Abstract
Background and Aims
Plant parasitism and arbuscular mycorrhizal (AM) associations have many parallels and share a number of regulatory pathways. Despite a rapid increase in investigations addressing the roles of AM fungi in regulating interactions between parasitic plants and their hosts, few studies have tested the effect of AM fungi on the initiation and differentiation of haustoria, the parasite-specific structures exclusively responsible for host attachment and nutrient transfer. In this study, we tested the influence of AM fungi on haustorium formation in a root hemiparasitic plant.
Methods
Using a facultative root hemiparasitic species (Pedicularis tricolor) with the potential to form AM associations, the effects of inoculation were tested with two AM fungal species, Glomus mosseae and Glomus intraradices, on haustorium initiation in P. tricolor grown alone or with Hordeum vulgare ‘Fleet’ (barley) as the host plant. This study consisted of two greenhouse pot experiments.
Key Results
Both AM fungal species dramatically suppressed intraspecific haustorium initiation in P. tricolor at a very low colonization level. The suppression over-rode inductive effects of the parasite's host plant on haustoria production and caused significant growth depression of P. tricolor.
Conclusions
AM fungi had strong and direct suppressive effects on haustorium formation in the root hemiparasite. The significant role of AM fungi in haustorium initiation of parasitic plants was demonstrated for the first time. This study provides new clues for the regulation of haustorium formation and a route to development of new biocontrol strategies in management of parasitic weeds.
Keywords: Root hemiparasite, Pedicularis tricolor, Orobanchaceae, arbuscular mycorrhizal fungi, initiation of haustoria, Hordeum vulgare ‘Fleet’, Glomus mosseae, Glomus intraradices
INTRODUCTION
Parasitic plants and arbuscular mycorrhizal (AM) fungi are two ubiquitous and important components in terrestrial ecosystems. Both groups impose direct impacts on their host plants as well as consequent influences on community structure of their ecosystems (Press and Phoenix, 2005; Bardgett et al., 2006; Smith and Read, 2008), though in very different ways. Despite their opposite influence on host plants (with parasitic plants often being parasitic and AM fungi mostly mutualistic), recent findings suggest that colonization by AM fungi and infection by root parasitic plants may be modulated by similar molecular mechanisms (Fernandez-Aparicio et al., 2010), and interesting parallels between plant parasitism and AM associations have been indicated (Akiyama and Hayashi, 2006; Bouwmeester et al., 2007; Fernandez-Aparicio et al., 2010; Xie et al., 2010). In addition, a limited but growing number of studies have found that the AM status of host plants significantly influences growth of parasitic plants (Davies and Graves, 1998; Lendzemo and Kuyper, 2001; Salonen et al., 2001; Gworgwor and Weber, 2003; Lendzemo et al., 2005; Lopez-Raez et al., 2011). However, most root parasitic plants do not form mycorrhizal associations (Atsatt, 1973; Brundrett, 2002) and no study has experimentally addressed direct interactions between parasitic plants and AM colonization.
Pedicularis (Orobanchaceae), a genus of photosynthetic, root hemiparasitic plants which was thought to be non-mycorrhizal (Harley and Harley, 1987), has been suggested to form associations with AM fungi (Lesica and Antibus, 1986; Kohn and Stasovski, 1990; Li and Guan, 2007). In a survey of parasitic habit and AM status of Pedicularis spp. from Yunnan Province, China, Li and Guan (2008) found that some Pedicularis spp. can be AM while retaining their parasitic habit. Pedicularis species are known to form haustoria on individuals of its own species (intraspecific haustoria) or even on soil debris (Piehl, 1963; Ren et al., 2010), indicating a primitive parasitic status. Hemiparasitic plants of the genus Pedicularis may therefore represent the earliest stage in the evolutionary transition to plant parasitism, evolving from autotrophy (generally AM) to heterotrophy (with a tendency to lose AM potential as they become more advanced and obligate parasites). Pedicularis thus provides an excellent system for analysing indirect (i.e. via host plants) as well as direct interactions between parasitic plants and AM fungi.
The extent to which parasitic plants depend on their host plants varies greatly (Press and Phoenix, 2005). However, all parasitic plants develop organs called haustoria, which are invasive structures connecting their vasculature with that of their hosts to allow the transfer of nutrients and water (Estabrook and Yoder, 1998). Initiation and proper development of haustoria is essential to successful parasitism (Westwood et al., 2010). Better understanding of tripartite interactions among parasitic plants, their host plants and AM fungi requires investigation of the effects of fungal inoculation on initiation and differentiation of haustoria.
