Abstract
Background:
Rauwolfia vomitoria has been used in Nigeria to manage psychiatric disorders despite orthodox medicine.
Aims:
This research was therefore aimed at comparing the effects of R. vomitoria, chlorpromazine and reserpine on social behaviour and pain in mice.
Materials and Methods:
Ninety male CD-1 mice (32 – 38g body weight) were grouped into 3 with 5 subgroups (n=6) each. Mice were given chlorpromazine (0.0, 0.25, 1.0, 2.0, 4.0 mg/kg i.p.), 30 minutes before testing and R. vomitoria (0.0, 0.25, 1.0, 2.0, 4.0 mg/kg, i.p.) and reserpine (0.0, 0.1, 0.4, 0.8, 1.6 mg/kg, i.p) 24 hours before testing. Nesting score assessed social behaviour while the tail flick and hot plate analgesiometers assessed pain.
Results:
Chlorpromazine dose-dependently decreased nesting score (F4,25 = 5.5660; p< 0.01), indicating decreased social behaviour (social loss) in the mice. Although R. vomitoria did not affect nesting score, reserpine decreased the nesting score (social loss). In the pain test, chlorpromazine did not alter tail flick latency but decreased hind paw lick latency in the hot plate at 2.0 and 4.0 mg/kg (p< 0.01), indicating increased pain sensitivity at these doses which may indirectly increase social withdrawal and thus aggravating depression. R. vomitoria however, increased tail flick and hind paw lick latencies in the hot plate test (p< 0.05) indicating decreased pain sensitivity. Reserpine, like R. vomitoria, increased latency of hind paw lick in the hot plate.
Conclusion:
R. vomitoria has a high potential as an antipsychotic and may have advantage over chlorpromazine; it is not necessary to isolate active components from this herb.
Keywords: Rauwolfia vomitoria, chlorpromazine, reserpine, social behaviour, pain
Introduction
The World Health Organisation (WHO) worldwide statistics in 2002 showed that about 154 million people suffer depression, 25 million people suffer schizophrenia, 91 million suffer from behavioural disorders associated with alcohol use and 15 million suffer from other drug use disorders[1]. In fact, Africa is at higher risk of these disorders because of Political crisis and wars, prevailing poor standard of living as well as moral decay and drug abuse. Other low and middle income earning populations of the countries in Asia are also at high risk[2].
Chlorpromazine (CPZ) is a first generation commonly used and readily available standard antipsychotic drug[3] listed as one of the most essential drugs by the WHO in 2003[4]. Although chlorpromazine has been used to treat both acute and chronic psychoses[1] it has been associated with side effects such as anti-dopaminergic extrapyramidal syndromes[5], dry mouth, blurred vision and urinary retention (anticholinergic)[6], neuroleptic dysphoria, blood pressure disturbances, temperature and muscle control (neuroleptic malignant syndrome), diminished libido, erectile impotence and ejaculation inhibition in male patients[7].
Despite advances in and availability of orthodox, there is still a high patronage of traditional/herbal medicine for treatment of mental and other disorders in Africa/Nigeria [8,9], in Japan[10] and the United States of America[11].
Rauwolfia vomitoria is a common herb used traditionally for psychiatric management in Nigeria[12]. Its extracts have anti-inflammatory effect[13], antipyretic effect[14], anti-diabetic effect[15] and anti-cancer effect (due to the β-carboline alkaloid, alstonine)[16]. R. vomitoria has been reported to be relatively safe with a LD50 of 17.5 g/kg[17]. R. vomitoria extract has been very well characterised using a combination of the high-performance liquid chromatography (HPLC) and the high-performance thin-layer chromatography (HPTLC) on normal- and reverse-phase Armsor[18] and the indole akaloids with yohimbane skeleton namely yohimbine, reserpine, rescinnamine, raucaffricine, ajmaline and ajmalicine identified as the biologically active[19].
Reserpine, one of the alkaloids of the Rauwolfia species, had been used for the management of hypertension, schizophrenia and psychiatric disorders[20] and even thought to be beneficial in cases of Huntington disease[21]. However, its use was discontinued because it caused extrapyramidal side effects such as orofacial dyskinesia and tremor[22].
This study was therefore aimed at comparing the effect of chlorpromazine, R. vomitoria and reserpine on social behaviour and pain sensitivity.
Materials and Methods
Ninety age-matched male CD-1 mice (32g – 38g body weight) purchased from Charles River laboratory, Halifax, Canada were housed singly in a standard animal facility of the Department of Psychology, Dalhousie University, Canada (temperature - 21 ± 2°C; humidity - 55 ± 5%; air changes – 10/hr; and a reversed 12/12 hour light/dark cycle). The animals had access to rodent laboratory chow 5001 from LabChows, Agriband Purina, Canada and clean tap water ad libitum. The mice were grouped into three: each group for Reserpine, R. vomitoria and Chlorpromazine treatment respectively. Each of these groups consisted of 5 sub-groups (n = 6). The research was approved by the Dalhousie University committee on laboratory animal care with protocol number 06-125 in June, 2007 and it was in accordance with the internationally accepted principles for laboratory animal use and care as found in the European Community guidelines (EEC Directive of 1986; 86/609/EEC).
