Abstract
Background and Aims
Plasticity in structural and functional traits related to water balance may determine plant performance and survival in ecosystems characterized by water limitation or high levels of rainfall variability, particularly in perennial herbaceous species with long generation cycles. This paper addresses whether and the extent to which several such seasonal to long-term traits respond to changes in moisture availability.
Methods
Using a novel approach that integrates ecology, physiology and anatomy, a comparison was made of lifetime functional traits in the root xylem of a long-lived perennial herb (Potentilla diversifolia, Rosaceae) growing in dry habitats with those of nearby individuals growing where soil moisture had been supplemented for 14 years. Traditional parameters such as specific leaf area (SLA) and above-ground growth were also assessed.
Key Results
Individuals from the site receiving supplemental moisture consistently showed significant responses in all considered traits related to water balance: SLA was greater by 24 %; roots developed 19 % less starch storing tissue, an indicator for drought-stress tolerance; and vessel size distributions shifted towards wider elements that collectively conducted water 54 % more efficiently – but only during the years for which moisture was supplemented. In contrast, above-ground growth parameters showed insignificant or inconsistent responses.
Conclusions
The phenotypic changes documented represent consistent, dynamic responses to increased moisture availability that should increase plant competitive ability. The functional plasticity of xylem anatomy quantified in this study constitutes a mechanistic basis for anticipating the differential success of plant species in response to climate variability and change, particularly where water limitation occurs.
Keywords: Alpine tundra, climate change, drought stress, functional anatomy, herb-chronology, hydraulic conductivity, intervascular xylem, phenotypic plasticity, Potentilla diversifolia, specific leaf area, starch, vessel size
INTRODUCTION
The ability to modify traits related to life history, structure and function (e.g. growth rate, reproductive effort, longevity, root : shoot ratio, water-use efficiency, hydraulic efficiency) largely determine the success of plant species (Valladares and Sanchez-Gomez, 2007; Matesanz et al., 2010). Particularly, perennial herbaceous species with long generation cycles growing in marginal and rapidly changing environments are challenged by recent and projected climate change. Climate change has and increasingly will affect the dynamics of herbaceous vegetation. Indeed, changes at the species, community and ecosystem level have been widely documented for many regions in the world (e.g. Jump and Penuelas, 2005; Hof et al., 2011). To understand the mechanistic basis for changes in herbaceous vegetation, studies grounded in long-term integration across plant life spans are necessary, yet conspicuously absent.
Phenotypic plasticity and genetic variability in structural and functional traits related to water balance may be particularly important for survival of herbaceous species in water-limited ecosystems and in systems characterized by high levels of rainfall variability. There are several mechanisms operating at different organizational levels by which a plant can dynamically adjust to temporal changes in soil moisture availability: e.g. by changing root : shoot ratios (Fay et al., 2003); by altering specific leaf area (SLA) (Wright et al., 2002); by adjusting permeability of cell membranes (Javot and Maurel, 2002); and by controlling stomatal conductance (Maseda and Fernandez, 2006).
Other suites of adjustments conferring drought tolerance and water use efficiency – that are the focus of this study – lie in the functional anatomy of the hydraulic system. Efficiency of the hydraulic system depends on a variety of factors, including vessel length (Comstock and Sperry, 2000), resistance of end wall plates (Hacke et al., 2006), pathway redundancy (Carlquist, 2001; Cai and Tyree, 2010), and the capacity to refill embolized vessels (Salleo et al., 2006). Vessel diameter also has a direct influence on plant function and performance. Wide xylem vessels conduct water more efficiently than narrow ones, since hydraulic conductivity increases with the radius at the fourth power (Poiseuille's law; Tyree and Ewers, 1991). However, wide vessels are also more vulnerable to dysfunction caused by emboli that form under strong negative water potential and block water transport. This may lead to trade-offs between safety and efficiency and/or survival and competitive ability (Hacke and Sperry, 2001; Maseda and Fernandez, 2006; Hacke et al., 2009). Consequently, during drought, roots and stems containing a high proportion of narrow xylem vessels may effectively transport more water than those with wide, but easily embolized vessels (Stevenson and Mauseth, 2004). Although there is a genetic component to vessel-size distribution in most species (e.g. Carlquist, 2001; Christensen-Dalsgaard et al., 2008), phenotypic plasticity in this trait would enable individuals and species to dynamically adjust to the wide range of moisture availability experienced during their lifetimes. The extent to which this facet of hydraulic architecture might vary with environmental conditions is largely unknown, however, and is the focus of this study.
Storage of non-structural carbohydrates (NSC) such as starch is yet another potentially dynamic mechanism of coping with temporal variability in soil moisture availability. These reserves may partly mitigate the risks of a more efficient, but riskier hydraulic architecture, because the hydrolysis of starch to sugar plays a central role in refilling embolized xylem vessels. Although the exact mechanism of vessel refilling is unknown, the resultant sugar seems to provide the energy and/or increase in osmotic potential needed to remove emboli (Salleo et al., 2006; Secchi and Zwieniecki, 2011). In addition, NSC reserves such as starch represent stored energy that would allow a plant to avert starvation when stomata are closed to prevent dehydration during dry periods (McDowell et al., 2008; Sala et al., 2010). Roots of herbaceous plants are rich in starch (Salisbury and Ross, 1992), particularly when growing at high altitudes (Daubenmire, 1941; Tolsma et al., 2007). However, its allocation in roots is not uniform; rather, starch is concentrated in parenchyma cells in the intervascular root xylem (Bell et al., 1996; Bell and Ojeda, 1999). The abundance of intervascular root xylem may thus be used as an indicator of a plant's need and capacity to store starch (Raven and Evert, 1992; Bowes, 1996). In a hydraulic context, these pools of energy increase a plant's potential resistance to drought stress. The development of intervascular xylem is always at the expense of the vascular xylem; and allocation to one or the other tissue may thus represent another set of safety versus efficiency and storage versus growth trade-offs (Chapin et al., 1990).
Here, we report results from a novel integrative approach that quantifies long-term phenotypic plasticity in vessel diameter and intervascular xylem development in roots of plants from two sub-populations of the perennial herb Potentilla diversifolia (varileaf cinquefoil; Rosaceae) growing at sites with contrasting soil moisture availability. Analysis of annual growth rings in persistent roots (a method termed ‘herb-chronology’, which is similar to dendrochronology, the study of annual growth rings in tree stems) is a way to quantify plant age (e.g. Dietz and Ullmann, 1997; von Arx and Dietz, 2006) and annual growth rates (Dietz and Ullmann, 1998; von Arx et al., 2006). We expanded herb-chronological analysis to include quantification of root xylem vessels and intervascular xylem abundance, and combined it with more traditional growth and eco-physiological parameters.