To our knowledge, there is only one report on the effects of AM fungi on haustorium formation. In their greenhouse experiment with a facultative root hemiparasitic species, Rhinanthus minor, Davies and Graves (1998) found that parasitic plants grown with an AM host produced twice as many haustoria as those grown with a non-mycorrhizal (NM) host. However, because the number of haustoria was presented per plant rather than number of haustoria per unit root, it is unclear whether the increased number was due to increased root biomass of the root parasite or increased incidence of haustorium formation per se. Furthermore, the authors did not test direct interactions between R. minor and AM fungi, as the parasitic species they used is a strictly NM species (Harley and Harley, 1987), as confirmed by their experiment.
In this study, we used Pedicularis tricolor, a facultative root hemiparasitic species with the potential to be AM (Li and Guan, 2008) and to form intraspecific haustoria (A.-R. Li, unpubl. res.), to test the effects of AM fungi on haustorium initiation and development. Specifically, we addressed the following questions. (1) Does inoculation with AM fungi affect formation of intraspecific haustoria in the absence of a host plant? (2) Does inoculation with AM fungi interfere with induction of haustoria in the presence of a host plant? Knowledge gained will shed light on how a primitive parasitic plant responds to AM colonization, will contribute to a better understanding of regulation of haustorium formation and may provide clues for development of new biocontrol strategies in management of parasitic weeds.
MATERIALS AND METHODS
Experimental design
The study consisted of two experiments. In expt 1, we tested the effect of different AM fungi on formation of intraspecific haustoria by Pedicularis tricolor Hand.-Mazz. in the absence of a host plant. Two species, Glomus mosseae (Nicol. and Gerd.) Gerdemann and Trappe (WFVAM45/BEG161) and Glomus intraradices Schenck and Smith (DAOM 181602, which has been recently reported to be Glomus irregulare (Stockinger et al., 2009)), were used separately. Pot cultures of the fungi are maintained at the Waite Campus of the University of Adelaide. Five individuals of P. tricolor were grown in each pot and six replicate pots were set up for each treatment. In expt 2, we tested the influence of an AM fungus on haustorium initiation in the presence and absence of a host plant of P. tricolor. We conducted a factorial pot cultivation experiment using Hordeum vulgare ‘Fleet’ (barley) as host plant and G. intraradices (as used in expt 1). The experimental design was as follows: (1) two P. tricolor plants, no AM fungus or host plant; (2) two P. tricolor plants, inoculated with AM fungus but no host plant; (3) two P. tricolor plants, no AM fungus but with one host plant; and (4) two P. tricolor plants, with AM fungus and one host plant. Two individuals of Pedicularis were used in expt 2 so that in treatments without host plant, the root hemiparasites would form intraspecific haustoria, which is a similar design to expt 1 (but with only one AM fungal species and reduced plant density). We did that to test the consistency of AM fungal effects on haustorium formation between different environmental conditions (as with the two experiments). There were six replicate pots per treatment.
Growth medium, plant materials and AM fungal inoculum
Growth medium
A mix of 10 % soil collected from the Waite Arboretum, University of Adelaide, Australia, and 90 % fine sand was used as growth medium in both experiments. Arboretum soil was sieved through a 2 mm sieve and then autoclaved along with fine sand at 121 °C (twice on separate days, 1 h each time). The soil mix had 2·6 mg kg−1 plant-available phosphorus by the resin extraction method (McLaughlin et al., 1994) and its pH (in 0·01 m CaCl2 solution) was approx. 6·0.
Plant materials
Seeds of P. tricolor were collected from Shangri-la, Yunnan Province of China, in September 2008 and had been kept in paper bags at 4 °C until used. To promote germination, the seeds were surface sterilized in 4·5 % commercial sodium hypochlorite for 10 min, rinsed thoroughly with running reverse osmosis (RO) water, soaked in 1000 mg L−1 gibberellic acid for 2 h and then stratified at 4 °C for 1 week. Germination was carried out on filter papers at 20 °C in total darkness for 6 d.
Barley (H. vulgare) was used as host plant for P. tricolor based on previous host preference cultivation experiments (A.-R. Li, unpubl. res.). Seeds of host species were surface sterilized in 4·5 % commercial sodium hypochlorite for 10 min, rinsed with RO water, and germinated on filter papers at 25 °C in total darkness for 3 d.