Preparation of Aqueous root bark extract of R. vomitoria
The plant R. vomitoria was identified in the botanical garden of the University of Calabar, Nigeria and a sample deposited in the University herbarium with the voucher number MIA 2004. The roots of the plant were harvested, washed and the root bark was peeled off and sun-dried before blending to fine powder which was stored in a cool dry place, away from light, until required for use. Preparation of the aqueous root bark extract of R. vomitoria was according to the method of Klyushnichenko et al[18]. This was done by mixing 5 g of the powdered root bark of R. vomitoria in 20 ml of distilled water using a sonicator (Ultrasonic cleaner, model no.75T, serial no. 02TS 42 470, VWR International, Westchester, PA) for 10 minutes. The mixture was then centrifuged at about 3,000 g for 5 minutes and the supernatant collected. Twenty ml of distilled water was added to the sediments and mixed again for 10 minutes using the sonicator, centrifuged and the supernatant collected a second time. This process was repeated a third time and the supernatant collected. The supernatant collected from the three cycles of mixing was cleaned of particle by suction filtration, first, using Whatmann no. 1 filter paper and then a second time using cellulose filter paper. The filtrate was evaporated to dryness at 30°C using a vacuum rotary evaporator (Caframo, VV 2000, Ohio) and water bath (Caframo, WB 2000). This extraction gave a percentage yield of about 0.1%.
Drug preparation and treatment
All drugs were prepared by dissolving in 0.9 % saline and were administered at the rate of 0.1 ml/10g body weight, intraperitoneally. The aqueous root bark extract of R. vomitoria was reconstituted to a stock concentration of 1 mg/ml, from which graded doses of 0.0 (0.9 % saline - control), 0.25, 1.0, 2.0, 4.0 mg/kg body weight were administered. A stock solution of 5 mg/ml was obtained from reserpine powder (R 0875 – 1G, Sigma-Aldrich Ltd, Canada) for administration at doses 0.0 (control), 0.1, 0.4, 0.8 and 1.6 mg/kg body weight. Chlorpromazine hydrochloride powder (C8138-5G, Sigma-Aldrich Ltd, Canada) was constituted to a stock concentration of 1 mg/ml, from which 0.0 (control), 0.25, 1.0, 2.0, 4.0 mg/kg body weight were administered. Treatment was for 5 days and the last treatment was 24 hour before behavioural testing for R. vomitoria and reserpine, while it was 30 minutes for chlorpromazine.
Behavoural assay
The tail flick test:
The automated Tail-flick apparatus (Tail-flick Analgesia meter, Columbus Instruments, Columbus, Ohio, USA) produced a radiant light under the base of a tail of a restrained mouse for a maximum duration of 15 seconds (cut-off point; to prevent tissue damage). The time (in seconds) it took for the mouse flick its tail away from the heat of the light was measured as latency of tail flick[23]. Mice were familiarized with the restrainers 20 minutes prior to testing and care was taken not to cause panic in the mice during the test. Mice were given one trial during testing[24,25].
The hot plate test
The hot-plate assay was performed according to the method of Eddy & Leimbach[26], which involved exposing mice to a hot surface, within a confined glass cage. The hot plate apparatus (Columbus Instruments, Columbus, Ohio, USA ) with a restrain cage was used. Animals were introduced into the hot plate after it had been pre-heated to 55°C. The cut off mark here was 30 seconds. A foot pedal connected to a counter was started to record the time it took for the mice to lick their hind paws (Latency of hind paw lick)[27,28].
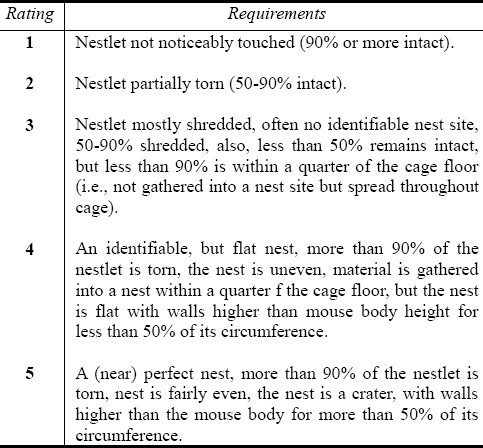
Nesting behaviour used by Bender et al[29] and Deacon[30] as an assay for social behaviour was employed in this study. Mice were housed individually and tested in their home cages. One hour before giving the mice nesting materials, all enrichment objects in the home cages of the mice were removed. About 3.0g of nesting material was supplied to each mouse in its home cage and allowed for 24 hours after which the nests were assessed using the rating scale supplied by Deacon[30] (Table 1). This was based on what was seen. Care was taken while bringing out the cage for observation as causing panic on the mouse could result in destroying the nest so built.
Table 1.
Nesting behaviour rating scale
Statistical analysis
Data among the groups with different concentrations of the treatment agents was analysed by one-way analysis of variance (ANOVA) followed by post hoc Student's Newma-Keuls test. Data were presented as means ± SEM (standard error of mean) P value less than 0.05 was considered statistically significant.
Results
Reserpine, at low to moderate doses (0.1 and 0.4 mg/kg), increased the tail flick latencies whereas at the higher doses (0.8 and 1.6 mg/kg) the tail flick latency did not differ significantly compared to control (Fig. 1). The hot-plate test, however, showed a dose-dependent increase in the latency of hind paw lick for all the doses of reserpine tested (Fig. 2).