We hypothesized that plants receiving supplemental soil moisture would exhibit dynamic adjustments, including (a) a shift towards wider vessels, (b) greater hydraulic conductivity, (c) a reduction in the abundance of intervascular xylem, and (d) an increase in SLA (Table 1). We also sought to determine whether any observed changes in functional traits would co-vary with other, more traditional growth parameters. Results are discussed in the context of how variation in the degree of this plasticity might determine which species are best suited to cope with climate variability and to adjust to climate change.
Table 1.
Overview of hypothesized and observed responses to higher moisture availability in traits related to water balance in Potentilla diversifolia plants on the Niwot Ridge alpine tundra LTER site (Colorado, USA)
| Trait* | Hypothesized | Observed |
|---|---|---|
| Percentage wide vessels | ↑ | ↑ |
| Potential maximum conductivity | ↑ | ↑ |
| Percentage intervascular xylem | ↓ | ↓ |
| Specific leaf area (SLA) | ↑ | ↑ |
* Traits developing after installation of a snow fence in 1993 that divided the population into a drier windward group and a moister lee-side group.
MATERIALS AND METHODS
Study site
This study was conducted on alpine tundra on either side of a snow fence (60 m long; 2·6 m tall) established on the Niwot (NWT) Ridge Long-Term Experimental Research site (105°35′W, 40°03′N) in summer 1993 (Fig. 1) (Walker et al., 1999). NWT is located in the Rocky Mountains Front Range, Colorado, USA. The snow fence is situated on a south–north-oriented ridge-top with negligible slope (0–2°) at 3528 m a.s.l. Mean (1951–1985) annual precipitation at NWT is 930 mm, but interannual variability is high and ranges from 540 to 1430 mm (Greenland, 1989). Precipitation during growing season (June–August; mean ± 1 s.d.; 1982–2007) is 209 ± 71 mm, usually arriving in brief convective storms. Two-thirds of all precipitation leaves the system as run-off and about one -third via evapotranspiration (Greenland, 1989). Air temperature (mean ± 1 s.d.; 1987–2007) is –9·6 ± 3·1 °C in January and 9·9 ± 2·1 °C in July. Winds are primarily westerly and perpendicular to the snow fence with an average speed of 11·5 ± 2·0 m s−1 in January and 4·3 ± 1·2 m s−1 in July. Since the snow fence was removed during the summer except for the supporting poles (Walker et al., 1999) (see Fig. 1) we do not expect any influence on ambient wind, light and temperature conditions during the growing season. Soils are Cryochrepts with a depth of approx. 2·0 m over granitic parent material. Soil organic C in the top 0·1 m ranges from 130 to 200 g kg−1 and soil N from 9 to 15 g kg−1. Vegetation consists of dry, moist and wet meadow communities. The dominant plant species are the gramminoids Kobresia myosuroides and Deschampsia caespitosa, and the forb Acomostylis rossii (NWT LTER, 2011).
Fig. 1.
Snow-fence study site at the Niwot Ridge alpine tundra LTER site, Colorado, USA: (A) panoramic view from south-east (August 2007); (B) aerial view (Google Earth™) in mid-May to early June 2002 showing the snow fence and the location of the belt transects (white and black rectangles, respectively) on the windward (westerly) and lee (easterly) side. Snow accumulation on the lee side reduces length of the growing season by approx. 16 %, but increases moisture availability.
Snow accumulates on the lee (east) side of the fence (Fig. 1B) and on average (1994–2007), persists through the first week of July, which is about 3 weeks later than snowmelt on the windward (west) side. As a result, the growing season on the lee side (approx. 100 d) is about 16 % shorter. Despite the considerable precipitation in many alpine tundra ecosystems, plant-available water in exposed locations may be depleted within 1 week of snowmelt (Oberbauer and Billings, 1981). In the moister lee side of the fence, soil moisture (mean ± 1 s.d.; 1994–2002) declines from 24·7 ± 7·3 % v/v (TDR at 0·15 m soil depth) in June to 18·1 ± 8·9 in July and 14·8 ± 8·8 % v/v in August. For the drier windward transect no soil moisture data are available; however, means (2000–2006) from a nearby, comparable location are 11·9 % v/v in July (TDR at 0·15 m soil depth). Soil temperature during the summer months (JJA 1995; mean ± 1 s.d.) is comparable on the lee (8·1 ± 2·9 °C) and windward sides (7·0 ± 1·4 °C; t-test, t = 0·995, P = 0·335). Plant productivity, soil organic matter and microbial activity are generally higher at the moister lee side than the drier windward side (Fisk et al., 1998; Williams et al., 1998; Litaor et al., 2002). All data in the above two paragraphs, if not cited otherwise, is courtesy of the NWT LTER site (see http://culter.colorado.edu/NWT/ and Acknowledgements for list of investigators).
Study species
Potentilla diversifolia Lehm. (Rosaceae) is a polycarpic, dicotyledonous perennial herb (forb) with a persistent main root that occurs in alpine and sub-alpine meadows across the western and north-eastern regions of North America. It forms rosettes and produces several semi-erect, branched stems that grow up to 30 cm tall at the study site. Growth rate is moderate and rooting depth intermediate. Potentilla diversifolia has low fertility and medium water requirements and grows in fine- to coarse-textured soils. Its drought tolerance is low, and it usually occurs in areas with an annual precipitation of 200–900 mm and a minimum frost-free period of 100 d (species characterization follows Oberbauer and Billings, 1981; Stinson, 2005).
Data collection and processing
In late August 2007, 22 individuals were collected from each side of the snow fence along transects, 10–14 m wide, parallel to the fence (Fig. 1B). The lee-side transect was placed at 1 m and the windward transect at 40 m distance from the snow fence. Sampling followed a stratified random design, wherein the first two plants encountered at 5-m intervals were selected. The number of shoots, maximum shoot length, number of flowers, number of rosettes, number of leaves, and maximum leaf length were recorded for each plant. Root stocks and three representative leaves were collected from each plant and bagged for functional root analysis and determination of SLA. SLA was computed as leaf area (measured on a CID-251 leaf area meter; CID Inc., Vancouver, WA, USA) divided by dry mass.