AM fungal inoculum
Inocula of G. mosseae and G. intraradices consisted of colonized root fragments, soil and spores, derived from pot cultures prepared with Medicago truncatula grown in 1:9 Arboretum soil–fine sand mix (pH approx. 6·0) or Trifolium subterraneum grown in 1:9 Mallala soil–sand mix (pH approx. 7·4), respectively. For NM treatments, non-inoculated pot cultures from the same pot culture batch as G. mosseae were used. Ten per cent (w/w) of each type of inoculum was used in the soil mix for both experiments.
To introduce microbes other than AM fungi in all treatments, 20 mL of soil filtrate was added to each pot. The filtrate was made from a mix of all the types of inocula (each approx. 50 g) used in the corresponding experiment, suspended in 1 L of RO water and then filtered through Whatman filter papers #1 and #42 (Facelli et al., 2010).
Planting and growth conditions
In expt 1, uniform newly germinated seeds of P. tricolor were planted directly into experimental pots. In expt 2, P. tricolor was pre-grown in nurse pots alone inoculated with G. intraradices or NM pot culture for 3 weeks and then transplanted into corresponding experimental pots at the same time as well-germinated barley seeds (average root length 6 cm).
The surface of the soil–sand mix was covered with autoclaved polyethylene beads to retain moisture, and pots were watered to weight with RO water every 2 d to maintain water content at around 10 % of oven-dry soil. Long Ashton nutrient solution minus phosphorus but with increased nitrogen (2 mm K2SO4, 1·5 mm MgSO4·7H2O, 4 mm CaCl·2H2O, 0·1 mm FeEDTA, 4 mm (NH4)2SO4, 16 mm NaNO4, 2·86 mg L−1 H3BO3, 1·81 mg L−1 MnCl2·4H2O, 0·5 mg L−1 ZnSO4·7H2O, 0·08 mg L−1 CuSO4·5H2O, 0·025mg L−1 NaMoO4·2H2O) was applied weekly (15 mL per pot) after transplanting. Pots were fully randomized on a single bench for each experiment and re-randomized at each watering to reduce position effects.
The experiments were conducted from mid-September to early November (Southern Hemisphere Spring) in an environmentally controlled glasshouse at the Waite Campus, University of Adelaide. Night–day temperature range in the glasshouse was 16–31·3 °C. During cloudy days, supplementary lights were turned on to increase irradiance, which was in the range of 450–1000 µmol m−2 s−1.
Harvest and sampling
Plants were harvested at 8 weeks after planting (expt 1) or transplanting (expt 2). In expt 2, plant height of P. tricolor was measured at 4 weeks after transplanting, when a difference was observed between AM-inoculated and NM plants. At harvest, the survival of P. tricolor plants was recorded. The shoots were cut at the soil surface and harvested separately from roots. Shoot dry weight per plant was determined after being oven-dried at 85 °C for 24 h. Thoroughly washed root samples were kept in 50 % ethanol until further analysis. Pedicularis roots were separated from those of their host plants (haustoria on host roots were carefully cut off with minimized host tissue and pooled with Pedicularis roots) under a stereomicroscope and fresh weights were determined after blotting with paper towels. Because roots of two Pedicularis plants proved to be impossible to separate from each other, roots from the same pot were treated as one sample.
For assessment of AM colonization and haustorium formation by Pedicularis in different treatments, a weighed sub-sample of root material <1 mm in diameter was taken, cleared in 10 % KOH and stained in a 5 % ink–vinegar solution (Vierheilig et al., 1998). Abuscular mycorrhizal colonization was recorded according to the magnified intersections method (McGonigle et al., 1990). At least 100 intersections per sample were observed and the incidence of AM structures was scored for hyphae, arbuscules/coils and vesicles. The percentage incidence of each structure over total intersections was calculated and the total percentage colonization was determined. The incidence of haustorium formation was recorded as the number of haustoria per gram root dry weight. The number of haustoria of the sub-sample was counted under a bright field microscope at a magnification of ×40. Haustoria with distinct xylem bridges were recorded as presumably functional haustoria (PFH; Li and Guan, 2008). The remainder of the root samples was oven-dried at 85 °C for 48 h and the dry weights were determined. The dry weight of a sub-sample used for checking AM colonization and haustorium formation was obtained from the ratio between the fresh weight and dry weight of the remainder and the fresh weight of the sub-sample.
