Abstract
Context:
Clear cell Hidradenocarcinoma is a rare carcinoma arising from sweat glands. It is an aggressive tumor that most metastasizes to regional lymph nodes and distant viscera; surgery with safe margins is the mainstay of treatment.
Case Report:
We report a case of 68-year-old woman who presented with an invasive clear cell hidradenocarcinoma situated in the left parotid area which recurred 5 months after surgery, this recurrence was managed successfully by high-dose irradiation of the tumor bed (66 Gy) and regional lymphatic chains (50 Gy), after a follow-up of more than 15 months, the patient is in good local control without significant toxicity.
Conclusion:
Post operative radiotherapy allows better local control and should be mandatory when histological features predictive of recurrence are present: positive margins, histology poorly differentiated, perineural invasion, vascular and lymphatic invasion, lymph node involvement, and extracapsular spread.
Keywords: Hidradenocarcinoma, sweat-gland, rare, surgery, recurrence, megavoltage external beam radiotherapy
Introduction
Hidradenocarcinoma (HDC) is a rare malignancy which arises from sweat glands, and it occurs conventionally de novo and rarely results in degeneration of a pre-existing hidradenoma[1], and wide surgery is the mainstay of treatment. The estimated recurrence rate is 50% despite aggressive surgical treatment[2], this justifies the use of an adjuvant treatment, based on external beam radiotherapy.
Case Report
A 68 year-old-woman presented with a lump in the parotid area measuring 1,5 cm in size, which progressed slowly over the next 2 years before treatment. Surgical excision was performed, pathologically this was a malignant clear cell hidradenocarcinoma with positive surgical margins, and re-excision of tumor bed was performed without leaving residual tumor.
Lesion recurred five months later, CT scan showed a tumor of parotid area measuring 3.6 cm and infiltrating overlying skin (Fig. 1). Subtotal parotidectomy has been realized 4 months later, surgical margins were positive with great residual tumor measuring 7cm, associated with ipsilateral fixed adenopathy measuring over 6 cm in size.
Fig. 1.
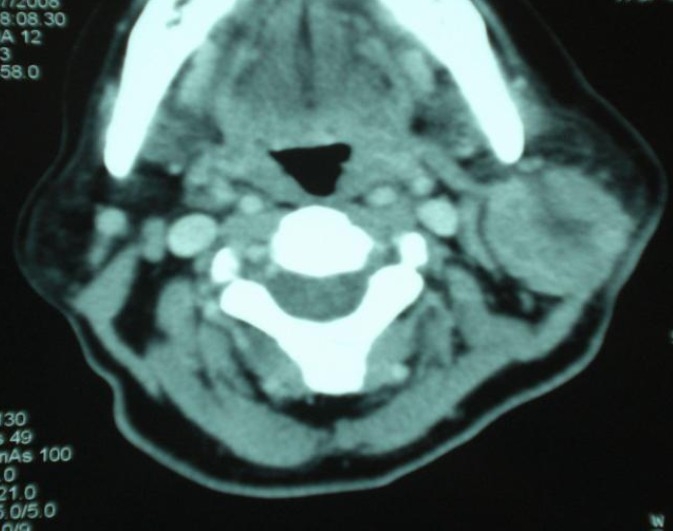
CT scan showing a tumor of the parotid area.
External beam radiotherapy has been realized, with opposed pair of large lateral opposing faciocervical fields that cover primary tumor bed, ipsilateral adenopathy and the upper neck lymphatics in one volume, at a total dose of 66 Gy, 2 Gy per fraction , 5 fractions a week , 33 fractions (Fig. 2). Lower neck lymphatics received prophylactic dose of 50 Gy, with an anteroposterior ipsilateral supraclavicular matched field. After a follow-up of more than 15 months the patient is in good local control without significant toxicity (Fig. 3).
Fig. 2.