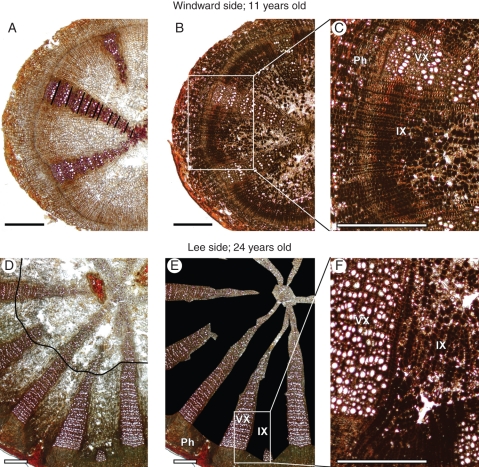
The proximal segment of the main root of each individual was preserved in a 50 % iso-propanol solution. Thin cross-sections (30 µm) were obtained from the proximal end of the main root (root collar) using a sledge microtome. Lignified structures – walls of xylem vessels and lignified walls of parenchyma cells in the vascular xylem in P. diversifolia – were stained reddish using phloroglucinol–HCl. Stained cross-sections were then photographed through the oculars of a transmitting microscope (Olympus BX51, ×20 magnification) using a standard digital camera (Nikon Coolpix 990; von Arx and Dietz, 2006). Multiple overlapping images were taken from each sample and stitched together using PTGui (New House Internet Services B.V., Rotterdam, The Netherlands) to obtain high-resolution images of the entire cross-sections. The stitching process also effectively removed spatial distortions introduced by the optical systems. Pixel-to-micron resolution was determined by a calibration function obtained from photographing a stage micrometer with different camera zoom levels. Intervascular xylem parenchyma was verified to be the main location of starch in a random sub-sample of five individuals from each side of the snow fence after applying Lugol's solution to thin sections, which colours grains of starch black (Fig. 2B, C, F; cf. Bell et al., 1996; Salleo et al., 2006).
Fig. 2.
Root cross-sections (30 µm) of Potentilla diversifolia plants growing on opposite sides of a snow fence at the Niwot Ridge alpine tundra LTER site (Colorado, USA) as captured through the oculars of a microscope. Lignified structures are stained reddish with phloroglucinol–HCl in (A), (D) and (E); starch-containing cells appear black after staining with Lugol's solution in (B), (C) and (F). (A) Individual sampled on the drier windward side, 11 years old; black markers indicate annual ring boundaries. (B, C) Another cross-section from the same individual; note that parenchyma cells containing starch mainly occur in the intervascular xylem (IX) and the phloem (Ph), but not in the vascular xylem (VX). (D) Individual sampled on the moister lee side, 24 years old; the black line indicates the transition from 1993 to 1994 when the snow fence was established. (E) Same cross-section as in (D) with intervascular xylem blacked out to illustrate boundaries between vascular and intervascular xylem; the percentage area of intervascular xylem in this cross-section is 67 % as compared with 77 % in the windward-side individual depicted in (A–C). (F) Enlargement of a portion of (E) shown in the same scale as (C); the vessel size distribution in this specific lee-side plant conducts water 64 % more efficiently than the one in the windward individual in (C), when only considering the period after establishment of the snow fence. Scale bars = 500 µm.
Plant age was determined by counting the number of annual rings in the digital images. In cases where the central part of the root had decomposed (n = 6 plants), radial axes were extrapolated and the root centre was assumed to occur where the radial axes intersected. A (conservative) minimum and (non-conservative) maximum number of missing rings was then estimated on the basis of the mean width of present rings and added to the count of present rings. The minimum and maximum number of annual rings was also estimated when several or all annual ring boundaries were indistinct. In both cases the mean of minimum and maximum number of annual rings was used as an estimate of plant age (von Arx et al., 2006). All xylem vessels, widths of annual rings and radial rays of intervascular xylem were quantified in the digital images of the entire cross-sections using ‘ROXAS’. ROXAS is an image analysis tool developed by the first author (von Arx and Dietz, 2005; von Arx, 2011) that is based on the image manipulation and registration capability of Image Pro Plus v4.5-v6.3 (Media Cybernetics, Silver Spring, MA, USA). Mean ring width was obtained either by ROXAS or, where some annual ring boundaries were indistinct, by dividing the mean xylem radius by estimated plant age (von Arx et al., 2006). The daily radial root growth rate was obtained by dividing mean ring width by the estimated average number of growing-season days (100 for the lee side during the post-snow fence period, 119 for all the others).
Data analysis
Age, means of all growth parameters, and of the abundance of intervascular xylem were compared between plants on opposite sides of the snow fence using a two-sample t-test (if variances were equal; ANOVA, P > 0·05) or a Welch two-sample t-test (if variances were unequal; ANOVA, P ≤ 0·05; R Development Core Team, 2010). Data of the two sub-populations were normally distributed in all instances (Shapiro–Wilk's test, P > 0·05), so no transformations were required before statistical testing.
The efficiency and safety of the hydraulic xylem characteristics of the two sub-populations were evaluated using three approaches. Hydraulic architecture was assessed by grouping the vessels of each plant into 50-μm2 classes and calculating the mean relative frequency of each vessel cross-sectional area class (hereafter simply termed ‘vessel size distribution’). The contribution of each vessel size class to overall hydraulic conductivity was expressed as Poiseuille's law for ideal capillaries (the fourth power of its mean radius; Tyree and Ewers, 1991) weighted by the relative frequency of the corresponding vessel size class (Mauseth and Stevenson, 2004). Relative cumulative conductivity curves were derived from this to assess the percentage of overall conductivity attained by vessels up to a specific size class. Significant differences between the two sub-populations were evaluated for all three approaches using a two-sample Kolmogorov–Smirnov test (Crawley, 2005). Only vessels >50 µm2 were considered in the analysis because narrower vessels could not be reliably detected with the chosen image resolution. Since the contribution of the narrowest vessels to an individual's overall conductivity is negligible (Mauseth and Stevenson, 2004; Fonti et al., 2010), we assumed that exclusion of vessels <50 µm2 would not impair any conclusion drawn from the results. Plant age, ring widths, daily growth rates, abundance of intervascular xylem, and the hydraulic xylem characteristics were analysed separately for the years before and after the snow fence was erected (1993) to verify whether the two sub-populations started out the same with respect to the considered traits in the pre-fence period. Among plants with partially indistinct annual ring boundaries, one to two annual rings on either side of the assumed 1993/1994 date were excluded from analysis to minimize the chance that xylem tissue formed during the pre-snow fence period would be mistakenly classified as tissue formed during the post-snow fence period (and vice versa). Furthermore, the first three years in each individual's life were omitted so as to confine our assessments to the adult stages of the plant life cycle and not confound these with structural attributes occurring during early developmental stages of the life cycle. All statistical analyses were performed in R version 2.12.1 (Crawley, 2005; R Development Core Team, 2010).