Data analysis
For the data from expt 1, one-way analysis of variance (ANOVA) was performed using Statistical Product and Service Solutions (SPSS) software (version 16·0; SPSS China Ltd, Shanghai, China), and Duncan's multiple range test was used for comparing the means. Data from expt 2 were analysed by PERMANOVA, a non-parametric method for ANOVA, as most of the data did not fulfil the assumptions of either normality or homogeneity of variances required for a parametric ANOVA. Pair-wise a posteriori comparisons of the means were done wherever necessary according to the User's Guide of this program (Anderson, 2005).
RESULTS
AM fungal colonization
No AM fungal colonization occurred in non-inoculated plants in either experiment. The colonization levels in P. tricolor roots were generally very low. In expt 1, the average colonization of P. tricolor by G. mosseae and G. intraradices was 3·4 and 13·7 % root length, respectively. In expt 2, the mean colonization of P. tricolor by G. intraradices was 4·9 % in the presence of barley and 15·5 % in its absence, despite high colonization in barley roots (91·4 %). Hyphae were virtually the only structures observed in P. tricolor rootlets. Barley roots developed extensive arbuscules (62·9 % root length) and vesicles (24·4 % root length), together with hyphae.
Effects of different AM fungi on formation of intraspecific haustoria and growth of P. tricolor (expt 1)
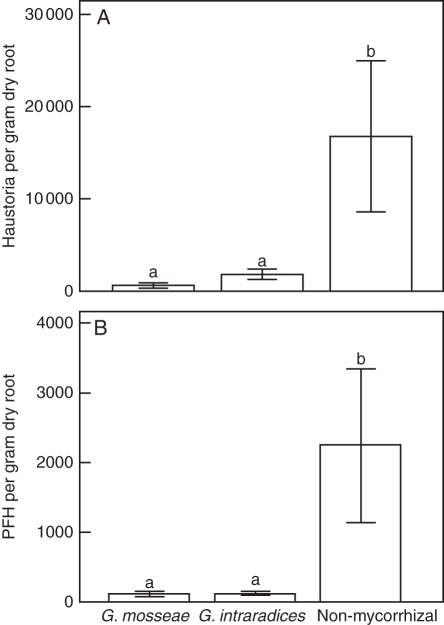
Numerous haustoria occurred on rootlets of P. tricolor, among which some were presumed to be functional (i.e. with a xylem bridge). Inoculation with either AM fungal species significantly reduced the total number of haustoria and number of PFH. There was no significant difference between the two AM fungal species in their influence on formation of intraspecific haustoria by P. tricolor (Fig. 1). No AM-related growth response was observed in this experiment (P = 0·412 for shoot dry weight per plant, and P = 0·861 for survival of P. tricolor).
Fig. 1.
Influence of two Glomus species on haustorium initiation and differentiation in Pedicularis tricolor in expt 1. (A) Total number of haustoria per gram of dry root. (B) Presumably functional haustoria (PFH) per gram of dry root. Data are presented as the mean ± s.e. of six replicate pots. Different letters indicate a statistically significant difference at the P < 0·05 level.
Effects of G. intraradices on haustorium formation and growth of P. tricolor in the absence/presence of a host plant (expt 2)
As in expt 1, intraspecific haustoria were formed in the absence of a host plant. Inoculation with G. intraradices strongly repressed initiation and differentiation of haustoria in P. tricolor, with or without the presence of barley (Table 1 and Fig. 2). Arbuscular mycorrhizal fungal-inoculated P. tricolor seedlings showed severe growth depression 4 weeks after transplanting (P < 0·001). At 4 weeks, plant heights of inoculated P. tricolor were 0·8 ± 0·1 cm in the presence of barley and 0·9 ± 0·1 cm in the absence of barley, while those in NM treatments were 1·8 ± 0·2 and 2·9 ± 0·2 cm, respectively. High mortality was observed in AM fungal-inoculated treatments at harvest (Fig. 2D). In contrast, barley had a negligible influence on the total number of haustoria and survival rate of P. tricolor, but significantly promoted the formation of PFH (Table 1 and Fig. 2). Significant interaction effects were detected between the host plant and AM inoculation on the formation of PFH (Table 1). As a result, suppressing effects of G. intraradices over-rode the promoting effects of barley on PFH formation (Figs 2B, C and 3).
Table 1.