Fig. 1.
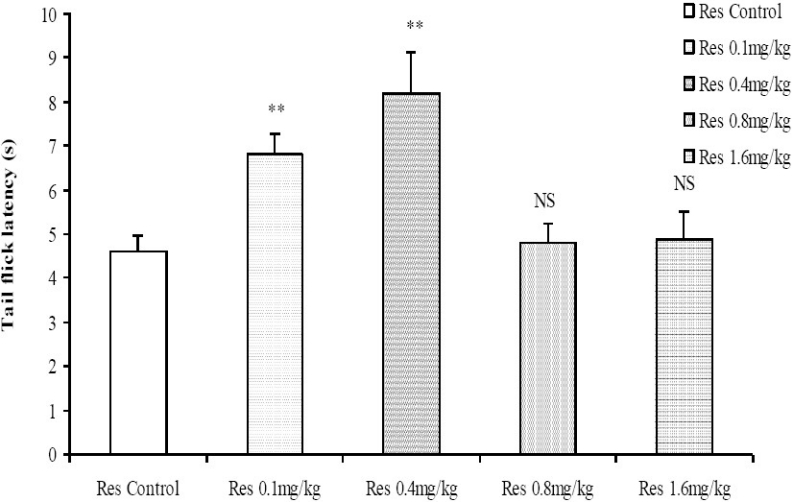
Comparison of tail flick latencies using the tail flick analgesia meter following administration of graded doses of reserpine. NS=Not significant compared to control, **= Significant at p < 0.01 compared to control, n = 6.
Fig. 2.
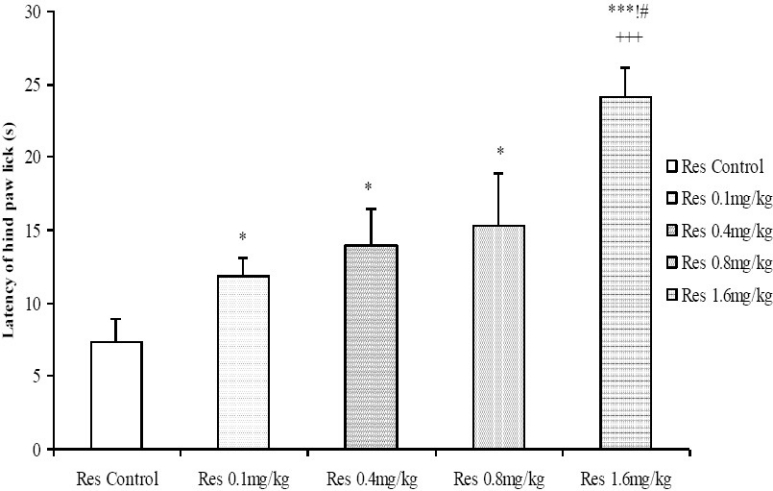
Comparison of Latency of hind paw lick in the Hot plate test following administration of graded doses of reserpine. *=Significant at p < 0.05 compared to control, ***=Significant at p < 0.001 compared to control, +++=Significant at p < 0.001 compared to 0.1 mg/kg reserpine, !=Significant at p < 0.05 compared to 0.4 mg/kg reserpine, #=Significant at p < 0.05 compared to 0.8 mg/kg reserpine, n = 6.
Moderate to high doses of the crude root bark extract of R. vomitoria (1.0, 2.0, 4.0 mg/kg) significantly increased the tail flick latencies in a dose related manner (Fig. 3). The results from the hot plate test also showed a similar trend, with longer latencies of hind paw lick for the 1.0, 2.0 and 4.0 mg/kg doses of the R. vomitoria root bark extract, although there did not seem to be a clearly defined dose-dependent relationship (Fig. 4).
Fig. 3.
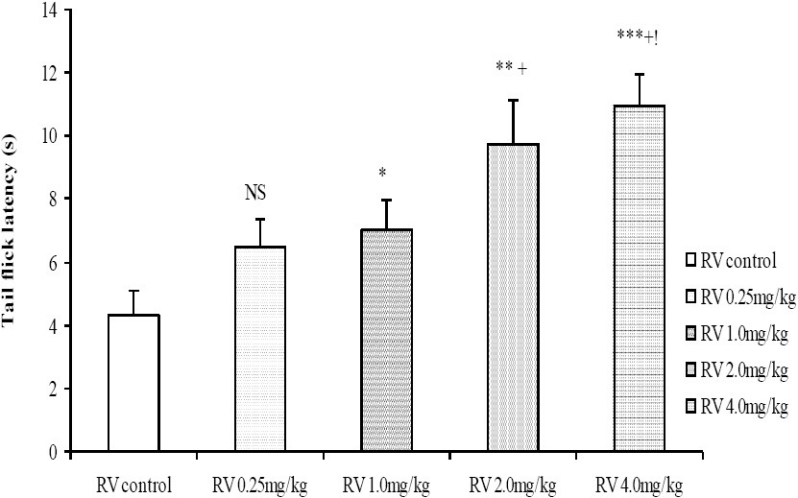
Comparison of tail flick latencies in the tail flick test following administration of graded doses of R. vomitoria root bark extract. NS=Not significant compared to control, *=Significant at p < 0.05 compared to control, **=Significant at p < 0.01 compared to control, ***= Significant at p < 0.001 compared to control, +=Significant at p <0.05 compared to 0.25 mg/kg Rauwolfia vomitoria, != Significant at p < 0.05 compared to 1.0 mg/kg Rauwolfia vomitoria, n = 6.