RESULTS
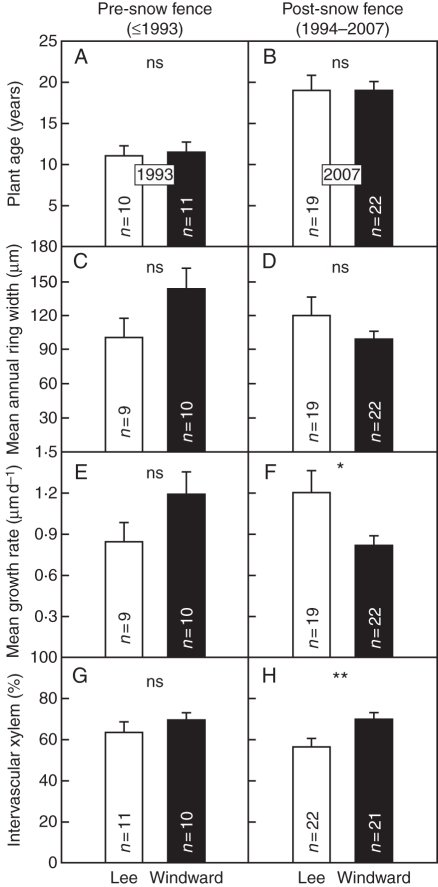
Plants sampled from the moister lee side and drier windward side of the snow fence were of statistically comparable age (Table 2 and Fig. 3A, B). Above-ground growth parameters did not differ consistently between plants from the opposite sides of the snow fence. Lee-side plants tended to have fewer rosettes and fewer, but significantly larger, leaves. While plants on the moister lee side produced significantly fewer, but longer, shoots, the number of flowers per plant was comparable in both sub-populations. Likewise, long-term mean growth (mean ring width) did not differ significantly between plants from the two sub-populations during the years before and after installation of the snow fence (Table 2 and Fig. 3C, D). However, since the effective growing season was typically approx. 16 % shorter on the moister lee side of the fence, the daily growth rate during the growing season was significantly higher in the lee-side plants than in the windward-side plants (Table 2 and Fig. 3E, F).
Table 2.
Summary of growth, structural and anatomical traits of Potentilla diversifolia plants collected from the drier windward and the moister lee side of a snow fence installed in 1993 at the Niwot Ridge alpine tundra LTER (Colorado, USA)
| Lee (mean ± 1 s.e.) | Windward (mean ± 1 s.e.) | t | d.f. | P | |
|---|---|---|---|---|---|
| Overall | |||||
| Plant age (years) | 19·0 ± 1·9 | 18·9 ± 1·1 | 0·049* | 35 | 0·961 |
| No. of rosettes | 3·7 ± 0·5 | 5·0 ± 0·6 | –1·703* | 42 | 0·096 |
| No. of leaves | 14·8 ± 1·8 | 19·6 ± 2·0 | –1·759* | 42 | 0·086 |
| Leaf length (×10−3 m) | 7·4 ± 0·4 | 6·6 ± 0·4 | 1·488 * | 42 | 0·144 |
| Leaf area (×10−6 m2) | 7·2 ± 0·7 | 4·0 ± 0·4 | 3·739† | 34·1 | < 0·001 |
| No. of shoots | 2·1 ± 0·3 | 3·8 ± 0·6 | –2·504† | 23·0 | 0·020 |
| Shoot length (×10−2 m) | 19·5 ± 1·3 | 13·5 ± 1·2 | 3·087* | 24 | 0·005 |
| No. of flowers | 7·3 ± 0·8 | 6·7 ± 1·2 | 0·405* | 26 | 0·689 |
| Specific leaf area (m2 kg−1) | 18·0 ± 0·8 | 14·6 ± 0·3 | 4·298† | 27·9 | < 0·001 |
| No. of intervascular xylem rays | 14·9 ± 1·2 | 15·9 ± 1·8 | –0·479* | 41 | 0·635 |
| Pre-snow fence (≤1993) | |||||
| Mean ring width (μm) | 101 ± 16 | 143 ± 19 | –1·700* | 17 | 0·107 |
| Intervascular xylem (%) | 64·4 ± 4·4 | 69·56 ± 3·8 | –0·880* | 19 | 0·390 |
| Post-snow fence (1994–2007) | |||||
| Mean ring width (μm) | 121 ± 16 | 98 ± 8 | 1·258† | 27·5 | 0·219 |
| Daily radial root growth (μm) | 1·2 ± 0·2 | 0·8 ± 0·1 | 2·203† | 24·8 | 0·037 |
| Intervascular xylem (%) | 56·9 ± 3·8 | 70·7 ± 2·8 | –2·874* | 41 | 0·006 |
Bold font highlights statistically significant (P < 0·05) differences between lee- and windward plants.
* Two-sample t-test.
† Welch two-sample t-test.
Fig. 3.
Comparison of mean (± 1 s.e.) age (A, B), annual ring width (C, D), growth rate per growing season day (E, F) and percentage of cross-sectional area characterized by intervascular xylem (G, H) for individual Potentilla diversifolia plants growing on the drier windward and moister lee side of a snow fence at the Niwot Ridge alpine tundra LTER site (CO, USA). (A), (C), (E) and (G) are for pre-snow fence years; (B), (D), (F) and (H) for post-snow-fence years. Note that mean age values in (A) and (B) do not represent means over the pre- and post-snow-fence periods as in the parameters of the other panels, but means just before installation of the snow fence in 1993 and at the sample date in 2007, respectively. n = number of individual plants; ns, not significant; *, P < 0·05; **, P < 0·01.
In contrast to the non-significant or inconsistent differences in most above-ground growth parameters, parameters directly related to plant water balance showed a consistent, significant response in the hypothesized directions (Table 1). SLA was significantly greater in lee-side plants (Table 2). The contribution of intervascular xylem to root cross-sectional area was statistically comparable in the two subpopulations in the years before the installation of the snow fence (Table 2 and Fig. 3G). However, in the years following snow-fence installation, plants on the moister lee side showed significantly reduced development of their intervascular xylem compared with plants on the drier windward side (Table 2 and Fig. 3H). This reflects the occurrence of narrower intervascular xylem rays on lee-side plants, as the number of intervascular xylem rays did not differ significantly (Table 2).
