Abstract
Background and Aims
Preliminary field observations in 2001 and 2002 suggested that Kingdonia uniflora (Circaeasteraceae, Ranunculales) exhibits heterodichogamy, an unusual kind of reproductive heteromorphy, hitherto unreported in Ranunculales and known from only one other genus in basal eudicots.
Methods
During several subsequent years flowers were observed in the field. Flowers were fixed in FAA and studied with microtome sections series and with the scanning electron microscope.
Key Results
The flowers proved to be heterodichogamous, with protandrous and protogynous morphs, which have a 1 : 1 ratio. Both morphs equally set fruit. Each year a single flower is formed at the tip of a rhizome or more rarely two flowers. The flowers are already open when they appear at the soil surface, before they are receptive and before pollen is dispersed. In both floral morphs the styles elongate early and the stigmas are positioned above the anthers before anthesis begins. In protogynous flowers the stigmas become receptive in this position; later the styles become reflexed and then the anthers dehisce. In contrast, in protandrous flowers the stamen filaments elongate during early anthesis such that the dehiscing anthers come to lie above the (still unreceptive) stigmas; after dehiscence of all anthers in a flower the styles begin to elongate and become receptive.
Conclusions
This is the first record of heterodichogamy in a representative of Ranunculales, in an herbaceous eudicot, and in a plant with uniflorous ramets. The occurrence of heterodichogamy in Kingdonia in which clonal reproduction appears to be dominant might be an adaptation to avoid mating between the ramets from a common mother individual (genet).
Key words: Kingdonia, Circaeasteraceae, Ranunculales, heterodichogamy, reproductive heteromorphy
INTRODUCTION
Circaeasteraceae are a small family of Ranunculales (Hoot and Crane, 1995; Oxelman and Lidén, 1995; Wang et al., 2009; APG III, 2009) with two monotypic eastern Asian genera, Circaeaster and Kingdonia. The biology of neither is well known in its natural habitat. Kingdonia uniflora is endemic in China. It is an endangered and nationally protected species and has a disjunct distribution in three isolated areas, Deqing of Yunnan, north-west of Sichuan to south of Gansu and the middle Mt Qinling of Shaanxi province, and occurs at an elevation of 2400–3800 m, whereby its altitudinal range does not exceed 400 m on any mountain (Lei et al., 2000a). There are a number of studies on the vegetative structure (Foster, 1959; Foster and Arnott, 1960; Hu and Lee, 1979; Ren and Hu, 1996; 1998; Ren et al., 1998b; Liu et al., 2000), reproductive structure (Diels, 1932; Foster, 1961; Hu and Tian, 1985; Kosuge et al., 1989; Ren et al., 2004), embryology (Mu, 1983, 1984; Ren et al., 1998a), palynology, (Nowicke and Skvarla, 1982; Zhang, 1983), pollination biology (He et al., 2006), genetic diversity (Ren et al., 2005), reproduction and conservation biology (Ren et al., 2003; Lei et al., 2000a, b; Li et al., 2009; 2010) and geographic distribution (Hu et al., 1964).
Kingdonia uniflora is a rhizomatous herb. Each year a single flower is produced by the terminal bud of a rhizome (Tamura, 1995, fig. 21; Ren et al., 2004, fig. 2a) or more rarely two flowers. The flowers are bisexual. They have a long pedicel. Floral organs are spirally arranged (Ren et al., 2004). All floral organs are free. The 5(–9) tepals are yellow-green, and have an open venation. Conventional petals are absent. However, 9–11 outer, nectariferous staminodes correspond to petals in other Ranunculales (see Endress, 2010a). In Kingdonia each staminode has a stalk and a dilated head with a nectariferous secretory groove on the ventral side. The anthers of the 5–8 stamens dehisce toward the upper side. The 5–6(–9) carpels are differentiated into ovaries, styles and stigmas.
Fig. 2.
