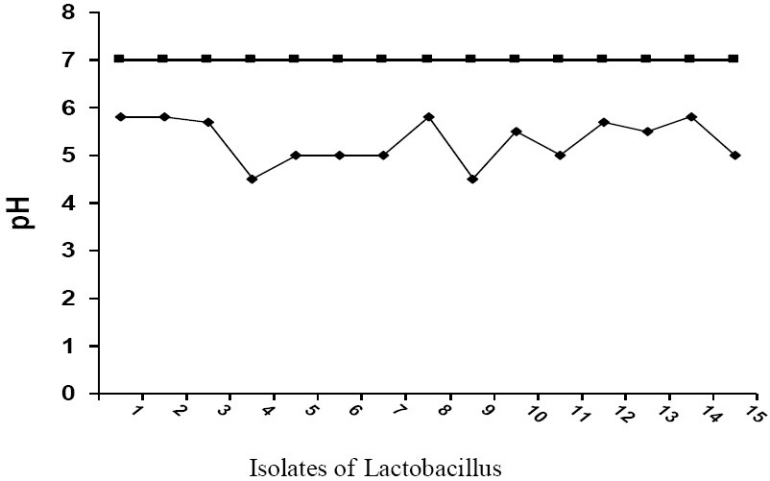
pH changes in broth after anaerobic cultivation of Lactobacilli at 37°C for 24 hours.
Effects of Antibiotics on Lactobacilli
Some antibiotics were used to show their effect on Lactobacilli isolates. It has been found that 93.3% of Lactobacilli isolates were resistant to vancomycin and ciprofloxacin, while the resistance for gentamycin was 53.3%. Some isolates were resistant to amoxicillin 40%, while most of these isolates were susceptible with higher degree to erythromycin 93.4% (Figure 2).
Fig. 2.
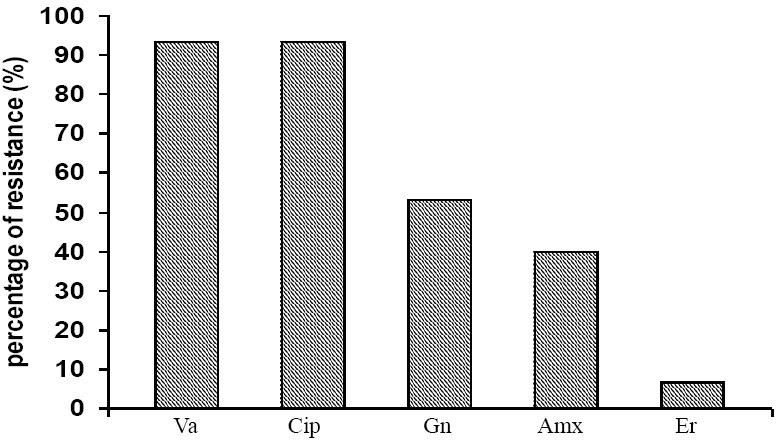
Antibiotics resistance of Lactobacilli isolates. Va: Vancomycin, Cip: Ciprofloxacin, Gn: Gentamycin, Amx: Amoxicillin, Er: Erythromycin.
Relationship Between Lactobacilli And Other Organisms Associated With Vaginitis
Results revealed that there was an inverse relationship between Lactobacilli presence and the organism that caused vaginitis. These results are attributed to the production of defense factors by Lactobacilli.
Discussion
The role of intrauterine device as predisposing factor in vaginitis was investigated. Results found that the incidence of vaginitis was higher among married women than unmarried women and the incidence of vaginitis among married women that with IUD was higher than married women without IUD. These findings were in agreement with that of[12], who found that about (87%) of women reported vaginitis or reproductive tract infection (RTI) due to IUD. It was also found that there were significant differences in alteration of vaginal discharge and presence of RTI-related symptoms among women used IUD in comparison to women not used IUD[12]. A homogenous, malodorous thin, grey discharge, which resembled non specific vaginitis occurred in high percentages of women with intrauterine contraceptive device about four times more common than those women without it. Also the normal lactobacilli dominated microbial vaginal flora was replaced by many other opportunistic pathogens and certain anaerobic species among women used IUD with discharge[13].
Out of 110 vaginal swabs (obtained from women suffering from vaginitis) five samples showed no bacterial growth. These negative bacterial cultures may be attributed to consumption of antibiotics by the patients, or the presence of another type of causative agents that might need a special techniques for their detection such as viruses, Chlamydia, and other agents.
