Abstract
Background and Aims
Leafy vegetable Brassica crops are an important source of dietary calcium (Ca) and magnesium (Mg) and represent potential targets for increasing leaf Ca and Mg concentrations through agronomy or breeding. Although the internal distribution of Ca and Mg within leaves affects the accumulation of these elements, such data are not available for Brassica. The aim of this study was to characterize the internal distribution of Ca and Mg in the leaves of a vegetable Brassica and to determine the effects of altered exogenous Ca and Mg supply on this distribution.
Methods
Brassica rapa ssp. trilocularis ‘R-o-18’ was grown at four different Ca:Mg treatments for 21 d in a controlled environment. Concentrations of Ca and Mg were determined in fully expanded leaves using inductively coupled plasma-mass spectrometry (ICP-MS). Internal distributions of Ca and Mg were determined in transverse leaf sections at the base and apex of leaves using energy-dispersive X-ray spectroscopy (EDS) with cryo-scanning electron microscopy (cryo-SEM).
Key Results
Leaf Ca and Mg concentrations were greatest in palisade and spongy mesophyll cells, respectively, although this was dependent on exogenous supply. Calcium accumulation in palisade mesophyll cells was enhanced slightly under high Mg supply; in contrast, Mg accumulation in spongy mesophyll cells was not affected by Ca supply.
Conclusions
The results are consistent with Arabidopsis thaliana and other Brassicaceae, providing phenotypic evidence that conserved mechanisms regulate leaf Ca and Mg distribution at a cellular scale. The future study of Arabidopsis gene orthologues in mutants of this reference B. rapa genotype will improve our understanding of Ca and Mg homeostasis in plants and may provide a model-to-crop translation pathway for targeted breeding.
Keywords: Biofortification, Brassica rapa ssp. trilocularis ‘R-o-18’, calcium, magnesium
INTRODUCTION
Calcium (Ca) and magnesium (Mg) are required in large amounts by both animals and plants (White and Broadley, 2003; Broadley and White, 2010; White and Brown, 2010). The effects on human health of a diet low in Ca include reduced bone density and increased risks of bone fracture and osteoporosis (Department of Health, 1991; Institute of Medicine, 1997). Dietary Mg deficiency is linked to hypertension, cardiovascular risk and pre-eclampsia. The global extent of Ca and Mg deficiency is difficult to define. However, >9 % of UK and US adults are thought to be at high risk of physiological disorder based on Ca and Mg intake data, with much greater numbers having sub-optimal dietary Ca and Mg intakes (Broadley and White, 2010).
Leafy vegetables are an important dietary source of Ca and Mg, even though they are not typically consumed in large quantities (Broadley and White, 2010). For example, food composition tables report that kale (Brassica oleracea var. acephala) contains 130 mg Ca 100 g−1 fresh weight (f. wt) and 34 mg Mg 100 g−1 f. wt (Food Standards Agency, 2002). Leaf Ca and Mg concentrations up to 2-fold higher than this have been recorded in wider genotypic screens of kale (Broadley et al., 2008). Broccoli (Brassica oleracea var. italica) florets are also high in Ca and Mg, e.g. 112 mg Ca 100 g−1 f. wt and 44 mg Mg 100 g−1 f. wt (Pennington and Fisher, 2010). Levels of Ca and Mg in leafy Brassica are typically higher than those observed in many other vegetables and fruit, e.g. Pennington and Fisher (2010) report values for tomato of 20 mg Ca 100 g−1 f. wt and 13 mg Mg 100 g−1 f. wt, for potatoes of 19 mg Ca 100 g−1 f. wt and 15 mg Mg 100 g−1 f. wt, and for citrus fruit of 33 mg Ca 100 g−1 f. wt and 11 mg Mg 100 g−1 f. wt.
Increased consumption of leafy Brassica could reduce the risk of inadequate intake of Ca and Mg for many individuals (Broadley and White, 2010). Even greater benefits may be possible if Ca and Mg concentrations in leaves are increased through Ca or Mg fertilization, or crop selection/improvement. Calcium and Mg fertilizers can be used to increase Ca and Mg concentrations in leafy vegetable crops. It is more difficult to increase Ca concentration of grain, seeds and fruit using this approach as Ca is relatively immobile in the phloem (Karley and White, 2009; White and Broadley, 2009). In contrast, Mg fertilizers can be used to increase Mg concentrations in grain, seed and fruit as Mg is mobile in the phloem. Excess Ca and Mg can be toxic to plants, and biofortification strategies must consider carefully the effects of altered Ca and Mg supply on the movement and sites of accumulation of these elements within the plant.
There is no current information on the distribution of Ca and Mg within Brassica leaves. However, scanning electron microscopy (SEM) combined with X-ray microanalyses has shown that Ca is accumulated in the leaves of Citrus jambhiri in palisade mesophyll > spongy mesophyll >> bundle sheath > adaxial/abaxial epidermis (Storey and Leigh, 2004), consistent with observations in other eudicot species, including several Brassicaceae (Storey and Leigh, 2004; Kerton et al., 2009; Conn and Gilliham, 2010). This contrasts with observations in cereals, in which Ca is preferentially accumulated in epidermal cells (Williams et al., 1993; Fricke et al., 1994; Karley et al., 2000). There are fewer previous studies for Mg than for Ca; however, vacuoles of palisade mesophyll cells in Arabidopsis thaliana have been observed to accumulate 15 mm higher Mg concentrations (and 57 mm higher Ca concentrations) than those of neighbouring adaxial epidermal cells (Conn et al., 2011a). Cell-specific accumulation of Ca and Mg correlates with altered cell-specific expression of tonoplast-localized Ca (AtCAX1) and Mg (AtMRS2-1 and AtMRS2-5) transporters (Conn et al., 2011a, b; 2012).