Fig. 4.
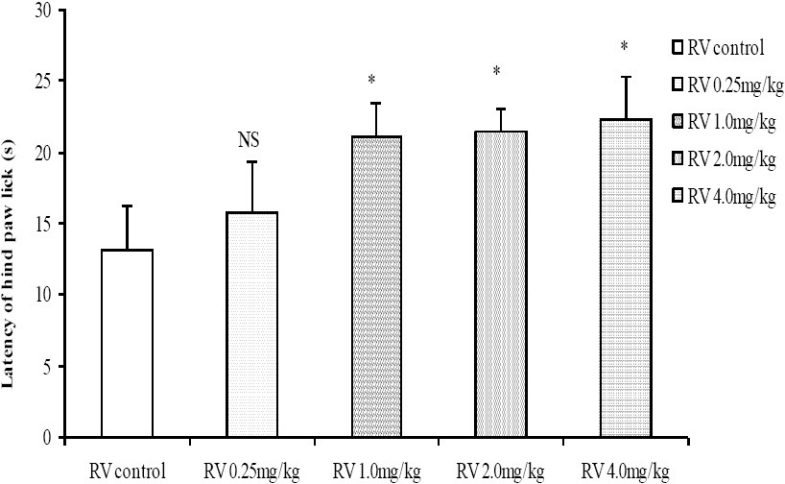
Comparison of Latency of hind paw lick in the Hot plate test following administration of graded doses of R. vomitoria root bark extract. NS=Not significant compared to control, *=Significant at p < 0.05 compared to control, n = 6.
Following administration of graded doses of chlorpromazine, the latency of tail flick did not differ significantly (Fig. 5). In the hot plate test however, the latency of hind paw lick was dose-dependently decreased at the moderate to high doses of chlorpromazine (2.0 and 4.0 mg/kg, i.p.; Fig. 6).
Fig. 5.
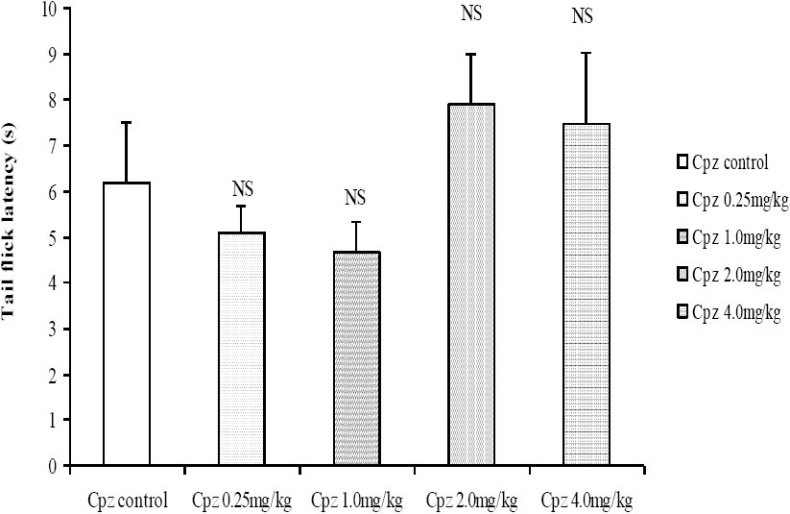
Comparison of Tail flick latencies in the Tail flick following administration of graded doses of chlorpromazine. NS= Not significant compared to control, n = 6.
Fig. 6.
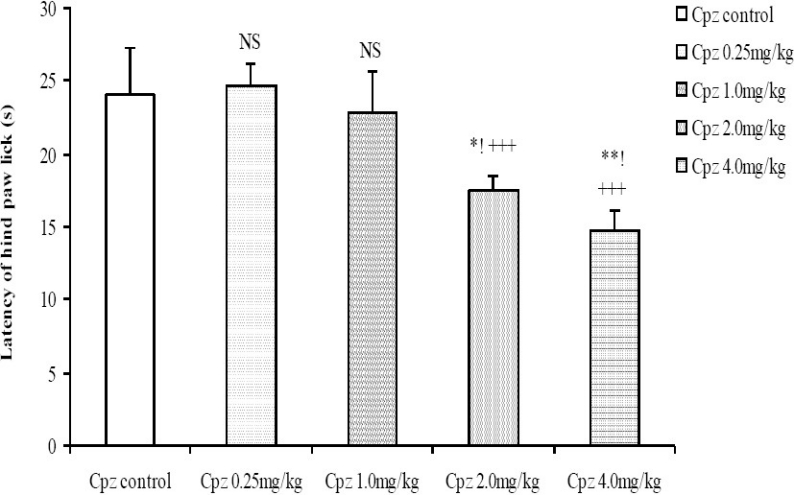
Comparison of Latency of hind paw lick in the Hot plate test following administration of graded doses of chlorpromazine. NS=Not significant compared to control, *= Significant at p < 0.05 compared to control, **=Significant at p < 0.01 compared to control, +++=Significant at p < 0.001 compared to 0.25 mg/kg chlorpromazine, !=Significant at p < 0.05 compared to 1.0 mg/kg chlorpromazine, n=6.