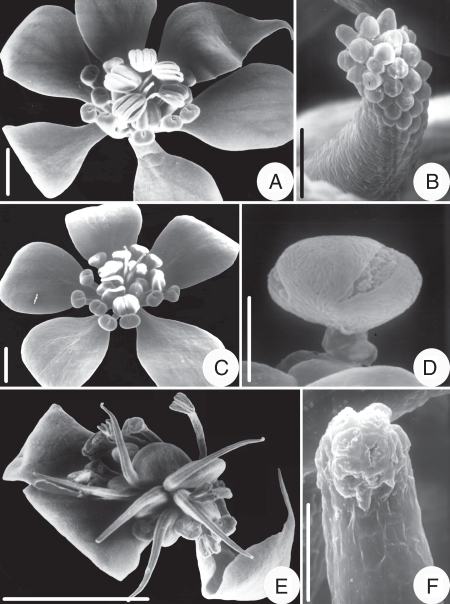
Kingdonia uniflora. Heterodichogamy seen with SEM. (A) Protandrous flower with anthers dehisced; (B) enlarged stigma of flower in (A), with turgescent papillae but no pollen; (C) protogynous flower; (D) enlarged part of a stigma of flower in(C), with a germinated pollen grain; (E) flower at the end of anthesis, with all styles reflexed and anthers dried; (F) enlarged stigma of a protogynous flower, with the stigmatic papillae collapsed after anther dehiscence. Scale bars: A, C, E = 1 mm; B, F = 200 µm; D = 10 µm.
In spite of these earlier studies, the flowering behaviour and breeding system of Kingdonia have not been previously studied in detail. The present paper is the result of field studies carried out over several years. Preliminary observations made in 2001 and 2002 suggested that K. uniflora is heterodichogamous. In detailed field studies carried out in seven additional years these results were confirmed. The aim of the present paper is to report the details in flower behaviour of this heterodichogamous herbaceous species with uniflorous ramets and to discuss its uniqueness among other heterodichogamous angiosperms.
MATERIALS AND METHODS
Field observations on the flowering of Kingdonia uniflora Balf.f. & W.W.Sm. were carried out in Xiabansi (34°00′32·44″N, 107 °47′31·87′E, 2800–3200 m a.s.l.), Mt Taibaishan, Shaanxi province each year from 2001 to 2005 and from 2008 to 2011. Only in 2003, 2004, 2005 and 2009 did the studied plants develop fruits. In the other years almost all flowers died due to late spring frost, i.e. sudden snow and reduced temperature after a period of warm days in early spring, at the beginning of anthesis. The mean temperature of the study area in April and May is 2·5 °C and 4·6 °C, respectively, at an elevation of 3000 m (Tang and Fang, 2006). Late spring frost in May or early June, the time of anthesis and early fruit development, occurs regularly to a smaller or larger extent. Plants were studied in three sub-populations. The lower sub-population extends from 2800 to 2830 m, in an area of about 20 × 240 m, and in forest of Abies fargesii and Rhododendron clementinae subsp. aureodorsale. The middle sub-population extends from 2890 to 2910 m, in an area of about 10 × 30 m, in mixed forest of Abies fargesii and Larix chinensis. The upper sub-population occurs at 3200 m, in an area of about 5 × 10 m, in Larix chinensis forest.
Observations were carried out at individual and population levels. At the individual level, the individuals were divided into two groups: group A contained individuals with a single flower and group B contained individuals with two flowers. The flowers of group A were tagged just after they came out of the ground, and the status of the stamens and carpels was recorded at intervals of 24 h. Observations at the population level were carried out in 2002. One hundred flowers in group A were collected randomly from 29 April to 3 May, and on 6 and 12 May, respectively, and fixed in FAA. The status of the stamens and carpels was checked with a stereomicroscope. To potentially decrease the amount of resampling of the flowers from the ramets that separated from a common mother individual (genet), the distance between two tagged or collected flowers was not less than 2·5 m.
Because many of the young fruits were killed by late spring frost, field observations were finished 3–4 d after fruit formation began.