Results of types and percentages of opportunistic bacterial isolates recovered from non-pregnant patients in this study were correlated with the results obtained by[14], whereas, the results of bacteria isolated from pregnant women were similar to those obtained by other investigators[15].
Fourteen isolates (18.9%) of S. aureus were isolated in this study from non pregnant compared with eight (25.8%) isolates from pregnant women. These findings were in agreement with that of Mumtoz et al.[16] who found that 24% of the patients with aerobic vaginitis were infected with S. aureus followed by enteric gram negative bacilli and other gram positive cocci. The percentages of Staphylococcus species (S. aureus, S. epidermidis, S. saprophyticus) in patients with vaginitis may reach to 62%[6].
Hillier et al.[17] studied the vaginal flora of some women and they found that the organisms commonly isolated in women with normal smears were lactobacilli, coagulase negative staphylococci, S. aureus, diphtheroids, Candida and GBS.
The isolation of E. coli in pregnant and non-pregnant patients with vaginitis in the present study was also reported by other researchers[18].
Regarding GBS isolates recovered from pregnant and non-pregnant patients with vaginitis (Tables 2, 3), other studies have proved that the rate of isolation of Streptococcus agalactiae from vaginal swabs ranged from (5-40%) due to difference in the sample sites and cultural methods employed[19].
The presence of K. pneumoniae in cases of vaginitis, may be attributed to taking of antibiotics by infected women (especially β-lactam antibiotics) which inhibit the growth of other opportunistic pathogens, while Klebsiella isolates are considered the most common resistant bacteria to most antibiotics by producing extended spectrum β-lactamase[20]. It also can be attributed to absence or decrease in numbers of lactobacilli and subsequently their defense factors.
P. aeruginosa had also been isolated from non pregnant women and from pregnant women. This bacteria had been isolated especially from women suffering from offensive odor, and from vaginal discharge, besides, from non pregnant women using intrauterine device. It is potentially opportunistic bacteria within the vagina. Such microorganism may become an increasing prevalent upon minor alterations of the vaginal environment. Investigators had isolated this bacteria from cases of vaginitis and showed that this bacteria was prevalent among non pregnant women using IUD[21].
Acinetobacter spp. were isolated only from non-pregnant women in this study (6.8%). In a local study in Hilla, Iraq, Acinetobacter was isolated from healthy vagina at a low rate[22]. Only 3 isolates of N. gonorrhoeae had been isolated in this study and mostly from women with infected husbands. These organisms are gram negative, intracellular diplococci organisms that are sexually-transmitted causing infection in the genital tract which may disseminate to other organs[23].
The isolation of Lactobacilli from all patients with vaginitis was also investigated and it was found that fifteen isolates of Lactobacilli had been isolated anaerobically most of them (11/15 isolates) were from non pregnant patients. Studies found that Lactobacillus colonized 71% of sexually active women[24]. Although it is known that Lactobacillus is the predominant vaginal microorganism (107-108 CFU/ml fluid) in healthy premenapausal women, the composition and dynamics of the diverse Lactobacilli populations have been poorly characterized[5].
Although the presence of Lactobacilli may be used as a sign of healthy vaginal environment, the possibility that specific strain or combinations of strains of Lactobacilli being harmful should not be ignored, especially due to their ability to produce substance capable of inhibiting other normal vaginal microorganisms[5]. Other studies concentrated on importance of this bacteria as normal vaginal microflora that had the ability to regulate the growth of other vaginal flora, therefore any disturbances in Lactobacilli were highly correlated with the presence of these opportunistic microorganisms[25].
In addition, the results showed that the presence of Lactobacilli together with other opportunistic pathogens may be due to several factors: effects of antibiotics, type of incubation (as some Lactobacilli species are unable to produce some defense factors under anaerobic incubation), and antagonism among Lactobacilli species to maintain dominance.
Regarding defense factors of lactobacilli, the literature of this subject in many researches showed that this bacteria plays an important role in protection of the vagina from colonization by pathogens and this could be attributed to two main mechanisms: blocking the attachment of pathogens to the vaginal epithelium and by production of substances that inhibit their multiplication[5]. However, not all Lactobacillus strains express these properties with the same intensity, on the contrary, there are substantial differences among species and among strains from a single species. The potential importance of Lactobacilli is to protect the vagina from diseases, therefore, it has been used nowadays as probiotic[5].