The nesting score following treatment with 0.1, 0.4 and 1.6 mg/kg reserpine was dose-dependently decreased compared to control (Fig. 7). The nesting score following treatment with graded doses of the crude root bark extract of R. vomitoria did not differ significantly from control even when it seemed higher at low to moderate doses (Fig. 8). The nesting score was dose-dependently decreased following the administration of chlorpromazine; p< 0.05 (Fig. 9). Thus, the nesting score at higher doses (0.1, 0.4 and 1.6 mg/kg, i.p.) of chlorpromazine was poorer compared to the lower doses.
Fig. 7.
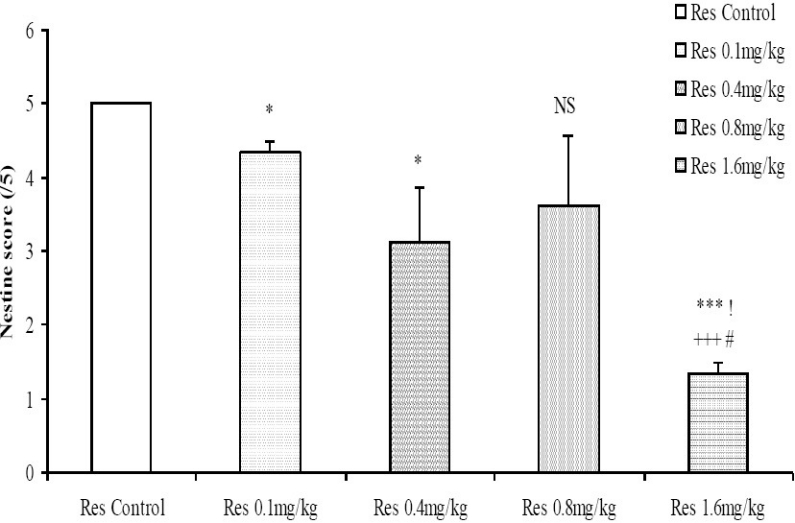
Comparison of nesting score following administration of graded doses of reserpine. NS=Not significant compared to control, *=Significant at p<0.05 compared to control, ***=Significant at p < 0.001 compared to control, +++=Significant at p < 0.001 compared to 0.1 mg/kg reserpine, !=Significant at p < 0.05 compared to 0.4 mg/kg reserpine, #=Significant at p < 0.05 compared to 0.8 mg/kg reserpine, n = 6.
Fig. 8.
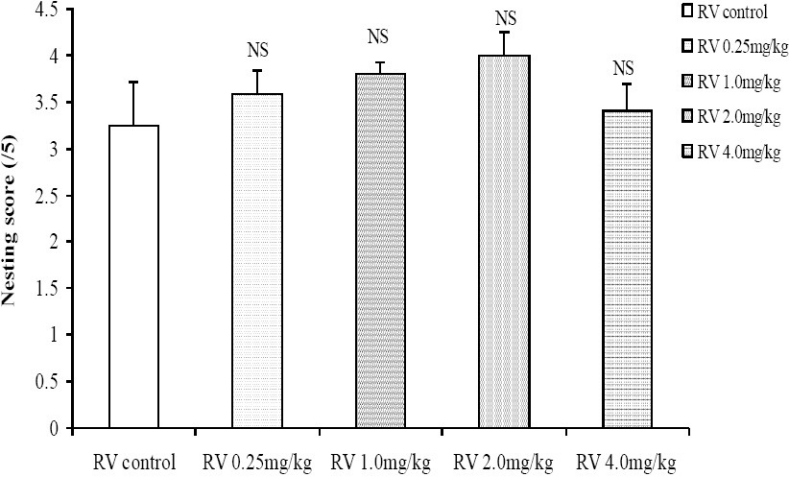
Comparison of nesting score following administration of graded doses of R. vomitoria root bark extract. NS= Not significant compared to control, n = 6.
Fig. 9.
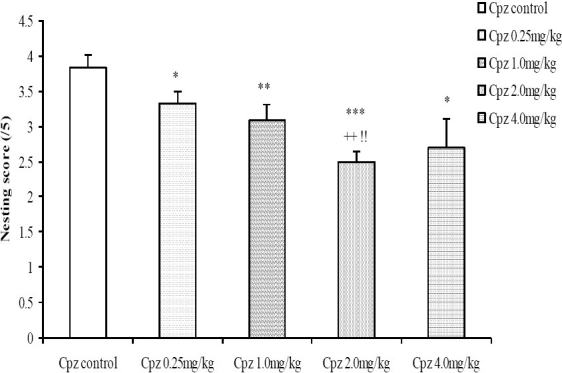
Comparison of nesting score following administration of graded doses of chlorpromazine. NS=Not significant compared to control, *=Significant at p < 0.05 compared to control, **=Significant at p < 0.01 compared to control, ***=Significant at p < 0.001 compared to control, ++=Significant at p < 0.01 compared to 0.25 mg/kg chlorpromazine, !!=Significant at p < 0.01 compared to 1.0 mg/kg chlorpromazine, n = 6.