PERMANOVA results (P-values) for total number of haustoria (H) and presumably functional haustoria (PFH) per gram dry root, percentage of PFH and number of surviving plants of Pedicularis tricolor at harvest in expt 2
| Source of variation | Total no. of H per g dry root | No. of PFH per g dry root | Percentage of PFH | No. surviving |
|---|---|---|---|---|
| Host | 0·2764 | 0·0205 | 0·0002 | 0·3407 |
| AM | 0·0001 | 0·0001 | 0·0001 | 0·0102 |
| Host × AM | 0·2899 | 0·0206 | 0·0002 | 0·5255 |
| d.f. residuals | 20 | 20 | 20 | 20 |
Significant values are highlighted in bold.
Fig. 2.
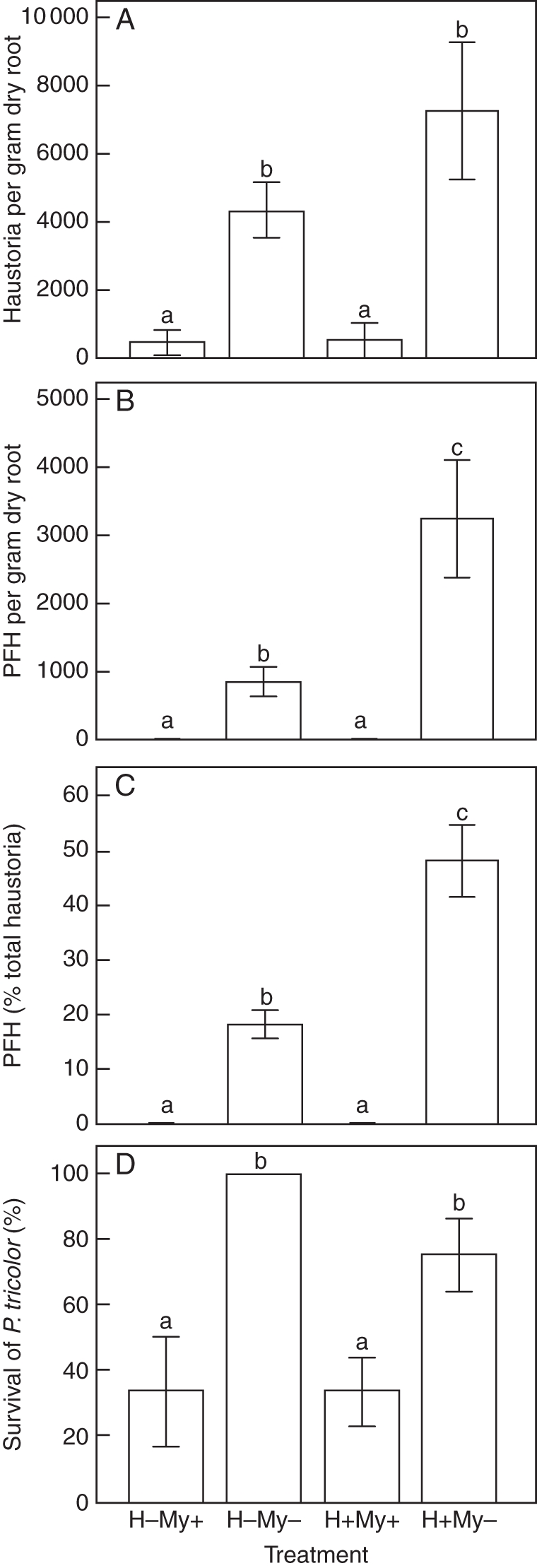
Influence of arbuscular mycorrhizal (AM) fungi (Glomus intraradices) and host plant (barley) on haustorium initiation and differentiation, and survival of transplanted Pedicularis tricolor in expt 2. (A) Total number of haustoria per gram of dry root. (B) Presumably functional haustoria (PFH) per gram of dry root. (C) PFH as a percentage of total haustoria. (D) Percentage survival of P. tricolor. Data are presented as the mean ± s.e. of six replicate pots. Different letters indicate a statistically significant difference at the P < 0·05 level. H–My + , no host plant of P. tricolor but with AM inoculation; H–My–, no host plant and without AM inoculation; H + My + , presence of a host plant and with AM inoculation; H + My–, presence of a host plant but without AM inoculation.
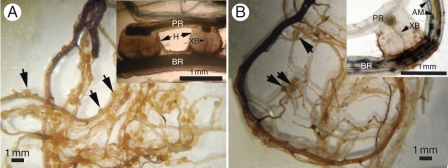
Fig. 3.