Photographs of flowers in different stages of anthesis were taken in the field with a Nikon Coolpix 990 digital camera. For scanning electron microscope studies, fixed flowers were dehydrated in ethanol and iso-amyl acetate series, treated with critical point drying in CO2, vacuum evaporation, and studied with a KYKY-2000 scanning electron microscope. The materials used for histology were dehydrated in an ethanol series, infiltrated with xylene and embedded in paraffin wax. The embedded material was sectioned at 10 µm thickness and stained with safranin and fast green.
RESULTS
Flowering
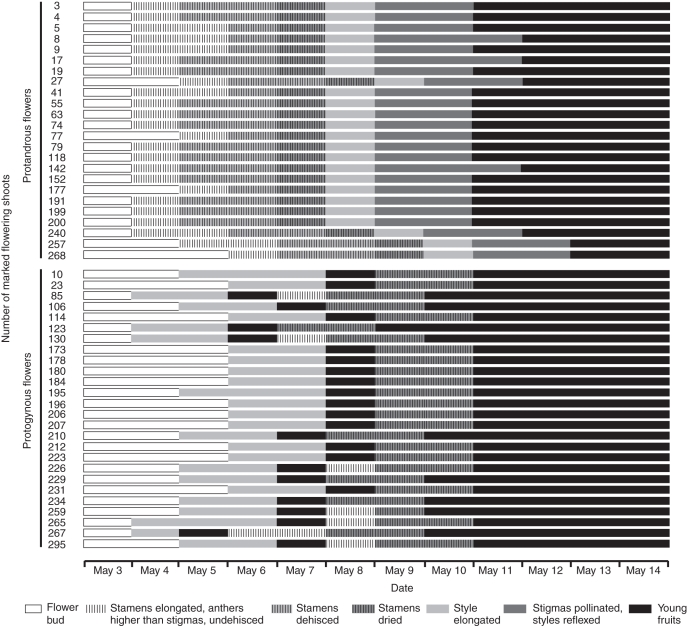
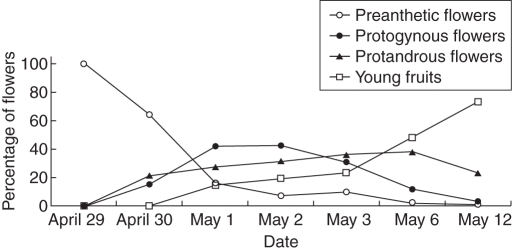
The young flowers of Kingdonia uniflora develop underground and open when they emerge above ground (Fig. 1A). Anthesis in the study area is from late April/early May to mid-May and the flowers in a population are largely, but not strictly, synchronous (Fig. 4). A surprising result of this study was that the species is heterodichogamous with protandrous and protogynous flowers.
Fig. 1.
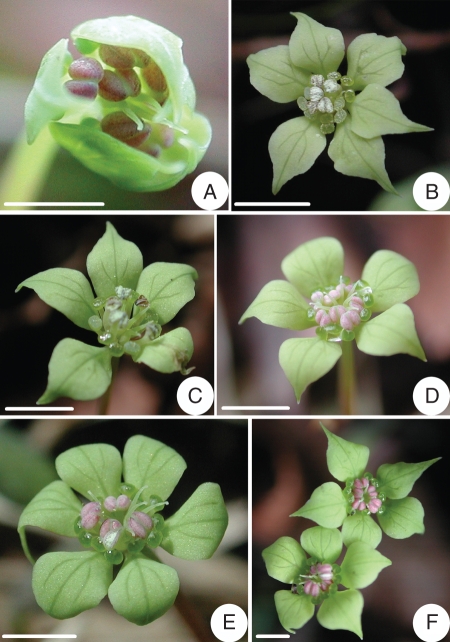
Kingdonia uniflora. Heterodichogamy. (A) Flower at early anthesis, with the stigmas higher than the anthers; (B) protandrous flower, with all anthers dehisced and stigmas at lower level than anthers; (C) protandrous flower, with all anthers dehisced and styles reflexed; (D) protogynous flower, with the styles reflexed but the anthers not yet dehisced; (E) protogynous flower, with the anthers beginning to dehisce; (F) two protogynous flowers of a single shoot; the styles of the upper flower beginning to reflex and anthers still undehisced, those of the lower flower reflexed and one stamen dehisced. Scale bars = 2 mm.