The animal models of physiological pain used in this study include the tail-flick and hot-plate tests[27]. The tail-flick response is thought to be a spinally mediated reflex while the hot-plate paw-shaking and paw licking responses are more complex and supraspinally organized behaviour. Longer latencies of tail flick and hind paw lick in the tail flick and hot plate tests (respectively) would indicate a higher pain threshold and therefore decreased pain perception or an analgesic effect. Conversely, shorter latencies of tail flick and hind paw lick indicate lower pain threshold and thus increased pain perception or hyperalgesic effect. The hot plate procedure, however, possesses an advantage over other methods of thermal stimulation such as the tail flick procedure because this test constitutes a more global estimate of nociceptive reactivity because it represents a complex pattern of willed behaviour rather than a simple reflex like the tail flick[28].
Since the hot plate test has an advantage over the tail flick test in terms of reliability, it is more appropriate to assert that reserpine at all doses tested (0.1, 0.4, 0.8 and 1.6 mg/kg) caused a dose-dependent decrease in nociceptive perception. It thus, produced an analgesic effect in the mice.
Similarly, the longer latencies observed following treatment with the root bark extract of R. vomitoria indicates a raised pain threshold and thus decrease in pain perception. The root bark extract of R. vomitoria at moderate to high doses therefore produced an analgesic effect in the mice. Although some derivatives of the Rauwolfia family had been earlier reported to have induced gastric pains[31], this occurred only through the oral route and it was peculiar to derivatives of the Rauwolfia plant not the whole extract. The report of Kutalek and Prinz[32] that the root bark extract of Rauwolfia vomitoria alleviated labour pains is in agreement with this study. The results from the hot plate test also showed a similar trend, with longer latencies of hind paw lick for the 1.0, 2.0 and 4.0 mg/kg doses of the R. vomitoria root bark extract although not dose-dependently.
The dose-dependent decrease in latency of hind paw lick in the hot plate test at the moderate to high doses of chlorpromazine (2.0 and 4.0 mg/kg) indicates that chlorpromazine caused a lowering of the pain threshold and thus an increase in pain perception (hyperalgesic effect) in mice. This result disagrees with earlier reports by Gordon and Campbell[33] who concluded that chlorpromazine is a useful adjunct in the control of intractable pain, but not without asserting that chlorpromazine of its own does not have any analgesic effect. The result in this study also negates the study of Shrestha et al[34] who compared the effect of intravenous chlorpromazine hydrochloride (25 mg) and intramuscular Ketorolac (60 mg) in treating acute migraine in humans using the Wong-Baker Faces Rating Scale. It is more likely that chlorpromazine may not have a very well defined analgesic effect but may only be providing the conducing atmosphere for other analgesic agent to function.
Nesting behaviour, a reflection of the social behaviour in mice, sheds light on important disorders of human social behaviour like schizophrenia, autism and Tourette's syndrome. Indeed, abnormal social behaviour exhibited in mice form a core deficit associated with autism spectrum disorder[35]. Mice in this case huddle together less and are unable to fluff up suitable beds from their nesting materials. A poor performance in the nesting task may indicate impairment in social relationship (social loss) in the mice and likelihood of the presence of autistic behaviour.
Reserpine (0.1, 0.4 and 1.6 mg/kg, i.p.) dose-dependently decreased nesting score compared to control. In corroboration with this study, reserpine-treated rats (1.0 mg/kg, i.p.) had exhibit altered social recognition memory abilities in earlier studies[36]. Earlier studies than this had also shown that reserpine induced self aggression in mice[37]. Therefore, this research confirmed reserpine to cause impairments in social interactions in mice and may induce social loss. Since social loss is considered to be one of the major precipitants of depression, prolonged usage of moderate to high doses of reserpine could cause depression.
The nesting score did not differ statistically when mice were treated with crude root bark extract of R. vomitoria. Therefore, the extract did not affect social behaviour and social interaction in the mice. The expected results were that R. vomitoria would increase social interaction because one it's very important alkaloid, alstonine, not only increases social interaction in normal mice, but also averts social deficits attributable to negative symptoms of schizophrenia[38].
Chlorpromazine (0.1, 0.4 and 1.6 mg/kg, i.p.) dose-dependently decreased the nesting score. Thus, higher doses of chlorpromazine caused greater degree of social loss when compared to lower doses. These results are consistent with the work of Li et al[39] who reported that chlorpromazine depressed nesting behaviour in mice.
Conclusion
Chlorpromazine at moderate to high doses (2.0 and 4.0 mg/kg, i.p.), increasing pain perception (hyperalgesic effect) whereas the crude aqueous root bark extract of Rauwolfia vomitoria dose-dependently decreased pain perception (analgesic effect). Reserpine similarly produced an analgesic effect in the mice.
Chlorpromazine at moderate to high doses (2.0 and 4.0 mg/kg, i.p.) caused an impairment in social behaviour, thus inducing social loss in the mice (implying possible exacerbated depression and social withdrawal in psychotic conditions). The root bark extract of R. vomitoria, however, did not affect social behaviour. On the contrary, reserpine (0.1, 0.4 and 1.6 mg/kg, i.p.), caused a dose-dependent impairment in social behaviour, as seen in poor nest building, hence social loss.