Effects of arbuscular mycorrhizal (AM) fungal inoculation on haustorium formation in Pedicularis tricolor. Haustoria are visible as swellings on roots, and some examples are shown with tailed arrows. (A) Presence of the host plant (barley) but without AM inoculation. The inset shows two haustoria (H) with a xylem bridge (XB) connecting a root of P. tricolor (PR) to a root of barley (BR). (B) Presence of the host plant (barley) with inoculation of Glomus intraradices. Note the lower density of haustoria compared with (A). The inset shows a haustorium with an XB connecting PR and BR. Colonization of barley by the AM fungus is also shown (AM).
DISCUSSION
Inoculation with AM fungi dramatically suppressed haustorium formation in both experiments, despite very low colonization of P. tricolor roots. The low AM colonization in P. tricolor may be due to poor suitability of the AM fungal species for the parasitic plant. A high AM colonization level in barley roots confirmed the high inoculum potential of the inoculum and appropriate environmental conditions for growth and development of these fungi. According to our previous studies, a single Pedicularis species may have a highly variable extent of AM colonization at different field sampling sites (range 19–68 %), indicating a significant influence of environmental factors as well as plant–fungal interactions (Li and Guan, 2007). Numerous studies have found that AM fungal identity affects colonization in the same plant species (Smith and Read, 2008; Facelli et al., 2010). Although we have previously confirmed that the predominant AM fungi associated with P. tricolor are Glomus spp. (A.-R. Li, unpubl. res.), further investigation is required to determine any AM fungal preferences in P. tricolor and whether AM fungi that colonize more effectively have similar effects to the fungi used in this study.
In both experiments, P. tricolor produced abundant intraspecific haustoria in the absence of a host plant. This indicates that inducing factors derived from host plants are not exclusively required for haustorium initiation in P. tricolor. However, the presence of a host plant played a key role in haustorium differentiation (shown as an increased percentage of PFH, Fig. 2C), as previously reported for other parasitic plants (Riopel and Musselman, 1979; Cameron and Seel, 2007). Intraspecific haustorium formation has been observed previously in facultative root hemiparasites, but usually numbers are lower than we observed here (Piehl, 1963; Atsatt et al., 1978; Yoder, 1997; Westwood et al., 2010). From an evolutionary point of view, the production of non-functional haustoria would confer little advantage to a parasitic plant and would exert a substantial energy cost (Stewart and Press, 1990). Suppression of intraspecific haustorium formation by AM fungi may be beneficial to the root parasite. Again, further investigations are required.
The suppression of haustorium formation was associated with significant growth depression in P. tricolor following AM inoculation in expt 2, but not in expt 1. The reasons for the difference and the underlying mechanisms require further investigation. They may include different ages of the plants at harvest, seasonally different growth conditions and the effects of transplanting in expt 2. In fact, AM-related growth depressions have been reported for non-parasitic plants even when the percentage colonization is low (Grace et al., 2009; Smith et al., 2009, 2011). In those cases, AM fungi suppressed phosporus uptake by the roots but supplied little phosporus because the colonization was too low. It will therefore be intriguing to test whether the phosporus uptake by roots of P. tricolor will be suppressed following AM inoculation.
To date there has been no empirical demonstration of AM fungi directly influencing haustorium formation in parasitic plants. Results from this study are a proof of concept, demonstrating that some AM fungal species can have a direct influence on haustorium formation in P. tricolor (Fig. 1 and Table 1). In contrast to a previous report on host-mediated promoting effects of Glomus spp. on haustorium formation in R. minor (Davies and Graves, 1998), inoculation with Glomus spp. strongly suppressed haustorium formation in P. tricolor and even over-rode the inductive effect of the presence of a host plant (Figs 2 and 3). The effects observed here may be caused by a direct interaction between AM fungi and P. tricolor, which is obviously not the case for the strictly NM R. minor. More work has to be done to reveal the underlying mechanisms.
Since haustoria are the exclusive structures of all parasitic plants via which nutrients and water are absorbed from host plants, the fact that inoculation with some AM fungi significantly suppresses formation of haustoria opens up the possibility of using these beneficial fungi in biocontrol of parasitic weeds. Furthermore, AM fungi have been found to reduce strigolactone production by host plants (Lopez-Raez et al., 2011) and hence repress seed germination of obligate parasitic plants whose germination often depends on the presence of strigolactones (Lendzemo and Kuyper, 2001; Gworgwor and Weber, 2003; Lendzemo et al., 2005). Arbuscular mycorrhizal fungi may therefore suppress infection of host plants by root parasitic plants at two very different stages, which would enhance biocontrol efficiency. Considering their mutualistic associations with most plant species (Smith and Read, 2008), using AM fungi as biocontrol agents in management of root parasitic weeds is much safer and more effective in terms of a sustainable practice than using pathogenic microbes. Furthermore, a thorough study of the underlying mechanisms by which AM fungi suppress haustorium initiation may inspire more effective strategies in management of parasitic weeds.