Fig. 4.
Kingdonia uniflora. Flowering of marked protandrous and protogynous flowers in 2008.
Male and female stages within a flower
The male stage was defined as beginning with anther dehiscence and ending with the drying of the anthers. The female stage is marked by turgescent stigmatic papillae; papillae collapse at the end of the female stage. Based on our observations in 2010 and 2011, in protogynous flowers the stigmas are receptive when the young flowers emerge above ground and are no longer receptive when the styles become reflexed. In protandrous flowers, the stigmas become receptive only when all stamens have dried and the styles begin to elongate. Thus, elongation of the styles is an easily recognizable marker for the beginning of the female stage. According to histological observations on 50 carpels, we found that the styles are erect (Fig. 1A) or slightly oblique before the egg cell is fertilized. Once the egg cell is fertilized the styles begin to become reflexed. Thus, reflexing of the styles was used as an easily recognizable marker for the end of the female stage. After anthesis, the styles continue to reflex and, in fruit, they become almost parallel with the achenes (see Ren et al., 2003, fig. 3:11).
Behaviour of single flowers and fruit development
Observations on flower behaviour and fruit set were carried out in 2008, 2010 and 2011. The flower behaviour was described on the basis of our observations in 2008 because there was no late spring frost at the time of anthesis, in contrast to 2010 and 2011. Recording of the flower behaviour was interrupted from 2 to 8 May in 2010 and from 13 to 16 May in 2011.
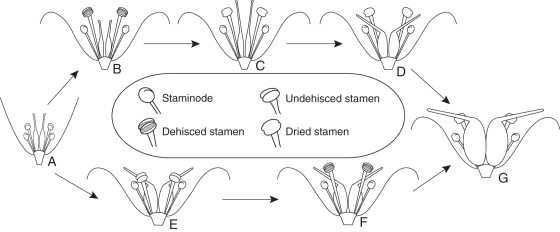
When the young shoot with the single flower emerged above ground, the tepals are already open. At this stage all flowers look the same. The styles are erect and the stigmas are positioned above the unopened anthers (Figs 1A and 3A).
Fig. 3.
Kingdonia uniflora. Diagram of heterodichogamy. (A) Flower at the beginning of anthesis. (B–D) Protandrous flowers: (B) male stage, with stamens dehisced and higher than stigmas; (C) female stage, with stigmas higher than dried anthers; (D) postanthetic flower, with anthers dried and styles reflexed. (E, F) Protogynous flowers: (E) late female stage, with styles reflexed and stamens not yet dehisced; (F) male stage. (G) Young fruit.
In protandrous flowers, the stamens elongate such that the anthers are positioned above the stigmas. This process lasted 1 d in 18 of the flowers studied and 2 d in six of the flowers (Fig. 4). The male stage starts with anther dehiscence (Figs 1B, 2A and 3B). It lasted 2 d in 18 flowers and 1 d in six flowers (Fig. 4). When all anthers in a flower have dried (drying of the anthers takes 1 d; Fig. 4), the styles are still erect (Fig. 2A, C) and there is no germinated pollen on the stigmas (Fig. 2B). The phase from stamen elongation to stamen drying lasted 4 d in 20 flowers, and 5 and 3 d in one flower each. Thus there is a non-functional day between the male and female stage. At the female stage the styles elongate (Fig. 1C) and become reflexed. This process lasted 2 d in 20 flowers and 3 d in four flowers (Fig. 4). At the end of anthesis all styles are reflexed (Fig. 3D) and the fruits begin to develop (Figs 2E and 3G).