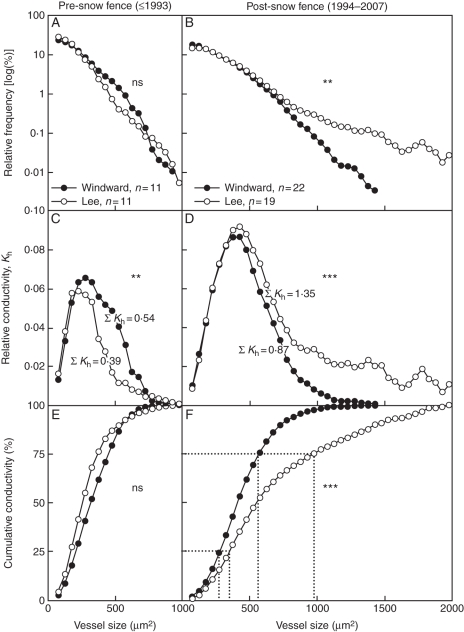
Vessel size distributions in the two sub-populations were comparable during the pre-snow-fence period (D = 0·158, P = 0·972; Fig. 4A). After installation of the snow fence and the attendant increases in soil moisture, vessel size distributions diverged significantly, with lee-side plants having greater maximum vessel sizes (2060 vs. 1389 µm2) and a higher frequency of wide vessels (D = 0·410, P = 0·003; Fig. 4B) than plants on the drier windward side. The contribution of each vessel size class to overall hydraulic conductivity also differed significantly between the two sub-populations for the pre- and the post-snow periods (D = 0·556, P = 0·004 and D = 0·641, P < 0·001, respectively; Fig. 4C, D). The vessel size class contributing most to overall conductivity was 275 µm2 and 225 µm2 in windward and lee-side plants, respectively, during the pre-snow-fence period. This increased to 425 µm2 for each group of plants during the post-snow-fence period, reflecting ontogenetic trend with increasing age. Plants on opposite sides of the snow fence were comparable with respect to cumulative conductivity during the pre-snow-fence period (D = 0·167, P = 0·964; Fig. 4E), becoming significantly different during the post-snow-fence period (D = 0·462, P < 0·001; Fig. 4F).
Fig. 4.
Mean xylem hydraulic properties of Potentilla diversifolia plants growing on the drier windward and moister lee side of a snow fence at Niwot Ridge (CO, USA): (A, B) relative frequency distribution of vessel size classes (simply termed ‘vessel size distribution’ in the text; note the logarithmic scale); (C, D) contribution of each vessel size class to overall hydraulic conductivity (denoted as Kh); (E, F) relative cumulative conductivity curves derived from vessel size distribution. (A), (C) and (E) are for pre-snow fence years; (B), (D) and (F) for post-snow-fence years. Dotted lines in (F) illustrate the remaining xylem conductivity that would be expected if embolisms rendered vessels greater than a specific size non-functional. See text for further explanations. A three-value simple moving average was used to smooth out curves for representation purposes, but not when statistical testing. n = number of individual plants; ns, not significant; **, P < 0·01; ***, P < 0·001.
The shift in vessel size distribution observed in plants on the moister lee side represents a dynamic change from a stress-avoidance hydraulic architecture to an architecture that would allow plants to take advantage of the increased soil-moisture availability by enabling them to conduct about 54 % more water than plants on the drier windward side (relative overall conductivity 1·35 vs. 0·87, cf. Fig. 4D). When taking into account the fact that lee-side plants contained more vessels (due to a greater abundance of vascular xylem as described above) and comparable vessel densities (131 ± 18 × 106 m−2 vs. 120 ± 21 × 106 m−2, respectively; Wilcoxon signed-rank test, P = 0·460) this difference potentially increases to 126 % in the post-snow-fence period. This greater abundance of vessels also means that were a lee-side plant hypothetically to lose its largest vessels to embolisms such that it was operating at 75 % capacity it could potentially still move more water than a windward plant at 100 % capacity. In summary, there was a pronounced shift in xylem structure from an architecture that would minimize embolisms and attendant stresses among plants occurring in the dry habitat to an architecture that would promote water transport and growth among plants in the moister habitat.
DISCUSSION
Plant species able to rapidly use water and nutrients and thus attain high growth rates usually are most successful in environments with an abundance of resources. In contrast, in environments where resources are scarce, more conservative or stress-tolerant strategies associated with slower growth rates prevail (Grime, 2001; Bornhofen et al., 2011). Recent and projected climate change may lead to a mismatch between the life-history strategy and range distributions of plant species if rapid changes occur within the lifetimes of individual plants or within a few generations (Jump and Penuelas, 2005). Long-term experiments like the one in this study are particularly useful to elucidate the capacity of a species to adjust and respond to changes in resource availability by dynamically shifting between traits conferring stress tolerance and competitive ability. A snow fence established in 1993 changed conditions on the lee side towards a higher availability of intermittently scarce water while reducing the length of the growing season and, though not explicitly considered here, possibly affecting productivity. This study illustrates the mechanisms by which Potentilla diversifolia plants phenotypically adjusted their hydraulic architecture in response to these experimentally induced changes in growing season conditions.
The fact that the considered vegetative and reproductive growth parameters of plants on the moister lee side of the snow fence exhibited no or inconsistent difference relative to that of plants on the drier windward side suggests that enhanced growth responses to snow-induced improvements in moisture availability were off-set by decreases in the number of growing season days. However, plants growing in the moister habitat created by the snow fence had consistently developed anatomical and morphological traits associated with release from drought stress (Table 2). These changes in hydraulic architecture apparently enabled plants to fully compensate for reductions in annual growth that might otherwise have accompanied a snow-induced shortening of the growing season. It thus appears that plasticity in root anatomy enabled P. diversifolia to dynamically switch from traits conferring drought-stress tolerance on drier habitats to traits conferring enhanced growth (via improved hydraulic conductivity) in moister habitats and thus dynamically adjust to directional changes in soil moisture availability.
Structural responses to changes in moisture availability
Plants growing at moister sites are often reported to have greater SLA relative to those on drier sites (e.g. Preston and Ackerly, 2003; Poorter et al., 2009) as was found in this study. The lower SLA observed in plants growing on the drier alpine habitat reflects allocation favouring greater leaf structural strength and resistance to prevailing dehydration stresses. In contrast, the higher SLA observed on plants growing in the moister habitat where the frequency or intensity of drought stress is presumably lower, reflects a leaf architecture that maintains its surface area for light capture, but with shorter payback time per gram of dry matter invested (Wright et al., 2002), thus freeing up biochemical resources for use elsewhere in the plant. This explains the nearly 50 % greater growth rates of lee-side plants (Table 2, Fig. 3F).