ACKNOWLEDGEMENTS
We thank Ms Rebecca Stonor for her excellent help with setting up the experiments and harvesting. The research was supported by the Natural Science Foundation of China (grant no. 30970288), the Natural Science Foundation of Yunnan Province (grant no. 2009CD114) and the Overseas Training Program of the Chinese Academy of Sciences for A.-R.Li.
LITERATURE CITED
- Akiyama K, Hayashi H. Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Annals of Botany. 2006;97:925–931. doi: 10.1093/aob/mcl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ. PERMANOVA: a FORTRAN computer program for permutational multivariate analysis of variance. New Zealand: Department of Statistics, University of Auckland; 2005. [Google Scholar]
- Atsatt PR. Parasitic flowering plants – how did they evolve. American Naturalist. 1973;107:502–510. [Google Scholar]
- Atsatt PR, Hearn TF, Nelson RL, Heineman RT. Chemical induction and repression of haustoria in Orthocarpus purpurascens (Scrophulariaceae) Annals of Botany. 1978;42:1177–1184. [Google Scholar]
- Bardgett RD, Smith RS, Shiel RS, et al. Parasitic plants indirectly regulate below-ground properties in grassland ecosystems. Nature. 2006;439:969–972. doi: 10.1038/nature04197. [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Roux C, Lopez-Raez JA, Becard G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends in Plant Science. 2007;12:224–230. doi: 10.1016/j.tplants.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Brundrett MC. Coevolution of roots and mycorrhizas of land plants. New Phytologist. 2002;154:275–304. doi: 10.1046/j.1469-8137.2002.00397.x. [DOI] [PubMed] [Google Scholar]
- Cameron DD, Seel WE. Functional anatomy of haustoria formed by Rhinanthus minor: linking evidence from histology and isotope tracing. New Phytologist. 2007;174:412–419. doi: 10.1111/j.1469-8137.2007.02013.x. [DOI] [PubMed] [Google Scholar]
- Davies DM, Graves JD. Interactions between arbuscular mycorrhizal fungi and the hemiparasitic angiosperm Rhinanthus minor during co-infection of a host. New Phytologist. 1998;139:555–563. [Google Scholar]
- Estabrook EM, Yoder JI. Plant–plant communications: rhizosphere signaling between parasitic angiosperms and their hosts. Plant Physiology. 1998;116:1–7. [Google Scholar]
- Facelli E, Smith SE, Facelli JM, Christophersen HM, Smith FA. Underground friends or enemies: model plants help to unravel direct and indirect effects of arbuscular mycorrhizal fungi on plant competition. New Phytologist. 2010;185:1050–1061. doi: 10.1111/j.1469-8137.2009.03162.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Aparicio M, Rispail N, Prats E, et al. Parasitic plant infection is partially controlled through symbiotic pathways. Weed Research. 2010;50:76–82. [Google Scholar]
- Grace EJ, Cotsaftis O, Tester M, Smith FA, Smith SE. Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytologist. 2009;181:938–949. doi: 10.1111/j.1469-8137.2008.02720.x. [DOI] [PubMed] [Google Scholar]
- Gworgwor NA, Weber HC. Arbuscular mycorrhizal fungi–parasite–host interaction for the control of Striga hermonthica (Del.) Benth. in sorghum [Sorghum bicolor (L.) Moench] Mycorrhiza. 2003;13:277–281. doi: 10.1007/s00572-003-0238-5. [DOI] [PubMed] [Google Scholar]
- Harley JL, Harley EL. A check-list of mycorrhiza in the British flora. New Phytologist. 1987;105:1–102. [Google Scholar]
- Kohn LM, Stasovski E. The mycorrhizal status of plants at Alexandra Fiord, Ellesmere Island, Canada, a high arctic site. Mycologia. 1990;82:23–35. [Google Scholar]
- Lendzemo VW, Kuyper TW. Effects of arbuscular mycorrhizal fungi on damage by Striga hermonthica on two contrasting cultivars of sorghum. Sorghum bicolor. Agriculture, Ecosystems and Environment. 2001;87:29–35. [Google Scholar]
- Lendzemo VW, Kuyper TW, Kropff MJ, van Ast A. Field inoculation with arbuscular mycorrhizal fungi reduces Striga hermonthica performance on cereal crops and has the potential to contribute to integrated Striga management. Field Crops Research. 2005;91:51–61. [Google Scholar]