The same doses of chlorpromazine which produced a hyperalgesic effect induced social loss, consistent with earlier studies on relationship between social behaviour and pain[40]. If this is applicable in humans, chlorpromazine may not be a very good antipsychotic even when it is the foremost and cheapest antipsychotic drug. Whole root bark extract of Rawolfia vomitoria therefore has a great potential as an antipsychotic because its effect is not due to the presence of reserpine alone.
Acknowledgments
Canadian Department for Foreign Affairs and International Trade, for awarding me the scholarship.
Staff of Brown Laboratory and Psychology Department, Dalhousie University, Halifax NS, Canada for being a wonderful scientific host.
Dr. M. U. Eteng of the Department of Biochemistry, University of Calabar for helping with the harvesting of plant materials.
Dr. M. I. Akpanabiatu for the introduction to the plant Rauwolfia vomitoria.
References
- 1.World Health Organization (WHO): Management of substance abuse: The bare facts. 2010. [Accessed October 2, 2010]. at http://www.who.int/mental_health/en .
- 2.Adams CE, Awad G, Rathbone J, thomley B. Chlorpromazine versus placebo for schizophrenia. Cochrane Database Syst Rev. 2007;2:284. doi: 10.1002/14651858.CD000284.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Chong MY, Tan CH, Fujii S, et al. Antipsychotic drug prescription for schizophrenia in East Asia: rationale for change. Psychiatry Clin Neurosci. 2004;58:61–67. doi: 10.1111/j.1440-1819.2004.01194.x. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). Essential medicines. WHO model list. 13th ed. 2007. [Accessed October 23, 2007]. at http://www.who.int/hac/techguidance/pht/essentialmed/en .
- 5.Peirre JM. Extrapyramidal symptoms with atypical antipsychotics: incidence, prevention and management. Drug Saf. 2005;28:191–208. doi: 10.2165/00002018-200528030-00002. [DOI] [PubMed] [Google Scholar]
- 6.Pilliai A, Terry AV, Jr, Mahandik SP. Differential effects of long-term treatment with typical and atypical antipsychotics on NeRVe growth factor (NGF) and Brain-derived neurotropic factor (BDNF) levels in rats striatum and hippocampus. Schizophr Res. 2006;82(1):95–106. doi: 10.1016/j.schres.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg HR. Inhibition of ejaculation by chlorpromazine. J Nerv Ment Dis. 1971;152(5):364–366. doi: 10.1097/00005053-197105000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Makanjuola ROA. Yoruba traditional healer in Psychaitry. Healers’ concept of the nature and aetiology of mental disorders. Afr J Med Med Sci. 1987;16:53–59. [PubMed] [Google Scholar]
- 9.Gureje O, Acha RA, Odejide OA. Pathways to Psychiatric care in Ibadan, Nigeria. Trop Med Int Health. 1995;47:125–129. [PubMed] [Google Scholar]
- 10.Kanba S, Yamada K, Mizushima H, Asai M. Use of herbal medicine for treating psychiatric disorders in Japan. J Neuropsychiatry Clin Neurosci. 1998;52:331–333. doi: 10.1111/j.1440-1819.1998.tb03260.x. [DOI] [PubMed] [Google Scholar]
- 11.Unützer J, Klap R, Sturm R, et al. Mental disorders and the use of alternative medicine: Results from a national survey. Am J Psychiatry. 2000;157:1851–1857. doi: 10.1176/appi.ajp.157.11.1851. [DOI] [PubMed] [Google Scholar]
- 12.Akpanabiatu MI, Umoh IB, Eyong EU. Influence of Rauwolfia vomitoria root bark on cardiac enzymes o normal Wistar albino rats. Recent Prog Med Plants. 2006;14:273–278. [Google Scholar]
- 13.Kweifio-Okai G, Bird D, Field B, et al. Anti-inflammatory activity of a Ghanaian antiarthritic herbal preparation: III. J Ethnopharmacol. 1995;46:7–15. doi: 10.1016/0378-8741(95)01222-y. [DOI] [PubMed] [Google Scholar]
- 14.Amole OO, Onabanjo AO. Reserpine: The effect and uses of Rauwolfia vomitoria. J Chemother. 1999;3:45–47. [Google Scholar]
- 15.Campbell JIA, Mortensen A, Molgaard P. Tissue lipid lowering-effect of a traditional Nigeian anti-diabetic infusion of Rauwolfia vomitoria foliage and Citrus aurantium fruit. J Ethnopharmacol. 2006;104:379–386. doi: 10.1016/j.jep.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Bemis DL, Capodice JL, Gorroocurn P, Katz AE, Buttyan R. Anti-prostate cancer activity of β-carboline alkaloid enriched extract from Rauwolfia vomitoria. Inter J Oncol. 2006;29:1065–1073. [PubMed] [Google Scholar]
- 17.Amole OO, Yemitan OK, Oshikoya KA. Anticonvulsant activity of Rauvolfia Vomitoria (Afzel) African Journal of Pharmacy and Pharmacology. 2009;3(6):319–322. [Google Scholar]
- 18.Klyushnichenko VE, Yakimov SA, Tuzova TP, et al. Determination of indole alkaloids from R. serpentina and R. vomitoria by high-performance liquid chromatography and high-performance thin-layer chromatography. J Chromatogr. 1995;704:357–362. [Google Scholar]