Changes in leaf structure evident in the year of sampling were accompanied by long-term changes in root structure. As hypothesized (Table 1), the proliferation of intervascular xylem was reduced in response to soil-moisture supplementation (e.g. Fig. 3C, D). Since storage of starch is one of the principal functions of intervascular xylem in roots (Bell et al., 1996; Bell and Ojeda, 1999), a reduction in allocation to these tissues among plants in moist habitats represents a shift towards a more competitive strategy in which a higher proportion of produced sugars are allocated to hydraulic efficiency and growth rather than storage functions (Metcalf et al., 2006). As with shifts in leaf structure, these changes in root anatomy contributed to the ability of plants to fully compensate for reductions in the number of growing season days on the snow-rich lee side. When starch resources are depleted during drought to prevent starvation or to refill embolized vessels (see Introduction), a high starch storage capacity as observed in windward-side plants may play a crucial role in enabling plants to endure periods of drought stress, therefore increasing the probability of long-term survival (Chapin et al., 1990). Plasticity in allocation to growth versus stress-tolerance functions would be key for enabling plants to adjust to changes in water availability that inevitably occur in most terrestrial ecosystems and successfully complete their life cycle (Matesanz et al., 2010).
The observed shift of vessel size distribution (Fig. 4A, B) fits well within the hypothesized concept of a safety/survival versus efficiency/competitive ability trade-off. Upon release from drought stress, lee-side plants shifted the distribution of vessel sizes towards larger diameter vessels under conditions where the risk of hydraulic system failure was reduced, thus enabling them to capitalize on the increased availability of soil moisture (e.g. Lovisolo and Schubert, 1998; but see Maherali et al., 2004). This explanation is in line with the observed increase in SLA, higher growth rates and the reduced investment in intervascular xylem in exchange for a proportionally greater investment in vascular xylem; and is evidence that optimizing the performance of an organism in response to environmental change involves concomitant adjustments of several traits (see also Markesteijn et al., 2011).
The investment in larger vessels by plants from the moister lee side may not have much of a down-side in terms of water transport in the sense that even if a substantial number of the largest vessels were lost to embolisms plants could still, hypothetically, move as much or more water than plants with size-class distributions shifted to smaller vessels. There may, however, be a loss of the metabolic investments made to larger vessels if they become non-functional, or an energetic cost associated with refilling wide embolized vessels. Threshold vessel sizes for maintaining 75 % of maximum conductivity differed markedly between plants from the two sub-populations (Fig. 4F), whereas vessel sizes required to maintain 25 % of maximum conductivity was comparable. This reflects the great influence of a few wide vessels on conductivity (Tyree and Ewers, 1991; Fonti et al., 2010), but may also reflect a greater plasticity in the production of wide (earlywood) vessels. The question as to whether earlywood or latewood vessels are more plastic is controversial (e.g. Woodcock, 1989; Fonti and Garcia-Gonzalez, 2008). From a functional viewpoint, our results suggest that investments in narrow vessels that ensure survival during drought periods are relatively fixed, whereas investments in wider vessels may be more plastic and dynamically responsive to resource availability. This view gains support from observations in trees that 5–20 % losses of conductance are quite common in a ‘native’ state, i.e. during normal transpiration rates (Tyree and Ewers, 1991), whereas severe wilting or death occurs when conductance losses approach 70–85 % (Tyree et al., 2002, 2003). Furthermore, vessel sizes contributing most to overall conductivity respond minimally to changes in moisture availability (Fig. 4C, D). The vessels contributing to this conductivity range may represent a stable optimization of sizes between those larger than the minima critical for survival, but smaller than those that would be at high risk failure because of embolism in the prevailing environment.
Plasticity in functional anatomy and climate change
In a climate change/climate variability context, the structural plasticity observed in this study – whether of phenotypic (most likely in this study) or genetic origin – may constitute an increasingly important adaptive feature (Matesanz et al., 2010; but see Hof et al., 2011). Although higher temperatures in spring and autumn could have a positive effect of prolonging the length of the growing season in tundra ecosystems, these benefits may not be realised if higher summer temperatures induce more plant stress by increasing atmospheric water demand and reducing the availability of water for plant growth while increasing leaf temperatures and potential transpiration rates (e.g. Jobbagy et al., 2002). Phenotypic adjustments to changes in water availability may be crucial to reproduction and survival while providing a (relative) competitive advantage over species with a smaller capacity for plasticity (Matesanz et al., 2010). Furthermore, depending on the time required to adjust functional anatomy, plants may be seriously challenged when faced with a increase in the projected frequency and magnitude of extreme climatic events (IPCC, 2007), This would be particularly so if short-term physiological responses are not sufficient to enable plants to survive long enough to adjust their morphology. Ultimately, species differences in functional plasticity are likely to drive changes in community composition (Berg and Ellers, 2010). A broader data set than the one collected here is required, however, to verify these considerations.
Conclusions and perspectives
The integrative approach taken here constitutes an example of a framework for connecting and integrating physiology, anatomy and ecology. To our knowledge, this is one of the first studies to provide empirical support for the safety versus efficiency concept of vessel size and its phenotypic plasticity in an ecologically relevant field situation (but see Herbette et al., 2010). A particularly important finding in our study was the existence of concomitant plastic responses of functional traits related to maintaining water balance in the face of changes in growing-season conditions. Our results suggest that, in environments where water limitations occur, metrics related to hydraulic properties may be better suited to assess functional responses to changes in moisture availability than traditional growth parameters (Fonti et al., 2010). This would be particularly so in the face of the multi-faceted changes in resource availability that will accompany climate change.
ACKNOWLEDGEMENTS
We thank W. D. Bowman for valuable discussions and permission to sample in the study area. Logistical support and data were provided by the NSF-supported Niwot Ridge Long-Term Ecological Research project (NSF DEB 0423662) and the University of Colorado Mountain Research Station (BIR 115097). Precipitation, air temperature and wind data were provided by M. Losleben, snow cover and soil temperature data by D. A. Walker, soil moisture data by T. Seasteadt. We thank D. Killick for providing laboratory facilities at the School of Anthropology (University of Arizona), C. Beretta for assistance in the laboratory and C. M. Hulsof for valuable comments on a previous version of this manuscript. This project was supported by a grant from Swiss National Science Foundation (PBEZA-117266) to G.v.A. and was conducted while M.K.H. was a Visiting Fellow at the Cooperative Institute for Environmental Studies, University of Colorado, Boulder.