The aim of this study was to determine the distribution of Ca and Mg in the leaves of a reference genotype of Brassica rapa (ssp. trilocularis 'R-o-18'), a rapid cycling, self-compatible, inbred, yellow sarson line, and to investigate the effect of altered exogenous Ca and Mg supply on this distribution. R-o-18 was selected as it has been used for TILLING (targeting induced local lesions in genomes) for functional analyses of genes (Stephenson et al., 2010; Ó Lochlainn et al., 2011). This study should therefore provide a baseline for downstream functional analysis of orthologues of genes that affect cell-specific accumulation of Ca and Mg in A. thaliana (cf. Conn et al., 2011a, b, 2012) in a crop species.
MATERIALS AND METHODS
Plant growth conditions
Brassica rapa L. ssp. trilocularis ‘R-o-18’ seed were sown on filter paper (Whatman No. 1, Fisher Scientific, Loughborough, UK) moistened with milli-Q water (18.2 mΩ cm) in 9 cm Petri dishes. The dishes were sealed with plastic film (Nescofilm®, Fisher Scientific), and incubated in the dark for 3 d at 20 °C before transferring to controlled environment (CE) conditions in a growth cabinet (Sanyo Gallenkamp, Loughborough, UK). The CE was set to 18 °C with a relative humidity of 70–85 % (day) and 90–95 % (night), and a 16 h photoperiod with 48 fluorescent tubes (36 Philips Master TL5 HO 49w/840 and 12 Philips Master TL5 HE 28w/840) providing 160 µmol m−2 s−1. One week after germination, seedlings were transplanted to a general purpose sphagnum peat and sand compost mix comprising: Shamrock Professional Range Medium Peat (pH 3.8–4.4) and horticultural grade silver sand (particle size <1 mm; J. Arthur Bowers, Lincoln, UK) at a ratio of 3:1 (v:v), supplemented with 10.18 g L−1 NH4H2PO4 (Krista® MAP, Yara UK, Immingham, UK). The compost mixes were supplemented with two Ca and two Mg concentration levels, giving four treatments in total. The ‘high’ (H) concentrations were 3.5 g CaCl2 L−1 compost and 3.04 g MgCl2 L−1 compost, and the ‘low’ (L) concentrations were 0.44 g CaCl2 L−1 compost and 0.20 g MgCl2 L−1 compost. The compost mix contributed at least 0.006 g L−1 water-soluble Ca and 0.004 g L−1 water-soluble Mg. Treatments are hereafter referred to in terms of Ca:Mg (L:L; L:H; H:L and H:H). Other chemicals (Sigma Aldrich, Dorset, UK, unless stated), were added to give the following concentrations (on a compost volume basis); 0.30 g L−1 Na2SO4 (VWR 102644v AnalaR grade); 0.629 g L−1 NH4NO3 (Nitraprill® horticultural grade, GrowHow, Chester, UK); 0.030 g L−1 FeNaEDTA; 2.24 g L−1 KOH; 1.8 mg H3BO3; 2.2 mg L−1 MnSO4·H2O; 0.29 mg L−1 ZnSO4·7H2O; 0.75 mg L−1 CuSO4·5H2O and 0.12 mg L−1 Na2MoO4·2H2O. Plants were sampled 21 d after transplanting. The experiment was repeated three times in three consecutive weeks by staged cultivation; each repeat was treated as a replicate.
Sample preparation and analysis
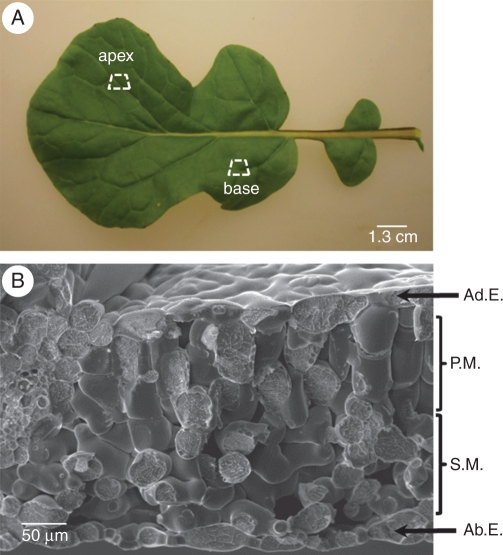
A fully expanded mature leaf was selected from one plant per replicate. Sections (approx. 7 × 5 mm) were cut and trimmed to symmetrical trapezoids from the leaf apex and base (Fig. 1A). Sections were mounted by their longest axis in vertical orientation on a slotted cryo-scanning electron microscope (cro-SEM) stub and held in position using a cryo-glue (OCT) compound (Tissue Tek®, Sakura, Tokyo, Japan). Stubs were plunged into pre-frozen liquid nitrogen (LN2), and transferred under vacuum to a cryochamber (Alto 2100 Gatan, Abingdon, UK) with the stage temperature cooled to –180 °C. Sections were fractured using a cold blade and the stage temperature was raised to –95 °C to sublimate any contaminating ice and to enhance features on the fractured surface. The stage heater was turned off and once the temperature recovered to approx. –160 °C, sections were coated with gold for 60 s. Prepared sections were then passed into the SEM (JSM LV6360 SEM, Jeol, Tokyo, Japan) and mounted on the stage with the temperature maintained at –160 °C. The fractured surfaces were examined at ×500 magnification with an accelerating voltage of 12 kV before setting parameters for microanalysis.