In protogynous flowers, the styles elongate continuously such that the stigmas become positioned above the anthers. This marks the beginning of the female stage. The female stage lasted 2 d in 22 flowers, 3 d in three flowers and 1 d in one flower (Fig. 4). At the end of the female stage the styles become reflexed, while the anthers are still closed (Figs 1D, 2C and 3E). This non-functional stage lasts about 1 d. Germinated pollen was found on all stigmas at this stage (Fig. 2D; see also He et al., 2006, fig. 1A, B). The endosperm begins to develop at this stage according to the histological observations. When the styles become oblique to the ovary, the anthers dehisce (Figs 1E and 3F) and the stigmatic papillae are collapsed at this stage (Fig. 2F). The male stage lasted 2 d in 25 flowers and only 1 d in one flower (Fig. 4). In seven flowers observed the stamens elongated in the male stage. This elongation process lasted 1 d in six flowers and 2 d in only one flower (Fig. 4).
Proportion of the two flower morphs in the populations studied
The proportion of the two flower morphs and fruit development in the studied populations was recorded in three different years (all from uniflorous shoots) as shown in Table 1.
Table 1.
Kingdonia uniflora: proportion of the two flower morphs and fruit setting in the populations studied
| Year | Flower morph | No. of tagged flowers | Tagged flowers (%) | No. of flowers setting fruit | Young fruits (%) |
|---|---|---|---|---|---|
| 2008 | Protandrous | 143 | 47·7 | 24 | 48·0 |
| Protogynous | 157 | 52·3 | 26 | 52·0 | |
| Total | 300 | 100 | 50 | 100 | |
| 2010 | Protandrous | 52 | 55·9 | 11 | 61·1 |
| Protogynous | 41 | 44·1 | 7 | 38·9 | |
| Total | 93 | 100 | 18 | 100 | |
| 2011 | Protandrous | 125 | 46·8 | 28 | 41·8 |
| Protogynous | 142 | 53·2 | 39 | 58·2 | |
| Total | 267 | 100 | 67 | 100 |
The ratio of protandrous to protogynous flowers in the population is close to 1 : 1 in all three years (two-sample t-test, t = 0·080, P = 0·940) and likewise that of fruit formation from protandrous and protogynous flowers (two-sample t-test, t = 0·083, P = 0·938).
Floral morphs in two-flowered shoots
In total we found 40 shoots (ramets) with two flowers (versus 660 tagged uniflorous ramets). The floral morph was always the same in a shoot (Fig. 1F). Flowering of the two flowers in a single shoot was synchronous or sometimes slightly asynchronous (from 1 to 3 d).
Approximate synchrony of flowering at population level
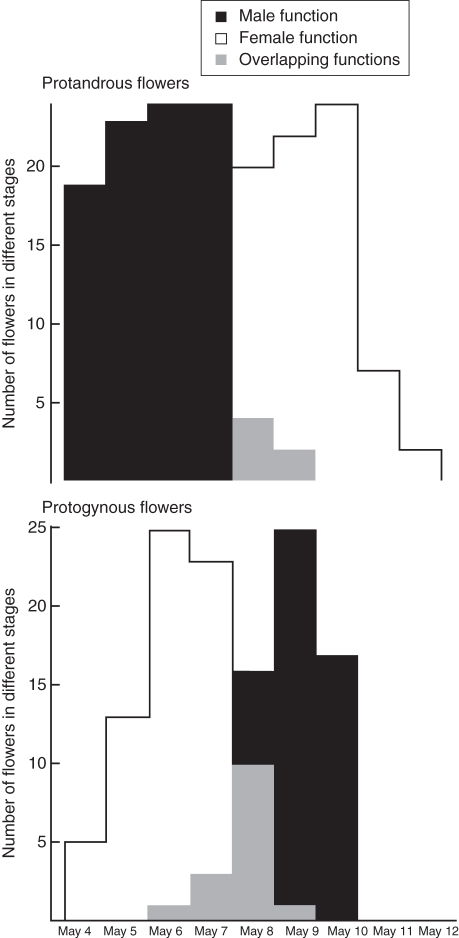
In 2002, the flowering period was from April 29 to mid-May in the studied population (Fig. 5). Anthesis of protogynous flowers was dominant from 2 to 4 May. The number of anthetic protandrous flowers increased slowly from 2–5 May and then decreased. In 2008, at the population level there was 4·3 % overlap between male and female functions in protandrous flowers and 12 % between female and male functions in protogynous flowers (Fig. 6).