The ability of Lactobacilli to produce hydrogen peroxide (H2O2) was investigated and the result showed that these isolates did not have the ability to produce H2O2. These results were in agreement with the results obtained by other researcher who found that only one isolate had the ability to generate H2O2 among all Lactobacilli isolated[26]. Therefore, the presence of other opportunistic pathogens in this study may correlated with the absence of H2O2 production by Lactobacilli However, the results were in contrast with Shopova[27], who found that 93.3% of women colonized by Lactobacilli were able to produce H2O2 and the remaining were non-producers represented as part of normal vaginal microflora.
The lack of H2O2 production by Lactobacilli may be attributed also to the type of incubation, and this finding was agreed with Eschenbach et al.[28] who postulated that anaerobic Lactobacilli did not produce H2O2 while facultative one could produce H2O2 and may represented a non specific antimicrobial mechanism of the normal vaginal ecosystem. Also, the presence of genital infection lead to decrease in the number of Lactobacilli that produce H2O2 and increase colonization of the vagina by Lactobacilli that do not produce it.
Four isolates of Lactobacilli showed potent bacteriocin activity against E. coli and S. aureus indicator isolates (Table 4). These results agreed with that mentioned by several authors who reported that some Lactobacilli exhibited potent antimicrobial activities in form of small, heat-stable, ribosomally-synthesized antimicrobial peptides called bacteriocin, these substances had bacteriocidal or bacteriostatic mode of action, generally, inhibiting microorganism that were closely related to the producing strains. They also found that bacteriocins produced by vaginal Lactobacilli could reduce the risk of vaginitis[29,30].
The production of lactic acid by vaginal Lactobacilli in the present study was recorded (Figure-1). This low pH is inhibitory for many potentially pathogenic organisms that colonize the vagina. This result agreed with that mentioned by Aroutcheva et al.[31] who found that changes in pH of the media was caused by secretion of lactic acid and other organic acids by lactobacilli. They also found that about 100% of lactic acid detected was produced by lactobacilli but the percentages differ according to species. The result also correlated with the result obtained by Simoes et al.[30] who found that after 22 hrs. of incubation of Lactobacilli, the pH decreased by 2 or 0.8 points.
Researches relating to relationship of Lactobacilli to genital microflora responsible for bacterial vaginosis found that the low vaginal pH is usually attributed to lactic acid producing Lactobacilli, they also found that during times of high oestrogen, large amount of glycogen are deposited in the vaginal epithelium and that the glycogen is anaerobically metabolized to lactic acid, so that the vaginal bacteria mainly lactobacilli were the primary source of lactic acid production[28].
Lactobacilli play an important role in maintaining the vaginal health by production of defense factors and some of these defense factors have an inhibitory effect on some opportunistic pathogens (S. aureus, E. coli), this fact was confirmed by results mentioned by Ronnquist. et al[32] who reported that the normal vagina of reproductive age women is predominately colonized with Lactobacilli which produce hydrogen peroxide, bacteriocins and lactic acid, these substances are able to lower the vaginal pH, which create a more hostile environment for bacteria other than Lactobacilli. If the number of Lactobacilli falls, the resulting increase in pH favors an overgrowth of anaerobic and facultative bacteria which can develop into virginities/vaginosis.
Hoyme and Saling[33] reported that the probiotic concept was also important to pregnant women by reducing the incidence of premature rupture of amniotic membranes which cause premature delivery and by the inverse association between Lactobacilli and group B streptococci and this very important for pregnant women since transfer of group B streptococci to neonates during delivery increases the risk of meningitis and other complications in newborns.
The role of Lactobacilli in preserving the vaginal health as being the predominant vaginal flora and mentioned that when Lactobacilli decreased due to many causes like douching, sexual practices and use of antibiotics; other pathogenic organisms would dominate. There is therefore, growing interest in the use of Lactobacilli of human origin as probiotics against genital infection. Various clinical attempts had been carried out to assess the ability of Lactobacilli in prevention as well as treatment of genital infection. A recent study showed that the use of Lactobacilli containing-vaginal tablets helped in treatment of 61% of women suffering from vaginal infection in comparison to 19% of placebo treated patients[34].
Effects of antibiotics on Lactobacilli in the present study was also investigated and results showed that most Lactobacilli isolates were resistant to vancomycin and ciprofloxacin (Figure 2). These results were in agreement with the results obtained by Coppola et al.[35], who found that 100% of lactobacilli isolate were resistant to both ciprofloxacin and vancomycin and this resistance was important in selection of potentially probiotic Lactobacilli. Several studies had recorded that there was an increase in the pattern of resistance of Lactobacilli species toward different inhibitors mainly protein synthesis (gentamycin, tobramicin) and nucleic acid inhibitors (mainly nalidexic acid and ciprofloxacin)[36].