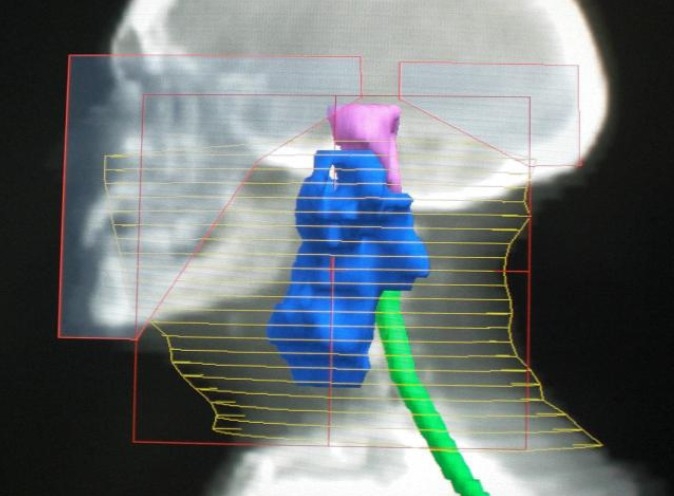
Digitally reconstructed radiography of the left lateral field.
Fig. 3.
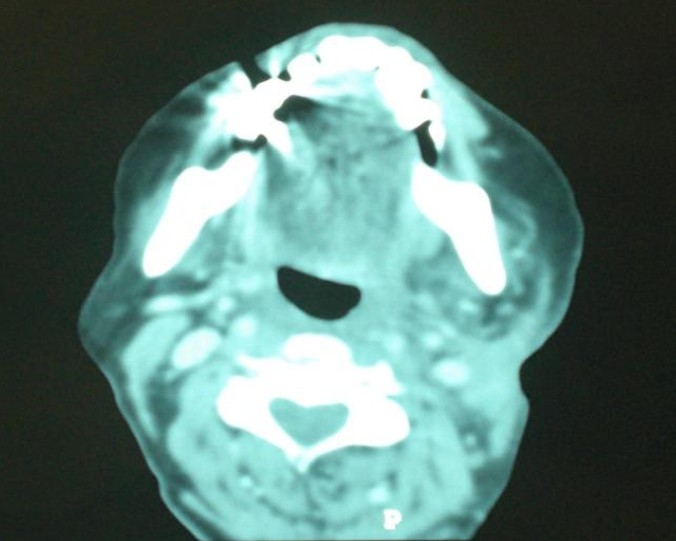
CT scan showing a radiological complete remission.
Morbidity was minimal, and the cosmetic result is excellent.
Discussion
HDC has been described for the first time by Keasby and Hadley in 1954[3], even today fifty cases have been reported in the literature[4]. It is a rare tumor reported most frequently on the head and neck and rarely on the distal extremities; the mean age of onset is 50 years, with a slight female predominance[5].
HDC can derive from preexisting clear cell hidradenomas but more commonly appear de novo, with the molecular events responsible for the pathogenesis currently unknown[1]. HDC usually present as a nodule, painless, firm or cystic, sometimes leaving a liquid which is serous or hemorrhagic. This nodule is usually single, and slowly growing[4]. The diagnosis is based on histopathological examination after biopsy or surgery, showing sweat intradermal proliferation inside a dense hyalinized stroma with nuclear pleomorphism and figures of mitosis[4,5], necrosis is common[5].
Surgery is the cornerstone of treatment, consisting of wide excision ensuring negativity of margins[6–7,10], the role of lymph node dissection is only discussed in the literature with anecdotal report. In the absence of known distant metastases, clinically involved nodal regions should be dissected and irradiated, while clinically uninvolved primary nodal drainage areas should be either dissected or irradiated[6–7,10]. The role of a selective neck dissection is still debated and there is no clear evidence to its usefulness[8].