Fig. 1.
(A) Fully expanded leaf of 21-day-old Brassica rapa (R-o-18). The apical and basal zones represent typical sample areas for sectioning. (B) Cryo-SEM image of a leaf section in which energy-dispersive X-ray spectroscopy (EDS) measurements were taken. Leaf cells were categorized as adaxial epidermis (Ad.E.), palisade mesophyll (P.M.), spongy mesophyll (S.M.) and abaxial epidermis (Ab.E.).
Microanalysis was performed using energy-dispersive X-ray spectroscopy (EDS) on an INCA 200 System (Oxford Instruments, High Wycombe, UK). Spectra were acquired from four clearly distinguishable cell types: adaxial epidermis, palisade mesophyll, spongy mesophyll and abaxial epidermis (Fig. 1B). X-rays were generated from a circular area (Ø approx. 2.075 µm) and collected for each spectrum over a set live time of 100 s. Ten spectra were taken from each cell type; thus, 960 spectra were sampled (i.e. 3 replicate plants × 4 Ca:Mg treatment levels × 2 leaf zones × 4 cell types × 10 spectra).
In addition, square sections of leaf tissue (approx. 2 × 2 mm) were cut from leaves and mounted flat on the SEM stub. After coating with gold for 60 s, the specimen was transferred to the cryo-SEM stage and images were obtained from the adaxial and abaxial surfaces. These were used for cell counts from a 250 µm2 frame at a magnification of ×250 and an accelerating voltage of 5 kV.
Semi-quantitative analysis of Ca and Mg
Absolute concentrations of Ca and Mg cannot be measured directly using EDS on fully hydrated, fractured surfaces and without the careful preparation of external standards made up using the typical cocktail of elements likely to be found in the growing fresh leaf. Moreover, to acquire the most meaningful data from EDS systems, specimens should ideally be flat, polished and homogeneous. It follows that fractured, frozen biological specimens are unable to meet these criteria. However, taking reasonable measures in instrument set-up and careful specimen preparation can go some way to satisfying the first two requirements.
The INCA Energy 200 software (Oxford Instruments) Point and Identify Module was used to target cell types across the fully hydrated frozen fractured surface. The spectra acquired are semi-quantitative results based on calculations using the system's internal standards for every element and can be used to compare relative concentrations. The cross hair tool was used for all spectra to limit variation of the interactive area, and minimal sublimation at a temperature of –95 °C was used to avoid movement of cell content (labile elements) or the generation of surface roughness. A KCl standard was used for Quant Optimization (QO) to check beam stability, peak position and resolution, and was repeated throughout the day as spectra were acquired. Microscope and acquisition parameters were maintained exactly as set up for the QO. The relative concentration is calculated using the ‘top-hat filtering’ system that separates the signals from the background in the spectrum by convoluting the Bremsstrahlung intensity with a digital function in the shape of a top-hat. The Gaussian peak shapes are left intact, measured with matrix corrections applied and the results calibrated against the internal standards.
Measuring biomass, leaf area and whole-leaf Ca and Mg concentration
The fresh weights of three fully expanded mature leaves from each plant were determined immediately after excision. The oldest leaf was used for EDS, and the second oldest leaf was used to determine leaf area (using triplicate measurements per leaf) using a LI-3000C Portable Area Meter (LI-COR Bioscience, Lincoln, NE, USA). The third oldest leaf (youngest fully expanded leaf) was used to determine total leaf Ca and Mg concentration by inductively coupled plasma-mass spectrometry (ICP-MS). This third leaf was oven-dried at 60 °C for 48 h in paper bags. Dry leaf material was homogenized using a pestle and mortar, and approx. 100 mg was sub-sampled and digested under closed-vessel microwave heating (45 min, 20 bar) in 2 mL of 70 % trace analysis grade (TAG) HNO3, 1 mL of H2O2 (Fisher Scientific) and 1 mL of milli-Q water (18.2 mΩ cm). The microwave system comprised a Multiwave 3000 platform with a 48-vessel 48MF50 rotor (Anton Paar GmbH, Graz, Austria). Samples were digested in perfluoroalkoxy (PFA) liners inserted into polyethylethylketone (PEEK) pressure jackets (Anton Paar GmbH). Digested samples were diluted to 15 mL with milli-Q water and stored at room temperature. Mineral analysis was conducted using ICP-MS. Briefly, samples were further diluted 1:10 with milli-Q water and analysed using an ICP-MS (X-Series II; Thermo Fisher Scientific Inc., Waltham, MA, USA). Internal standards included Sc (50 ng mL−1) and Ir (5 ng mL−1) in 2% TAG HNO3. External multielement calibration standards (Claritas-PPT grade CLMS-2, SPEX Certi-Prep Ltd, Stanmore, Middlesex, UK) included aluminium, arsenic, barium, bismuth, cadmium, cobalt, chromium, caesium, copper, iron, manganese, molybdenum, nickel, lead, rubidium, selenium, strontium, uranium, yttrium and zinc, in the range 0–100 µg L−1, and Ca, Mg, potassium and sodium in the range 0–100 mg L−1. Data were corrected using blank digestions.
Statistical analysis
All data analyses were conducted using GenStat (V.13.3.0.5165, VSN International, Hemel Hempstead, UK) using replicate as a blocking term. All treatment factors were considered in the analysis of variance (ANOVA), i.e. Ca:Mg supply (four levels), leaf zone (two levels) and cell type (four levels).