Fig. 5.
Kingdonia uniflora. Change of percentage of preanthetic flowers (buds), protandrous and protogynous anthetic flowers, and young fruits observed during the flowering period (data from 2002).
Fig. 6.
Kingdonia uniflora. Separation of male and female functions in the two mating types at the population level (data from 2008). Upper panel: protandrous flowers; lower panel: protogynous flowers. Grey shading indicated flowers with overlapping sex functions.
DISCUSSION
Dichogamous flowers, with their functional male and female phase separated in time, are common in angiosperms. Synchronous dichogamy, with anthesis synchronized among the flowers of an individual or population, is also known from a number of angiosperms. Much less often recorded in angiosperms is heterodichogamy. A special type of synchronous dichogamy, it involves two genetic morphs, exhibiting protandrous and protogynous flowers. The flowers behave equally within a morph but differently between the morphs, such that at a given time only flowers in the functional male or functional female phase are present in a given individual of a population (Renner, 2001). Dichogamy and, even more so, heterodichogamy, are considered as mechanisms to reduce selfing (Darwin, 1877; Gleeson, 1982; Cruden, 1988). Heterodichogamy usually occurs in species in which an individual produces numerous flowers in a flowering season. It was therefore surprising to find heterodichogamy in Kingdonia uniflora, in which each rhizome (ramet) produces only one flower per year (rarely two). However, as each rhizome probably represents a ramet of a more or less highly branched individual (genet) resulting from clonal spreading, a genet may in fact produce a number of flowers per season.
In the review by Renner (2001), heterodichogamy was mentioned for 11 families and 17 genera (Annonaceae, Eupomatiaceae, Lauraceae, Zingiberaceae, Trochodendraceae, Betulaceae, Juglandaceae, Rhamnaceae, Sapindaceae, Thymelaeaceae and Amaranthaceae). One of the families, Eupomatiaceae, is not strictly heterodichogamous (see Endress, 1984) but may represent an interesting intermediate evolutionary stage between synchronous dichogamy and heterodichogamy. In the meantime, heterodichogamy has been recorded in genera of three additional families: Hernandia (Hernandiaceae; Endress and Lorence, 2004), Kingdonia (Circaeasteraceae; this study), and, perhaps, Kirkia (Kirkiaceae; Immelmann, 1984; Bachelier and Endress, 2008).
To our knowledge, Kingdonia is the first record of heterodichogamy in Ranunculales, and only the second in basal eudicots. The other known case in basal eudicots is Trochodendron aralioides (Trochodendraceae) (Chaw and Chan, 1988; Glimmann, 1990; Chaw, 1992). Also in basal angiosperms heterodichogamy appears to be rare. The only cases found so far are some Lauraceae (Stout, 1927; Kubitzki, 1982; Kubitzki and Kurz, 1984), Hernandia nymphaeifolia (Hernandiaceae) (Endress and Lorence, 2004; Endress, 2010b), and, perhaps, Annona squamosa (Annonaceae) (Wester, 1910).
The heterodichogamy type of Kingdonia is similar to that in Trochodendron in that it functions at the level of an entire flowering season and is based on bisexual flowers, whereas in the other examples here mentioned it functions at the level of a daily rhythm and in Hernandia, in addition, in inflorescences with both male and female flowers. Two features stand out in Kingdonia. First, whereas most angiosperm species in which heterodichogamy has been recorded are woody plants, Kingdonia is herbaceous, a feature shared only with Alpinia (Zingiberaceae) (Li et al., 2001a, b). Second, what distinguishes Kingdonia from all other cases known as yet is that each ramet produces only one (or rarely two) flowers in one flowering period. The hitherto studied heterodichogamous species have multi-flowered inflorescences (additional references, not yet mentioned in the Discussion: Müller, 1875; Knuth, 1906; Wood, 1934; Skutch, 1945; Galil and Zeroni, 1967; Gabriel, 1968; de Jong, 1976; Thompson and Romberg, 1985; Pendleton et al., 1988, 2000; Dommée et al., 1990; 1995; McCarthy and Quinn, 1990; Polito and Pinney, 1997; Asai, 2000; Li et al., 2001a, b; Sato, 2002; Kimura et al., 2003; Bai et al., 2006; Renner et al., 2007).