Certain histopathology criteria should encourage consideration of postexcisional radiotherapy, the features, such as dermal lymphatic invasion, nerve-sheath involvement, deep structure infiltration, positive resection margins, highly anaplasic morphology, and extracapsular lymph node extension, may identify a high risk of recurrence[9,10]. Harari and colleagues reported complete remissions after external beam radiotherapy for sweat gland tumors with positive margins after surgery[10], the dose and technique of radiotherapy are not consensual. In the work of Harari et al, primary surgical beds were treated with 70 Gy, using a combination of photons and electrons, and regional lymphatic chains with 50 Gy. Hyperfractionation schemes were used in two patients to minimize late normal tissue effects[10].
There are both acute and long-term sequelae of radiation therapy for head and neck cancer that occur because of effects on normal tissues. Complications vary according to primary site and are dose dependent. Some common adverse effects include mucositis, xerostomia, trismus, hearing loss, and facial nerve dysfunction. Severe late complications include the risk of soft tissue necrosis, osteoradionecrosis, orocutaneous fistula, blindness, and second malignancies[11]. With the advent of new techniques of radiotherapy, such side effects are less frequent and better tolerated.
Conclusion
It seems obvious that the natural history of this aggressive tumor characterized by local recurrences mandates the use of radiotherapy after surgery for better local control.
Acknowledgments
All authors declare that they have no competing interests.
References
- 1.Gouiaa N, Abbes K, Fakhfekh I, et al. Hidradénocarcinome développé sur un hidradénome préexistant. Annales de dermatologie et de vénéréologie. 2008;135:714–715. doi: 10.1016/j.annder.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Elmoutaoui L, Disky A, Zouhair K, et al. Hidradénome nodulaire malin: une nouvelle observation. Ann Dermatol Venereol. 2007;134:91–92. [Google Scholar]
- 3.Keasbey LE, Hadley GG. Clear cell adenocarcinoma; report of three cases with widespread metastases. Cancer. 1954;7:934–52. doi: 10.1002/1097-0142(195409)7:5<934::aid-cncr2820070519>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Toulemonde A, Croue A, Rodien P, Verret JL. Hidradénome nodulaire malin et hidradénomes nodulaires multiples chez un malade hypogonadique. Ann Dermatol Venereol. 2006;133:1005–1008. doi: 10.1016/s0151-9638(06)71088-2. [DOI] [PubMed] [Google Scholar]
- 5.Requena L, Kutzner H, Hurt MA, et al. Pathology and genetics of skin tumors. Lyon: IARC Press; 2006. Malignant tumours with apocrine and eccrine differentiation. World Health Organisation classification of tumors. [Google Scholar]
- 6.Wick MR, Goellner JR, Wolfe JT, et al. Adnexal carcinomas of the skin. Cancer. 1985;56:1147–1162. doi: 10.1002/1097-0142(19850901)56:5<1147::aid-cncr2820560532>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Yugueros P, Kane WJ, Goellner JR. Sweat gland carcinoma: a clinicopathologic analysis of an expanded series in a single institution. Plast Reconstr Surg. 1998;102:705–710. doi: 10.1097/00006534-199809030-00014. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain RS, Huber K, White JC, et al. Apocrine gland carcinoma of the axilla. Am J Clin Oncol. 1999;22:131–135. doi: 10.1097/00000421-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Hall J, Kneey G, A’Hern RP, et al. Sweat-gland Tumours: A Clinical Review of Cases in One Centre Over 20 Years. Clin Oncol. 2006;18:351–359. doi: 10.1016/j.clon.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Harari PM, Shimm DS, Bangert JL, et al. The role of radiotherapy in the treatment of malignant sweat gland neoplasms. Cancer. 1990;65:1737–17340. doi: 10.1002/1097-0142(19900415)65:8<1737::aid-cncr2820650813>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Terhaard CH. Salivary glands. In: Halperin EC, Perez CA, Brady LW, editors. Principles and Practice of Radiation Oncology. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]