LITERATURE CITED
- von Arx G. ROXAS – a tool for the quantitative analysis of xylem anatomy. 2011 http://www.wsl.ch/roxas . [Google Scholar]
- von Arx G, Dietz H. Automated image analysis of annual rings in the roots of perennial forbs. International Journal of Plant Sciences. 2005;166:723–732. [Google Scholar]
- von Arx G, Dietz H. Growth rings in the roots of temperate forbs are robust annual markers. Plant Biology. 2006;8:224–233. doi: 10.1055/s-2005-873051. [DOI] [PubMed] [Google Scholar]
- von Arx G, Edwards PJ, Dietz H. Evidence for life history changes in high altitude populations of three perennial forbs. Ecology. 2006;87:665–674. doi: 10.1890/05-1041. [DOI] [PubMed] [Google Scholar]
- Bell TL, Ojeda F. Underground starch storage in Erica species of the Cape Floristic Region – differences between seeders and resprouters. New Phytologist. 1999;144:143–152. [Google Scholar]
- Bell TL, Pate JS, Dixon KW. Relationships between fire response, morphology, root anatomy and starch distribution in south-west Australian Epacridaceae. Annals of Botany. 1996;77:357–364. [Google Scholar]
- Berg MP, Ellers J. Trait plasticity in species interactions: a driving force of community dynamics. Evolutionary Ecology. 2010;24:617–629. [Google Scholar]
- Bornhofen S, Barot S, Lattaud C. The evolution of CSR life-history strategies in a plant model with explicit physiology and architecture. Ecological Modelling. 2011;222:1–10. [Google Scholar]
- Bowes BG. A colour atlas of plant structure. London: Manson Publishing; 1996. [Google Scholar]
- Cai J, Tyree MT. The impact of vessel size on vulnerability curves: data and models for within-species variability in saplings of aspen, Populus tremuloides Michx. Plant, Cell & Environment. 2010;33:1059–1069. doi: 10.1111/j.1365-3040.2010.02127.x. [DOI] [PubMed] [Google Scholar]
- Carlquist S. Comparative wood anatomy: systematic, ecological, and evolutionary aspects of dicotyledon wood. Berlin: Springer; 2001. [Google Scholar]
- Chapin FS, Schulze ED, Mooney HA. The ecology and economics of storage in plants. Annual Review of Ecology and Systematics. 1990;21:423–447. [Google Scholar]
- Christensen-Dalsgaard KK, Ennos AR, Fournier M. Are radial changes in vascular anatomy mechanically induced or an ageing process? Evidence from observations on buttressed tree root systems. Trees – Structure and Function. 2008;22:543–550. [Google Scholar]
- Comstock JP, Sperry JS. Theoretical considerations of optimal conduit length for water transport in vascular plants. New Phytologist. 2000;148:195–218. [Google Scholar]
- Crawley MJ. Statistics: an introduction using R. Chichester, UK: John Wiley & Sons; 2005. [Google Scholar]
- Daubenmire RF. Some ecologic features of the subterranean organs of alpine plants. Ecology. 1941;22:370–378. [Google Scholar]
- Dietz H, Ullmann I. Age-determination of dicotyledonous herbaceous perennials by means of annual rings: exception or rule? Annals of Botany. 1997;80:377–379. [Google Scholar]
- Dietz H, Ullmann I. Ecological application of ‘herbchronology’: comparative stand age structure analyses of the invasive plant Bunias orientalis L. Annals of Botany. 1998;82:471–480. [Google Scholar]
- Fay PA, Carlisle JD, Knapp AK, Blair JM, Collins SL. Productivity responses to altered rainfall patterns in a C-4-dominated grassland. Oecologia. 2003;137:245–251. doi: 10.1007/s00442-003-1331-3. [DOI] [PubMed] [Google Scholar]
- Fisk MC, Schmidt SK, Seastedt TR. Topographic patterns of above- and belowground production and nitrogen cycling in alpine tundra. Ecology. 1998;79:2253–2266. [Google Scholar]
- Fonti P, Garcia-Gonzalez I. Earlywood vessel size of oak as a potential proxy for spring precipitation in mesic sites. Journal of Biogeography. 2008;35:2249–2257. [Google Scholar]
- Fonti P, von Arx G, García-González I, et al. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytologist. 2010;185:42–53. doi: 10.1111/j.1469-8137.2009.03030.x. [DOI] [PubMed] [Google Scholar]
- Greenland D. The climate of Niwot Ridge, Front Range, Colorado, U.S.A. Arctic and Alpine Research. 1989;21:380–391. [Google Scholar]
- Grime JP. Plant strategies, vegetation processes, and ecosystem properties. Chichester, UK: John Wiley & Sons; 2001. [Google Scholar]
- Hacke UG, Sperry JS. Functional and ecological xylem anatomy. Perspectives in Plant Ecology, Evolution and Systematics. 2001;4:97–115. [Google Scholar]
- Hacke UG, Sperry JS, Wheeler JK, Castro L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiology. 2006;26:689–701. doi: 10.1093/treephys/26.6.689. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Jacobsen AL, Pratt RB. Xylem function of arid-land shrubs from California, USA: an ecological and evolutionary analysis. Plant, Cell & Environment. 2009;32:1324–1333. doi: 10.1111/j.1365-3040.2009.02000.x. [DOI] [PubMed] [Google Scholar]
- Herbette S, Wortemann R, Awad H, Huc R, Cochard H, Barigah TS. Insights into xylem vulnerability to cavitation in Fagus sylvatica L.: phenotypic and environmental sources of variability. Tree Physiology. 2010;30:1448–1455. doi: 10.1093/treephys/tpq079. [DOI] [PubMed] [Google Scholar]
- Hof C, Levinsky I, Araújo MB, Rahbek C. Rethinking species’ ability to cope with rapid climate change. Global Change Biology. 2011;17:2987–2990. [Google Scholar]
- Solomon S, Qin D, Manning M, editors. IPCC. Summary for policymakers Climate change 2007: the physical science basis. Cambridge: Cambridge University Press; 2007. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- Javot H, Maurel C. The role of aquaporins in root water uptake. Annals of Botany. 2002;90:301–313. doi: 10.1093/aob/mcf199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobbagy EG, Sala OE, Paruelo JM. Patterns and controls of primary production in the Patagonian steppe: a remote sensing approach. Ecology. 2002;83:307–319. [Google Scholar]