It was found that there was high percentage of Lactobacilli resistance to aminoglycosids (gentamycin, streptomycin, kanamycin). Although aminoglycosides have broad spectrum of activity, however, they are generally inactive against gram positive anaerobes and this is thought to occur because of membrane impermeability due to their characteristic multiple cationic charges. The low percentage of Lactobacilli resistance to amoxicillin in the present study was agreed with the result obtained by Herra et al.[37] who found that Lactobacilli resistance to amoxicillin was at low percentage and the Lactobacilli were mildly resistant to amoxicillin.
Regarding erythromycin, the result showed that the Lactobacilli were highly susceptible to erythromycin and this correlated with the result obtained by Wilks et al.[38] who found that Lactobacilli were highly susceptible and all isolates were inhibited by this drug. The antibiotic resistance among Lactobacilli species and strains could be attributed to many factors including enzymatic inactivation, decrease intracellular drug accumulation or presence of gene that confer antibiotic resistance. However, few studies had been done on antimicrobial resistance of Lactobacilli for their non pathogenic nature as anaerobic commensals.
The effect of antimicrobial therapy on Lactobacilli has been studied, and it was found that the antibiotic had an observable effect against the presence of Lactobacilli, these antibiotics can indiscriminately destroy both beneficial and pathogenic bacteria in the body, also the use of antibiotics could create an imbalance of the microflora with very negative effect, they cause the microflora to remove from epithelial cells of the vagina, thus allowing the attachment and proliferation of potentially putrefactive pathogenic organisms such as S. aureus, E. coli, and Candida. So, the use of antibiotics by the patients to treat virginities should be very selective in order not to kill lactobacilli. Although antimicrobial agents are generally effective at eradicating the infection but, there was a high incidence of recurrence, the patient quality of life is affected and many women become frustrated by cycle of reported antimicrobial agent, whose effectiveness is diminished due to increasing development of microbial resistance, in addition, the use of antimicrobial agent is not only select resistance bacteria but, it can also disturb the balance of the body by killing friendly bacteria leading to urogenital tract infection[39].
Results of the study also found that there was an inverse relationship between presence of Lactobacilli and the organisms causing virginities. These results are attributed to the production of defense factors by Lactobacilli. These findings agreed with those obtained by several researchers who found that Lactobacilli constitute an important part of the urogenital tract microbiota and this indigenous bacteria considered as a natural resistant factor against potential pathogenic microorganisms and provide colonization resistant factor against opportunistic pathogens by producing autogenic regulation factors like lactic acid, hydrogen peroxide and bacteriocins[40].
It was found that Lactobacilli produce a variety of substances such as bacteriocins which is toxic to other bacterial species, in addition acidification of the vagina due to lactic acid production is also protective while the production of hydrogen peroxide play the most important role against anaerobes and thus Lactobacilli producing H2O2 provide a protective role against virginities and acquisition of sexually transmitted infections[39].
Conclusion
This study concluded that there was an inverse relationship between Lactobacilli presence and the organism that caused vaginitis due to production of defense factors by Lactobacilli. The types of antibiotics used to treat vaginitis must be very selective in order not to kill the beneficial bacteria (Lactobacilli) that help in preservation of vaginal health and ecosystem as being one of the probiotic bacteria.