RESULTS
The aim of this study was to characterize the distribution of Ca and Mg in the leaves of a reference B. rapa genotype at a tissue level, and to determine the effects of altered exogenous Ca and Mg supply on this distribution. There were no significant effects of Ca or Mg supply on leaf area or adaxial or abaxial cell numbers (data not shown). The leaf zones and cell types analysed are illustrated in Fig. 1, and raw data are provided in Supplementary Data Table S1.
Whole-leaf Ca and Mg concentrations
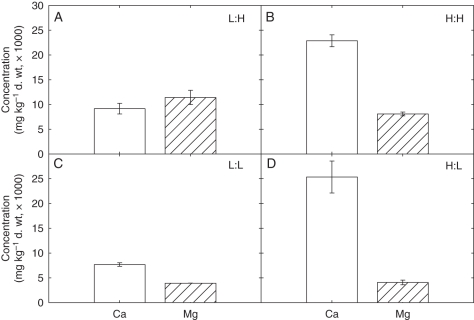
Leaf Ca and Mg concentrations increased at high Ca (H:L and H:H) and high Mg (L:H and H:H) supply, respectively (Fig. 2). At high Ca supply, there was an inhibitory effect of Mg supply on leaf Ca concentration, based on a least significant difference (l.s.d.) test (P < 0.05). At low Ca supply, leaf Ca concentration increased slightly when Mg supply was high, although this was not significant (P > 0.05). At high Mg supply, there was a highly significant reduction in leaf Mg concentration when Ca supply was also high (L:H > H:H; P < 0.001). At low Mg supply, there was no significant effect of Ca supply on leaf Mg concentration.
Fig. 2.
Concentrations of Ca and Mg in fully expanded leaves of 21-day-old Brassica rapa (R-o-18) measured by inductively coupled plasma-mass spectrometry (ICP-MS). Plants were grown at low (L) or high (H) exogenous Ca and Mg supply to give four different Ca:Mg ratios (units given per litre of compost, where H = ‘high’ and L = ‘low’): (A) L:H, 0.44 g CaCl2 L−1, 3.04 g MgCl2 L−1; (B) H:H, 3.5 g CaCl2 L−1, 3.04 g MgCl2 L−1 (C) L:L, 0.44 g CaCl2 L−1, 0.20 g MgCl2 L−1; (D) H:L, 3.5 g CaCl2 L−1, 0.20 g MgCl2 L−1. Data are the means of three replicates (± s.e.m.).
Distribution of Ca and Mg within leaves
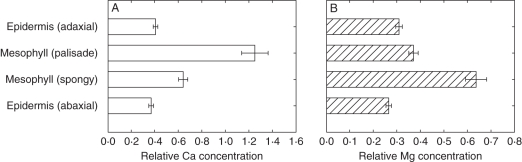
There were no significant differences in Ca accumulation between apical and basal leaf zones when data were analysed across all four Ca:Mg treatments (Fig. 3A; Table 1). In contrast, there was greater Mg accumulation in the basal than in the apical leaf zones (Fig. 3B, P < 0.01). There were differences between cell types in their Ca and Mg concentrations when data were analysed across all four Ca:Mg treatments (Fig 4; Table 1; P < 0.001). Thus, mesophyll cells accumulated more Ca and more Mg than epidermal cells. Palisade mesophyll cells accumulated more Ca than spongy mesophyll cells (Fig. 4A, P < 0.001). In contrast, spongy mesophyll cells accumulated more Mg than palisade mesophyll cells (Fig. 4B, P < 0.001). There was a greater relative difference in Ca accumulation between mesophyll and epidermal cells than for Mg.
Fig. 3.
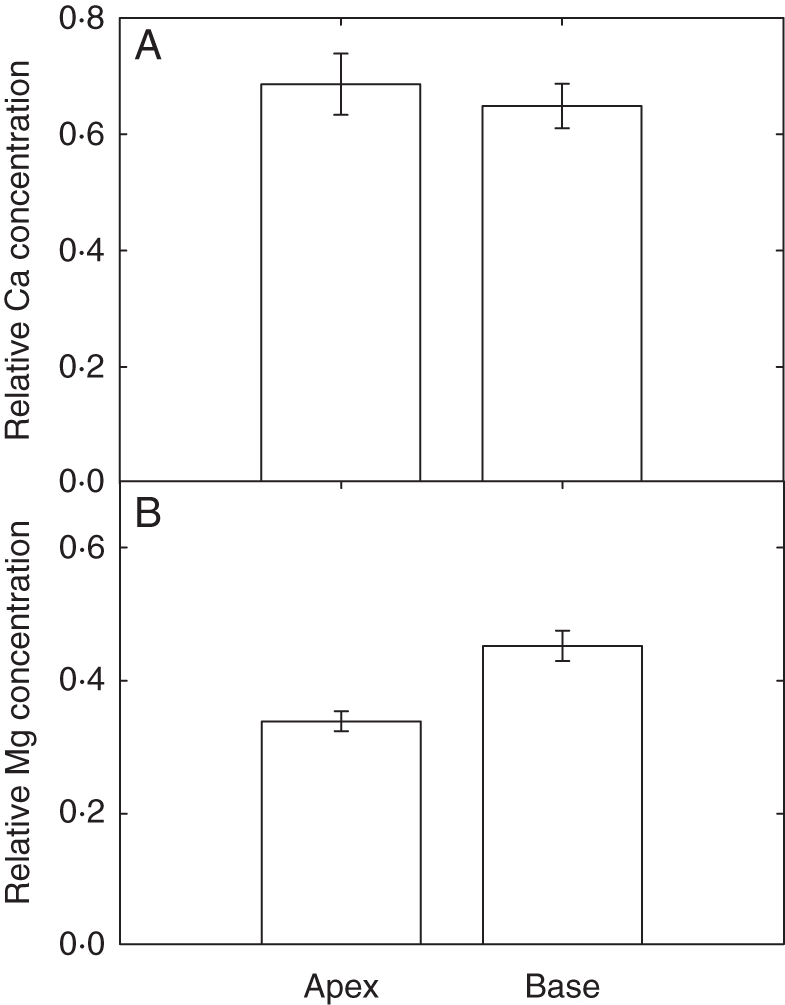
Relative concentrations of (A) Ca and (B) Mg at the apex and base of fully expanded leaves of 21-day-old Brassica rapa (R-o-18). Data represent the means (± s.e.m.) across all spectra (n = 10), cell types (n = 4) and Ca:Mg levels (n = 4) for three replicates (i.e. n = 480 in total).