- Jump AS, Penuelas J. Running to stand still: adaptation and the response of plants to rapid climate change. Ecology Letters. 2005;8:1010–1020. doi: 10.1111/j.1461-0248.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- Litaor MI, Seastedt TR, Walker DA. Spatial analysis of selected soil attributes across an alpine topographic/snow gradient. Landscape Ecology. 2002;17:71–85. [Google Scholar]
- Lovisolo C, Schubert A. Effects of water stress on vessel size and xylem hydraulic conductivity in Vitis vinifera L. Journal of Experimental Botany. 1998;49:693–700. [Google Scholar]
- McDowell N, Pockman WT, Allen CD, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- Maherali H, Pockman WT, Jackson RB. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology. 2004;85:2184–2199. [Google Scholar]
- Markesteijn L, Poorter L, Paz H, Sack L, Bongers F. Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant, Cell & Environment. 2011;34:137–148. doi: 10.1111/j.1365-3040.2010.02231.x. [DOI] [PubMed] [Google Scholar]
- Maseda PH, Fernandez RJ. Stay wet or else: three ways in which plants can adjust hydraulically to their environment. Journal of Experimental Botany. 2006;57:3963–3977. doi: 10.1093/jxb/erl127. [DOI] [PubMed] [Google Scholar]
- Matesanz S, Gianoli E, Valladares F. Global change and the evolution of phenotypic plasticity in plants. Annals of the New York Academy of Science. 2010;1206:35–55. doi: 10.1111/j.1749-6632.2010.05704.x. [DOI] [PubMed] [Google Scholar]
- Mauseth JD, Stevenson JF. Theoretical considerations of vessel diameter and conductive safety in populations of vessels. International Journal of Plant Sciences. 2004;165:359–368. [Google Scholar]
- Metcalf CJE, Rees M, Alexander JM, Rose K. Growth-survival trade-offs and allometries in rosette-forming perennials. Functional Ecology. 2006;20:217–225. [Google Scholar]
- NWT LTER. Niwot Ridge Long-Term Ecological Research Site. 2011 http://culter.colorado.edu/NWT/ [Google Scholar]
- Oberbauer SF, Billings WD. Drought tolerance and water-use by plants along an alpine topographic gradient. Oecologia. 1981;50:325–331. doi: 10.1007/BF00344971. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R. Tansley review. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. (with erratum in 183: 1222) [DOI] [PubMed] [Google Scholar]
- Preston KA, Ackerly DD. Hydraulic architecture and the evolution of shoot allometry in contrasting climates. American Journal of Botany. 2003;90:1502–1512. doi: 10.3732/ajb.90.10.1502. [DOI] [PubMed] [Google Scholar]
- Raven PH, Evert RF. Biology of plants. New York, NY: Worth Publishers; 1992. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. http://www.r-project.org/ [Google Scholar]
- Sala A, Piper F, Hoch G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytologist. 2010;186:274–281. doi: 10.1111/j.1469-8137.2009.03167.x. [DOI] [PubMed] [Google Scholar]
- Salisbury FB, Ross CW. Plant physiology. Belmont, CA: Wadsworth; 1992. [Google Scholar]
- Salleo S, Trifilo P, Lo Gullo MA. Phloem as a possible major determinant of rapid cavitation reversal in stems of Laurus nobilis (laurel) Functional Plant Biology. 2006;33:1063–1074. doi: 10.1071/FP06149. [DOI] [PubMed] [Google Scholar]
- Secchi F, Zwieniecki MA. Sensing embolism in xylem vessels: the role of sucrose as a trigger for refilling. Plant, Cell & Environment. 2011;34:514–524. doi: 10.1111/j.1365-3040.2010.02259.x. [DOI] [PubMed] [Google Scholar]
- Stevenson JF, Mauseth JD. Effects of environment on vessel characters in cactus wood. International Journal of Plant Sciences. 2004;165:347–357. [Google Scholar]
- Stinson KA. Effects of snowmelt timing and neighbor density on the altitudinal distribution of Potentilla diversifolia in western Colorado, USA. Arctic, Antarctic and Alpine Research. 2005;37:379–386. [Google Scholar]
- Tolsma AD, Read SM, Tolhurst KG. Roots of Australian alpine plant species contain high levels of stored carbohydrates independent of post-fire regeneration strategy. Australian Journal of Botany. 2007;55:771–779. [Google Scholar]
- Tyree MT, Ewers FW. The hydraulic architecture of trees and other woody-plants. New Phytologist. 1991;119:345–360. [Google Scholar]
- Tyree MT, Vargas G, Engelbrecht BMJ, Kursar TA. Drought until death do us part: a case study of the desiccation-tolerance of a tropical moist forest seedling-tree, Licania platypus (Hemsl.) Fritsch. Journal of Experimental Botany. 2002;53:2239–2247. doi: 10.1093/jxb/erf078. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Engelbrecht BMJ, Vargas G, Kursar TA. Desiccation tolerance of five tropical seedlings in Panama: relationship to a field assessment of drought performance. Plant Physiology. 2003;132:1439–1447. doi: 10.1104/pp.102.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares F, Sanchez-Gomez D. Ecophysiological traits associated with drought in Mediterranean tree seedlings: individual responses versus interspecific trends in eleven species. Plant Biology. 2007;8:688–697. doi: 10.1055/s-2006-924107. [DOI] [PubMed] [Google Scholar]
- Walker MD, Walker DA, Welker JM, et al. Long-term experimental manipulation of winter snow regime and summer temperature in arctic and alpine tundra. Hydrological Processes. 1999;13:2315–2330. [Google Scholar]
- Williams MW, Brooks PD, Seastedt T. Nitrogen and carbon soil dynamics in response to climate change in a high-elevation ecosystem in the Rocky Mountains, USA. Arctic and Alpine Research. 1998;30:26–30. [Google Scholar]
- Woodcock DW. Climate sensitivity of wood-anatomical features in a ring-porous oak (Quercus-macrocarpa) Canadian Journal of Forest Research/Revue Canadienne de Recherche Forestiere. 1989;19:639–644. [Google Scholar]
- Wright IJ, Westoby M, Reich PB. Convergence towards higher leaf mass per area in dry and nutrient-poor habitats has different consequences for leaf life span. Journal of Ecology. 2002;90:534–543. [Google Scholar]