- 19.Azeem SW, Khan MA, Ahmed I. Characterisation of oxidation products of Rauwolfia alkaloids. Pak J Pharm Sci. 2005;18(1):33–35. [PubMed] [Google Scholar]
- 20.Lopez-Munoz F, Bhatara VS, Alamo C, Cuenca E. Historical approach to reserpine discovery and its introduction in psychiatry. Actas Esp Psiquiatr. 2004;32(6):387–395. [PubMed] [Google Scholar]
- 21.Aminoff MJ. Pharmacologic management of Parkinsonism and other movement disorders. In: Katzung B G, editor. Basic and clinical pharmacology. 9th edition. Singapore: McGraw-Hill; 2004. [Google Scholar]
- 22.Naidu PS, Singh A, Kulkarin SK. Effect of Withania somnifera root extract on reserpine-induced orofacial dyskinesia and cognitive dysfunction. Phytother Res. 2006;20:140–146. doi: 10.1002/ptr.1823. [DOI] [PubMed] [Google Scholar]
- 23.Campbell VC, Taylor RE, Tizabi Y. Antinociceptive effects of alcohol and nicotine: Involvement of the opioid system. Brain Research. 2007;1097:71–77. doi: 10.1016/j.brainres.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 24.Huber JD, Campos CR, Egleton RD, et al. Conjugation of low molecular weight poly(ethylene glycol to biphalin enhances antinociceptive profile. J Pharm Sci. 2003;92:1377–1385. doi: 10.1002/jps.10406. [DOI] [PubMed] [Google Scholar]
- 25.Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine defence and reward differ between adolescent and adult mice. J Pharmacol Experiment Therap. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- 26.Eddy NB, Leimbach D. Synthetic analgesic. II. Dithienylbutenyl and dithienylbutylamines. J Pharmacolog Experiment Therap. 1953;107:385–393. [PubMed] [Google Scholar]
- 27.Ito S, Okuda-Ashitaka E, Minami T. Central and peripheral roles of prostaglandins in pain and their interactions with novel neuropeptides nociceptin and nocistatin. Neuroscience Research. 2001;41(4):299–332. doi: 10.1016/s0168-0102(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 28.Vogel HG, Vogel WH. New York: Springer-verlag; 2002. Drug discovery and evaluation. [Google Scholar]
- 29.Bender BS, Cottey R, Bell W, Taylor S. Body temperature and nesting behavior following influenza challenge in mice: Effects of age. Mech Ageing Dev. 1996;86:l–9. doi: 10.1016/0047-6374(95)01675-9. [DOI] [PubMed] [Google Scholar]
- 30.Deacon RMJ. Assessing nest building in mice. Nat Protoc. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 31.Kolarski V, Petrova-Shopova K, Vasileva E, Petrova D, Nikolov S. Erosive gastritis and gastroduodenitis-clinical, diagnostic and therapeutic studies. Vutr Boles. 1987;26(3):56–59. [PubMed] [Google Scholar]
- 32.Kutalek R, Prinz A. African Medicinal Plants. In: Yaniv Z, Bachrach U, editors. Handbook of medicinal plants. New Delhi: CBS publishers; 2007. [Google Scholar]
- 33.Gordon RA, Campbell M. The use of chlorpromazine in intractable pain associated with terminal carcinoma. Can Med Assoc J. 1956;75:420–421. [PMC free article] [PubMed] [Google Scholar]
- 34.Shrestha M, Singh R, Moreden J, Hayes JE. Ketorolac vs.chlorpromazine in the treatment of acute migraine without aura: A prospective, randomized, double-blind trial. Arch Intern Med. 1996;156(15):1725–1728. [PubMed] [Google Scholar]
- 35.DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviours, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav Brain Res. 2008;18I7(2):207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prediger RD, Da Cunha C, Takahashi RN. Antagonistic interaction between adenosine A2A and dopamine D2 receptors modulates the social recognition memory in reserpine-treated rats. Behaviou Pharmacol. 2005;16(4):209–218. doi: 10.1097/01.fbp.0000166825.62130.9a. [DOI] [PubMed] [Google Scholar]
- 37.Sharma R, Manchanda SK, Nayar U. Role of opioid receptors in self-aggression in rats. Indian J Physiol Pharmacol. 1991;35(3):165–169. [PubMed] [Google Scholar]
- 38.Linck VM, Herrmann AP, Goerck GC, et al. Putative antipsychotic alstonine reverses social interaction withdrawal in mice. 2008;32(6):1449–52. doi: 10.1016/j.pnpbp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Morrow D, Witkin JM. Decreases in nestlet shredding inn mice by serotonin uptake inhibitors: comparison with marble burying. Life Sciences. 2006;78:1933–1939. doi: 10.1016/j.lfs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Farabollini F, Giordanoa G, Carlia Tonic pain and social behavior in male rabbits. Behav Brain Res. 1988;31(2):169–175. doi: 10.1016/0166-4328(88)90020-4. [DOI] [PubMed] [Google Scholar]