Table 1.
Analyses of variance (ANOVA) of relative concentrations of Ca and Mg, according to four treatment levels, four cell types and two leaf zones, with ten observations per experimental unit
| Calcium |
Magnesium |
|||||||
|---|---|---|---|---|---|---|---|---|
| Source of variation | n | d.f. | Sum of squares | P | L.s.d.P = 0.05 | Sum of squares | P | L.s.d.P = 0.05 |
| Treatment | 240 | 3 | 165.3 | <0.001 | 0.140 | 40.8 | <0.001 | 0.052 |
| Cell type | 240 | 3 | 119.3 | <0.001 | 0.140 | 20.0 | <0.001 | 0.052 |
| Leaf zone | 480 | 1 | 0.3 | 0.455 | 0.099 | 3.1 | <0.001 | 0.037 |
| Treatment/cell type | 60 | 9 | 119.2 | <0.001 | 0.280 | 22.3 | <0.001 | 0.103 |
| Treatment/leaf zone | 120 | 3 | 1.9 | 0.368 | 0.198 | 5.0 | <0.001 | 0.073 |
| Cell type/leaf zone | 120 | 3 | 1.1 | 0.596 | 0.198 | 3.9 | <0.001 | 0.073 |
| Treatment/cell type/leaf zone | 30 | 9 | 4.6 | 0.571 | 0.395 | 2.7 | <0.001 | 0.146 |
| Residual | 928 | 564.9 | 77.1 | |||||
| Total | 959 | 976.8 | 174.8 | |||||
Least significant differences (l.s.d.P = 0.05) between means are indicated.
Fig. 4.
Relative concentrations of (A) Ca and (B) Mg in four different cell types of fully expanded leaves of 21-day-old Brassica rapa (R-o-18). Data represent the means (± s.e.m.) across all spectra (n = 10), leaf zones (n = 2) and Ca:Mg levels (n = 4) for three replicates (i.e. n = 240 in total).
Distribution of Ca and Mg within leaves in response to altered Ca and Mg supply
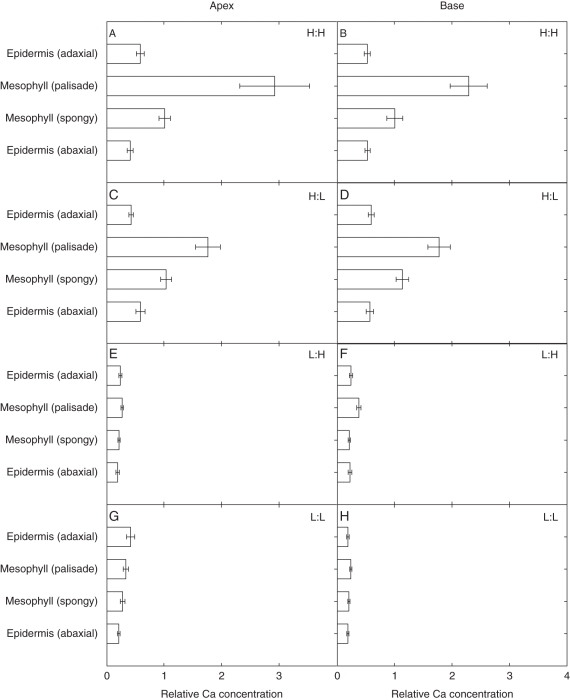
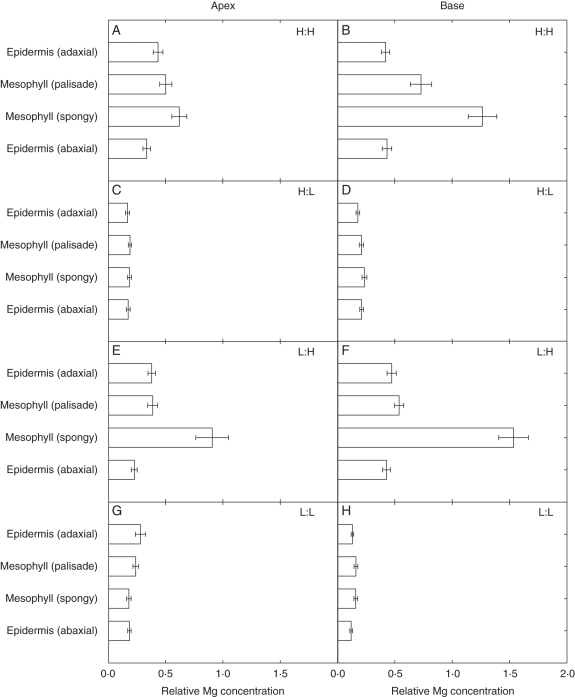
When exogenous Ca supply was high (H:L and H:H), Ca accumulated preferentially in palisade mesophyll cells (Fig. 5A–D). When the exogenous supply of Ca was low (L:L and L:H), Ca concentrations were lower in all cell types (Fig. 5E–H). Mesophyll cells showed the greatest response to Ca supply, i.e. differences in Ca distribution between mesophyll and epidermal cells at high Ca supply were more pronounced. However, Ca still accumulated to a greater extent in the palisade mesophyll tissues than in the spongy mesophyll or epidermal cells at low Ca supply in the basal region of the leaf (Fig. 5). When exogenous Mg supply was high (L:H and H:H), Mg accumulated preferentially in spongy mesophyll cells (Fig. 6A–F). When the exogenous supply of Mg supply was low (L:L and H:L), Mg concentrations were lower in all cell types (Fig. 6C–H). Mesophyll cells showed the greatest response to Mg supply. At the leaf base, high Ca supply increased Mg accumulation in the palisade mesophyll (Fig. 6B, F). Conversely, high Ca supply decreased Mg accumulation in the spongy mesophyll.