References
- 1.Egan ME, Lipsky MS. Diagnosis of vaginitis. Am Fam Physician. 1999;68(5):1095–104. [PubMed] [Google Scholar]
- 2.Joesoef MR, Schmid GP, Hillier SL. Bacterial vaginosis: Review of treatment options and potential clinical indications for therapy. Clin Infect Dis. 1999;28(Suppl. 1):557–65. doi: 10.1086/514725. [DOI] [PubMed] [Google Scholar]
- 3.Stenchever MA. 4th ed. Mosby Inc; 2001. Comprehensive gynaecology; pp. 668–678. [Google Scholar]
- 4.Hawes SE, Hillier SL, Benedetti J, et al. Hydrogen peroxide-producing Lactobacilli and acquisition of vaginal infections. J Infect Dis. 1996;174:1058–1063. doi: 10.1093/infdis/174.5.1058. [DOI] [PubMed] [Google Scholar]
- 5.Andreu A. Lactobacillus as probiotic for preventing urogenital infection. Rev Med Microbiol. 2004;15:1–6. [Google Scholar]
- 6.Forbes BA, Daniel FS, Alice SW. 12th ed. USA: Mosby Elsevier Company; 2007. Baily and Scott's diagnostic microbiology. [Google Scholar]
- 7.Eschenbach DA, Davick PR, Williams BL, et al. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27:251–256. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A1-Qassab AO, Al-khafaji ZM. Effect of different conditions on inhibition activity of enteric lactobacilli against diarrhea-causing enteric bacteria. J Agric Sci. 1992;3(1):18–26. [Google Scholar]
- 9.Boskey ER, Cone RA, Whaley KJ, Moench TR. Origen of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Eur Soc Human Reprod Embryol. 2001;16(9):1809–1813. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 10.8th ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2003. National Committee for Clinical Laboratory Standards (NCCLS). Performance standards for disc susceptibility tests. Approved standard M2-A8. [Google Scholar]
- 11.1. Vol. 30. Wayne, PA. USA: National Committee for Clinical Laboratory Standards; 2010. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. Approved standard M100-S20. [Google Scholar]
- 12.Samar G. Effects of intrauterine device on reproductive tract infection RTI in the Northenweat bank. M.Sc thesis, Al-Najah National university. 2005 [Google Scholar]
- 13.Kivijärvi A, Järvinen H, Grönroos M. Microbiology of vaginitis associated with intrauterine contraceptive device. Internat J Obst Gynecol. 2005;91:917–923. doi: 10.1111/j.1471-0528.1984.tb03709.x. [DOI] [PubMed] [Google Scholar]
- 14.Donder GG, Annei Eugene B, Alfons B, Geert D, Benard S. Definition of a type of abnormal Vaginal flora is distinct from bacterial vaginosis, aerobic vaginitis. BJOG. 2002;109(1):34–43. doi: 10.1111/j.1471-0528.2002.00432.x. [DOI] [PubMed] [Google Scholar]
- 15.Curzik D, Drazancis A, Hrogvic Z. Non specific aerobic vaginitis and pregnancy. Fetal Diagn Ther. 2001;16(3):187–92. doi: 10.1159/000053906. [DOI] [PubMed] [Google Scholar]
- 16.Mumtoz S, Ahmed M, Aftab I, Akhtar N, UlHassan M, Hamid A. Aerobic vaginal pathogens and their sensitivity pattern. J Ayub Med Coll Abbottabad. 2008;20(1):113–117. [PubMed] [Google Scholar]
- 17.Hillier SL, Krohn MA, Rabe LK. The normal vaginal flora. H2O2-producing Lactobacilli and BV in pregnant women. Clin Infect Dis. 1993;16(4):5273–5281. doi: 10.1093/clinids/16.supplement_4.s273. [DOI] [PubMed] [Google Scholar]
- 18.Michael B, Grossman G, Martin L. Aerobic microflora of the lower reproductive tract in non pregnant adult females and it's correlation with their life styles and enteric flora. J Cornell Medical Center. 1991 [Google Scholar]
- 19.Zhu YZ, Yang YH, Zhang XL. Vaginal colonization of group B Streptococcus: A study in 267 cases of factory women. Chung Hua Liu Hsing Ping Hsuen Tsa Chinh. 1996;17(1):17–19. [PubMed] [Google Scholar]
- 20.Bedenic B, Randegger CC, Stobberingh E, Hachler H. Molecular epidemiology of extended-spectrum β-lactamase from Klebsiella pneumoniae strains isolated in Zagreb. Croatia. Eur. J Clin Microbiol Infect Dis. 2001;20:505–508. doi: 10.1007/pl00011293. [DOI] [PubMed] [Google Scholar]
- 21.Takeyama K, Kunishima Y, Matsukawa M, Takahashi S, Hirose T, Kobayashi N, Kobayashi I, Tsukamoto T. Multidrug-resistant Pseudomonas aeruginosa isolated from urine of patients with urinary tract infection. J Infect Chemother. 2002;8:59–63. doi: 10.1007/s101560200007. [DOI] [PubMed] [Google Scholar]