- Lesica P, Antibus RK. Mycorrhizal status of hemiparasitic vascular plants in Montana, USA. Transactions of the British Mycological Society. 1986;86:341–343. [Google Scholar]
- Li AR, Guan KY. Mycorrhizal and dark septate endophytic fungi of Pedicularis species from northwest of Yunnan Province, China. Mycorrhiza. 2007;17:103–109. doi: 10.1007/s00572-006-0081-6. [DOI] [PubMed] [Google Scholar]
- Li AR, Guan KY. Arbuscular mycorrhizal fungi may serve as another nutrient strategy for some hemiparasitic species of Pedicularis (Orobanchaceae) Mycorrhiza. 2008;18:429–436. doi: 10.1007/s00572-008-0196-z. [DOI] [PubMed] [Google Scholar]
- Lopez-Raez JA, Charnikhova T, Fernandez I, Bouwmeester H, Pozo MJ. Arbuscular mycorrhizal symbiosis decreases strigolactone production in tomato. Journal of Plant Physiology. 2011;168:294–297. doi: 10.1016/j.jplph.2010.08.011. [DOI] [PubMed] [Google Scholar]
- McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytologist. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin MJ, Lancaster PA, Sale PG, Uren NC, Peverill KI. Comparison of cation–anion exchange resin methods for multielement testing of acidic soils. Australian Journal of Soil Research. 1994;32:229–240. [Google Scholar]
- Piehl MA. Mode of attachment, haustorium structure, and hosts of Pedicularis canadiensis. American Journal of Botany. 1963;50:978–985. [Google Scholar]
- Press MC, Phoenix GK. Impacts of parasitic plants on natural communities. New Phytologist. 2005;166:737–751. doi: 10.1111/j.1469-8137.2005.01358.x. [DOI] [PubMed] [Google Scholar]
- Ren YQ, Guan KY, Li AR, Hu XJ, Zhang L. Host dependence and preference of the root hemiparasite, Pedicularis cephalantha Franch. (Orobanchaceae) Folia Geobotanica. 2010;45:443–455. [Google Scholar]
- Riopel JL, Musselman LJ. Experimental initiation of haustoria in Agalinis purpurea (Scrophulariaceae) American Journal of Botany. 1979;66:570–575. [Google Scholar]
- Salonen V, Vestberg M, Vauhkonen M. The effect of host mycorrhizal status on host plant–parasitic plant interactions. Mycorrhiza. 2001;11:95–100. [Google Scholar]
- Smith FA, Grace EJ, Smith SE. More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytologist. 2009;182:347–358. doi: 10.1111/j.1469-8137.2008.02753.x. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. 3rd edn. London: Academic Press; 2008. [Google Scholar]
- Smith SE, Jakobsen I, Gronlund M, Smith FA. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiology. 2011;156:1050–1057. doi: 10.1104/pp.111.174581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GR, Press MC. The physiology and biochemistry of parasitic angiosperms. Annual Review of Plant Physiology and Plant Molecular Biology. 1990;41:127–151. [Google Scholar]
- Stockinger H, Walker C, Schussler A. ‘Glomus intraradices DAOM197198’, a model fungus in arbuscular mycorrhiza research, is not Glomus intraradices. New Phytologist. 2009;183:1176–1187. doi: 10.1111/j.1469-8137.2009.02874.x. [DOI] [PubMed] [Google Scholar]
- Vierheilig H, Coughlan AP, Wyss U, Piche Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied and Environmental Microbiology. 1998;64:5004–5007. doi: 10.1128/aem.64.12.5004-5007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood JH, Yoder JI, Timko MP, dePamphilis CW. The evolution of parasitism in plants. Trends in Plant Science. 2010;15:227–235. doi: 10.1016/j.tplants.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Xie XN, Yoneyama K, Yoneyama K. The strigolactone story. Annual Review of Phytopathology. 2010;48:93–117. doi: 10.1146/annurev-phyto-073009-114453. [DOI] [PubMed] [Google Scholar]
- Yoder JI. A species-specific recognition system directs haustorium development in the parasitic plant Triphysaria (Scrophulariaceae) Planta. 1997;202:407–413. doi: 10.1007/s004250050144. [DOI] [PubMed] [Google Scholar]