Fig. 5.
Relative concentrations of Ca in four different cell types of fully expanded leaves of 21-day-old Brassica rapa (R-o-18) at two leaf zones, the apex and base. Plants were grown at four different Ca:Mg ratios (units per litre of compost, where H = ‘high’ and L = ‘low’): (A, B) H:H, 3.5 g CaCl2 L−1, 3.04 g MgCl2 L−1; (C, D) H:L, 3.5 g CaCl2 L−1, 0.20 g MgCl2 L−1; (E, F) L:H, 0.44 g CaCl2 L−1, 3.04 g MgCl2 L−1; (G, H) L:L, 0.44 g CaCl2 L−1, 0.20 g MgCl2 L−1. Data represent the means (± s.e.m.) across all spectra (n = 10), for three replicates (i.e. n = 30 in total).
Fig. 6.
Relative concentrations of Mg in four different cell types of fully expanded leaves of 21-day-old Brassica rapa (R-o-18) at two leaf zones, the apex and base. Plants were grown at four different Ca:Mg ratios (units per litre of compost, where H = ‘high’ and L = ‘low’): (A) L:H, 0.44 g CaCl2 L−1, 3.04 g MgCl2 L−1; (B) H:H, 3.5 g CaCl2 L−1, 3.04 g MgCl2 L−1 (C) L:L, 0.44 g CaCl2 L−1, 0.20 g MgCl2 L−1; (D) H:L, 3.5 g CaCl2 L−1, 0.20 g MgCl2 L−1. Data represent the means (± s.e.m.) across all spectra (n = 10), for three replicates (i.e. n = 30 in total).
DISCUSSION
Whole-leaf Ca and Mg concentrations and their response to altered exogenous Ca and Mg supply
At the whole-leaf level, exogenous Ca and Mg supply had no major effect on short-term plant growth; the selected ‘L:L’ conditions of 0.44 g CaCl2 L−1 compost and 0.20 g MgCl2 L−1 compost appeared to be adequate for plant growth to the 3 week stage (data not shown). There was, however, a pronounced effect of exogenous Ca and Mg supply on leaf accumulation of these elements. Thus, leaf Ca and Mg concentrations both varied by approx. 3-fold; leaf Ca concentration ranged from 0.8 to 2.5 % dry weight (d. wt), and leaf Mg concentration ranged from 0.4 to 1.2% d. wt (Fig. 2). These concentrations overlap leaf Ca and Mg concentration ranges reported previously for Brassica, i.e. where large numbers of genotypes have been surveyed under single exogenous supply as part of wider genetic characterizations. For example, in a mapping population of field-grown B. rapa, leaf Ca concentration ranged from 1.0 to 2.6% d. wt and leaf Mg concentration ranged from 0.2 to 0.5% d. wt (Wu et al., 2008). In a diversity panel of compost-grown B. oleracea (n = 355), shoot Ca concentration ranged from 1.7 to 3.3% d. wt and shoot Mg concentration ranged from 0.4 to 0.8% d. wt (Broadley et al., 2008). Although genotypic variation in leaf Ca and Mg concentration is highly heritable, ‘environmental’ variation in leaf Ca and Mg concentration due to altered exogenous supply of Ca and Mg is greater than variation due to genotype. It is therefore clear that exogenous Ca and Mg could be managed, using fertilizers, to alter the Ca and Mg composition of leafy vegetables and thereby improve human diets (Broadley and White, 2010). It is also clear that genetic strategies to improve the Ca and Mg composition of leafy vegetables must be considered within an appropriate agronomic framework for Ca and Mg fertilizer usage.
Distributions of Ca and Mg in Brassica leaves are similar to observations in A. thaliana and other eudicot species.
Distributions of Ca and Mg within Brassica leaves were estimated using SEM/EDS, a technique reviewed by Conn and Gilliham (2010). Since the direct quantification of leaf Ca and Mg concentration using EDS on cryo-fractured samples is not an option, the semi-quantitative ‘relative concentration’ was used. For Ca, there were no significant differences in Ca accumulation between apical and basal leaf zones, at either low or high Ca supply. This contrasts with studies on coriander (Coriandrum sativum) in which Ca accumulation was localized at the leaf centre and which provided evidence that leaf Ca dynamics were not driven solely by bulk flow of soluble Ca2+ in the leaf apoplast due to transpiration, as had been hypothesized previously in cereals (Kerton et al., 2009). Differences in zonal Ca accumulation in coriander could be due to altered rates of transport to the vacuoles of bundle sheath mesophyll cells abutting the vascular system and/or altered Ca metabolism, e.g. the immobilization of Ca in biominerals such as Ca-oxalate. However, differential zonal Ca accumulation due to these processes was not seen in B. rapa. There was greater Mg accumulation in the basal than in the apical leaf zones in B. rapa. However, comparable studies on Mg are not available for other plant species, and so it is not clear if is this is a general or species-specific observation.