- 22.Al-Shukri M. M.Sc. Thesis, College of Science. Babylon University; 2003. A molecular and clinical study of some strains of Acinetobacter isolates from patients in Hilla province. [Google Scholar]
- 23.Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann NY Acad Sci. 1991;622:355–375. doi: 10.1111/j.1749-6632.1991.tb37880.x. [DOI] [PubMed] [Google Scholar]
- 24.Antonio MAD, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species, the dermographic and microbiological characteristic of women colonized by these species. J infect Dis. 1999;180:1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert GG, Bosmans E, Dekeersmacker A, Vereechen A, Spitz B. Pathogenicity of abnormal vaginal bacterial flora. Am J Obstet Gynecol. 2000;182(4):872–878. doi: 10.1016/s0002-9378(00)70338-3. [DOI] [PubMed] [Google Scholar]
- 26.Dick LTM, Silvester M, Lawson PA, Collins MD. Lactobacillus fornicalis sp. nov., isolated from the posterior fornix of the human vagina. Int J Syst Evol Microbiol. 2000;50:1253–1258. doi: 10.1099/00207713-50-3-1253. [DOI] [PubMed] [Google Scholar]
- 27.Shopova E. Hydrogen peroxide producing Lactobacillus species in healthy women and women with bacterial vaginosis. Akush Ginekol (Safiia) 2003;24(Suppl. 1):12–15. [PubMed] [Google Scholar]
- 28.Eschenbach DA, Thwin SS, Patton DL, et al. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin Infect Dis. 2000;30:901–907. doi: 10.1086/313818. [DOI] [PubMed] [Google Scholar]
- 29.Aroutcheva A, Simoes JA, Faro S. Relationship of Lactobacilli to genital microflora responsible for bacterial vaginosis. Int J Gynecol Obstet. 2000;70(Suppl 1):17. [Google Scholar]
- 30.Simoes JA, Aroutcheva A, Faro SF. Relationship of Lactobacilli to genital microflora responsible for bacterial vaginosis. Am J Obstet Gynecol. 2000;185(5):486–490. [Google Scholar]
- 31.Aroutcheva A, Gariti D, Simon M, et al. Defense factors of vaginal Lactobacilli. Am J Obstet Gynecol. 2001;185:375–379. doi: 10.1067/mob.2001.115867. [DOI] [PubMed] [Google Scholar]
- 32.Ronnquist PD, Forsgren-Bursk UB, Grahn-Hakansson EE. Lactobacilli in the female genital tract in relation to other genital microbes and vaginal pH. Acta Obstet Gynaecol Scand. 2006;85:726–735. doi: 10.1080/00016340600578357. [DOI] [PubMed] [Google Scholar]
- 33.Hoyme UB, Saling E. Efficient prematurity prevention is possible by pH-self measurement and immediate therapy of threatening ascending infection. Obstet Gynaecol Reprod Boil. 2004;115:148–153. doi: 10.1016/j.ejogrb.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 34.Mastromarino SP, Macchia S, Meggiorini L, Trinchieri U, Mosca L, Perluigi M. Effectiveness of Lactobacillus containing vaginal tablets in the treatment of symptomatic bacterial vagnosis. Clinc Microbial Infect. 2009;15:67–74. doi: 10.1111/j.1469-0691.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- 35.Coppola R, Succi M, Tremonte P, Reale A, Salzano G, Sorrentio E. Antibiotic susceptibility of Lactobacillus rhamnosus. Strains isolated from Parmigiano Reggiano cheese. BMJ. 2005;85:193–204. doi: 10.1016/j.femsle.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 36.Mandar R, Lijunkene K, Haf H, Karaki T, Miklsaur M. Antimicrobial susceptibility of intestinal Lactobacilli of healthy children. Scand J Infect Dis. 2001;33(5):344–349. doi: 10.1080/003655401750173940. [DOI] [PubMed] [Google Scholar]
- 37.Herra CM, Cafferkey MT, Keane CT. The in vitro susceptibilities of vaginal Lactobacilli to four broad spectrum antibiotics, as determined by the agar dilution and E-test methods. J Antimicrob Chemother. 1995;35:775–783. doi: 10.1093/jac/35.6.775. [DOI] [PubMed] [Google Scholar]
- 38.Wilks M, Wiggins R, Whiley A, et al. Identification and hydrogen peroxide (H2O2) production of vaginal Lactobacilli from pregnant women at high risk of preterm birth and relation with outcome. JCM. 2004;42(2):713–717. doi: 10.1128/JCM.42.2.713-717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reid G. Probiotic Lactobacilli for urogenital health in women. J Clin Gastroenterol. 2008;42(3):234–236. doi: 10.1097/MCG.0b013e31817f1298. [DOI] [PubMed] [Google Scholar]
- 40.Sethi S, Singh G, Sharma M. Probiotic role of Lactobacilli in vaginal health. Indian J Med Res. 2009;129:628–630. [PubMed] [Google Scholar]