The most striking observation from the present study is that high exogenous Ca and Mg supply results in accumulation of Ca and Mg in mesophyll cells. This is consistent with previous reports of cell-specific accumulation of Ca and Mg in the leaves of A. thaliana (Conn et al., 2011a, b) and for Ca in other eudicots including Citrus jambhiri (Storey and Leigh, 2004) and C. sativum (Kerton et al., 2009). However, it is noteworthy that cell-specific differences in Ca and Mg accumulation at low exogenous Ca and Mg supply were not seen in any of these studies, and so clearly the phenomenon is under strong environmental control. In a study of three nickel (Ni) hyperaccumulator species of Brassicaceae [Alyssum bertolonii, Alyssum lesbiacum and Thlaspi (Noccaea) goesingense], there was also evidence of greater accumulation of Ca in mesophyll cells compared with epidermal cells, despite very high Ca concentrations in epidermal cell walls (Küpper et al., 2001). However, there was no significant difference in Mg accumulation between mesophyll and epidermal cells in these hyperaccumulators. This could be due a low Mg supply, as seen in B. rapa in this study, or due to their accumulation pattern being affected by the perturbed Ni status of their leaves.
What are the underlying reasons for cell-specific differences in accumulation of Ca and Mg?
Recently, the preferential accumulation of Ca and Mg in leaf mesophyll cells has been studied at the molecular genetic level in A. thaliana, and a strong link between accumulation patterns and expression of tonoplast-localized Ca and Mg transporter genes has been observed (Conn et al. 2011a, b, 2012). For example, the expression of a tonoplast-localized Ca/H+ antiporter (AtCAX1) is higher in mesophyll cells (Conn et al., 2011b). Studies on loss-of-function mutants in AtCAX1 have revealed a complex process, which is dependent on exogenous Ca supply, and which involves a functional redundancy between AtCAX1 and AtCAX3. Thus, at low exogenous Ca supply, cax1/cax3 has no visible Ca phenotype, whereas at higher exogenous Ca supply, cax1/cax3 has gross alterations to leaf Ca partitioning including reduced mesophyll vacuolar Ca concentrations and increased apoplastic Ca concentrations, with epidermal Ca concentrations remaining unaffected. Additional tonoplast-localized transporters, including AtTPC1 and AtMCA1, may also have a role in cell-specific accumulation of Ca; however, these transporters are not abundant in the mesophyll (Conn et al., 2012). Similarly, loss-of-function mutants in the tonoplast-localized Mg2+ transporters AtMRS2-1 and AtMRS2-5 also show less Mg accumulation in the mesophyll, but again only at high exogenous Mg:Ca supply (Conn et al., 2011a, 2012). Clearly, cell-specific accumulation of Ca and Mg in B. rapa is also linked to exogenous Ca and Mg supply. In addition, there is also evidence of a synergistic effect of Mg supply on Ca accumulation at the tissue and whole-leaf levels (Figs 2 and 5), but it is inappropriate to infer too much from just two treatment levels in the supply of each element. The recent availability of a genome sequence for B. rapa (‘Chiifu-401-42’, a Chinese cabbage; Wang et al., 2011) and a TILLING population in ‘R-o-18’ (Stephenson et al., 2010) should now enable the function of orthologues of candidate transport proteins from A. thaliana to be studied, including their role under altered exogenous Ca and Mg supply.
Conclusions
The distribution of leaf Ca and Mg in B. rapa depends critically on exogenous Ca and Mg supply. At low supply, there is no evidence of preferential accumulation in different cell types; Ca and Mg are allocated to tissues in equivalent quantities, which presumably corresponds to minimal requirements for growth and functioning. At high supply, Ca and Mg are accumulated preferentially in mesophyll cells, with Ca and Mg accumulating preferentially in palisade and spongy mesophyll cells, respectively. This phenotype is consistent with observations of other eudicots, notably in the Brassicaceae. Based on recent functional insights from A. thaliana, it seems likely that many mechanistic processes will underlie these phenotypes. Orthologues of candidate proteins from A. thaliana should now be studied in Brassica to test for conserved mechanisms and their regulation by exogenous Ca and Mg supply. Such studies will improve our understanding of Ca and Mg homeostasis in plants and could provide a model-to-crop translation pathway for agronomically informed breeding to improve dietary Ca and Mg intakes.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported primarily by the UK Biotechnology and Biological Sciences Research Council (BBSRC), Grant BB-G013969-1, and the Ministry of Education of Spain Programa Nacional de Movilidad de Recursos Humanos del Plan Nacional de I-D + i 2008–2011 (AGR ex2009-1044 to J.J.R.). The James Hutton Institute's contribution was supported by the Rural and Environment Science and Analytical Services Division (RESAS) of the Scottish Government through Workpackage 7.2 (2011-2016). The Rothamsted Research contribution was supported by strategic funding from the BBSRC.
LITERATURE CITED
- Broadley MR, White PJ. Eats roots and leaves. Can edible horticultural crops address dietary calcium, magnesium and potassium deficiencies? Proceeding if the Nutrition Society. 2010;69:601–612. doi: 10.1017/S0029665110001588. [DOI] [PubMed] [Google Scholar]
- Broadley MR, Hammond JP, King GJ, et al. Shoot calcium and magnesium concentrations differ between subtaxa, are highly heritable, and associate with potentially pleiotropic loci in Brassica oleracea. Plant Physiology. 2008;146:1707–1720. doi: 10.1104/pp.107.114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn S, Gilliham M. Comparative physiology of elemental distribution in plants. Annals of Botany. 2010;105:1081–1102. doi: 10.1093/aob/mcq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn SJ, Conn V, Tyerman SD, et al. Magnesium transporters, MGT2/MRS2-1 and MGT3/MRS2-5, are important for magnesium partitioning within Arabidopsis thaliana mesophyll vacuoles. New Phytologist. 2011a;190:583–594. doi: 10.1111/j.1469-8137.2010.03619.x. [DOI] [PubMed] [Google Scholar]
- Conn SJ, Gilliham M, Athman S, et al. Cell-specific vacuolar calcium storage mediated by CAX1 regulated apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. The Plant Cell. 2011b;23:240–255. doi: 10.1105/tpc.109.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn S, Berninger P, Broadley MR, et al. Exploiting natural variation to uncover candidate genes that control element accumulation in Arabidopsis thaliana. New Phytologist. 2012;193:859–866. doi: 10.1111/j.1469-8137.2011.03977.x. [DOI] [PubMed] [Google Scholar]
- Department of Health. Report on health and social subjects: 41. Dietary reference values for food energy and nutrients for the United Kingdom. London: Her Majesty's Stationery Office (HMSO); 1991. [PubMed] [Google Scholar]
- Food Standards Agency. McCance and Widdowson's the composition of foods. 6th Summary edn. Cambridge: Royal Society of Chemistry; 2002. [Google Scholar]
- Fricke W, Leigh RA, Tomes AD. Concentration of inorganic and organic solutes extracts from individual epidermal, mesophyll and bundle-sheath cells of barley leaves. Planta. 1994;192:310–316. [Google Scholar]
- Gilliham M, Maclin D, Bradleigh JH, et al. Calcium delivery and storage in plant leaves: exploring the link with water flow. Journal of Experimental Botany. 2011;62:2233–2250. doi: 10.1093/jxb/err111. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, The National Academies Press; 1997. [PubMed] [Google Scholar]
- Karley AJ, White PJ. Moving cationic minerals to edible tissues: potassium, magnesium, calcium. Current Opinion in Plant Biology. 2009;12:291–298. doi: 10.1016/j.pbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Karley AJ, Leigh RA, Sanders D. Where do all the ions go? The cellular basis of differential ion accumulation in leaf cells. Trends in Plant Science. 2000;5:465–470. doi: 10.1016/s1360-1385(00)01758-1. [DOI] [PubMed] [Google Scholar]
- Kerton M, Newbury HJ, Hand D, et al. Accumulation of calcium in the centre of leaves of coriander (Coriandrum sativum L.) is due to an uncoupling of water and ion transport. Journal of Experimental Botany. 2009;60:227–235. doi: 10.1093/jxb/ern279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H, Lombi E, Zhao F-J, et al. Cellular compartmentation of nickel in the hyperaccumulators Alyssum lesbiacum, Alyssum bertolonii and Thlaspi goesingense. Journal of Experimental Botany. 2001;52:2291–2300. doi: 10.1093/jexbot/52.365.2291. [DOI] [PubMed] [Google Scholar]
- Ó Lochlainn S, Amoah S, Graham NS, et al. High Resolution Melt (HRM) analysis is an efficient tool to genotype EMS mutants in complex crop genomes. Plant Methods. 2011;7 doi: 10.1186/1746-4811-7-43. 43. http://dx.doi.org/10.1186/1746-4811-7-43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington JAT, Fischer RA. Food component profiles for fruit and vegetable subgroups. Journal of Food Composition and Analysis. 2010;23:411–418. [Google Scholar]
- Stephenson P, Baker D, Girin T, et al. A rich TILLING resource for studying gene function in Brassica rapa. BMC Plant Biology. 2010;10 doi: 10.1186/1471-2229-10-62. 62. http://dx.doi.org/ 10.1186/1471-2229-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey R, Leigh RA. Processes modulating calcium distribution in citrus leaves. An investigation using X-ray microanalysis with strontium as a tracer. Plant Physiology. 2004;136:3838–3848. doi: 10.1104/pp.104.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H, Wang J. The genome of the mesopolyploid crop species Brassica rapa. Nature Genetics. 2011;43:1035–1039. doi: 10.1038/ng.919. [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR. Calcium in plants. Annals of Botany. 2003;92:487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Broadley MR. Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist. 2009;182:49–84. doi: 10.1111/j.1469-8137.2008.02738.x. [DOI] [PubMed] [Google Scholar]
- White PJ, Brown PH. Plant nutrition for sustainable development and global health. Annals of Botany. 2010;105:1073–1080. doi: 10.1093/aob/mcq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ML, Thomas BJ, Farrar JF, et al. Visualising the distribution of elements within barley leaves by energy dispersive X-ray image map (EDX maps) New Phytologist. 1993;125:367–372. doi: 10.1111/j.1469-8137.1993.tb03888.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Yuan Y-X, Zhang X-W, et al. Mapping QTL for mineral accumulation and shoot dry biomass under different Zn nutritional conditions in Chinese cabbage (Brassica rapa L. ssp. pekinensis) Plant and Soil. 2008;310:25–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.