In protandrous flowers of K. uniflora, the styles elongate and the stigmas come to lie at a higher level than the stamens after release of all pollen, a position favourable to receive pollen from other flowers. Therefore the separation between the male and female stages is represented by the elongation of the styles, which takes approx. 1 d. For protogynous flowers, the separation between the female and male stages is represented by the period between when the styles become reflexed to the opening of the stamens, a period of also approx. 1 d.
Although the number of flowers differed from year to year, the ratio of protandrous and protogynous flowers was always approx. 1 : 1. This was also true for the ratio of protandrous and protogynous flowers developing fruit. Such 1 : 1 ratio of protandrous and protogynous flowers occurs in all the heterodichogamous species studied and may indicate a simple dominant–recessive Mendelian factor (Gleeson, 1982; Thompson and Romberg, 1985; Renner, 2001). The fact that the two flowers of biflorous shoots in K. uniflora always have the same mating type also indicates this genetic stability.
It has generally been assumed that heterodichogamy evolved from synchronous dichogamy (Darwin, 1877; de Jong, 1976; Gleeson, 1982; Renner, 2001; Endress, 2010b) and may lead to dioecy (Darwin, 1877; de Jong, 1976; Kubitzki and Kurz, 1984; Lloyd and Webb, 1986; Pendleton et al., 1988, 2000; Dommée et al., 1990; Gleiser et al., 2008; Tal, 2009). However, a critical study in Acer showed no transition from heterodichogamy to dioecy (Renner et al., 2007).
Sexual reproduction appears to be limited in Kingdonia because 66–86 % of the fruits aborted (Ren et al., 2003), and no seedlings were found in the field (Hu et al., 1964; Ren et al., 2003). Ramet number increases mainly by fragmentation of a rhizome into two or more parts (Lei et al., 2000b). Thus clonal development appears to be the main reproduction method. For such a clonal and uniflorous species, mating between two ramets which separated from a mother individual (genet) is equivalent to geitonogamy.
Conclusions
Heterodichogamy may be an adaptation to promote outbreeding for the apparently mainly clonally spreading K. uniflora, even if the plants produce mainly uniflorous shoots. The fact that 20 genera (from 14 families) which are known to have heterodichogamy occur sporadically in very different major clades, including basal angiosperms, monocots, basal eudicots and core eudicots (Renner, 2001; this study), indicates that heterodichogamy evolved independently in different clades. However, it may be expected that heterodichogamy occurs more widely than currently known. It may just not have been noticed because it cannot be easily seen if the flowering behaviour of a plant is not studied in detail. Future studies in Kingdonia should address the question of the proportion of ramets and genets in a population with molecular markers.
ACKNOWLEDGEMENTS
This project was supported by the National Key Project of Scientific and Technical Supporting Programs funded by the Ministry of Science and Technology of China (no. 2008BAC39B05).
LITERATURE CITED
- APG III. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121. [Google Scholar]
- Asai T. Dichogamy in fullmoon maple (Acer japonicum Thunb.) Bulletin of the Hokaido Forestry Research Institute. 2000;37:27–40. [Google Scholar]
- Bachelier JB, Endress PK. Floral structure of Kirkia (Kirkiaceae) and its position in Sapindales. Annals of Botany. 2008;102:539–550. doi: 10.1093/aob/mcn139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai W-N, Zeng Y-F, Liao W-J, Zhang D-Y. Flowering phenology and wind-pollination efficacy of heterodichogamous Juglans mandshurica (Juglandaceae) Annals of Botany. 2006;98:397–402. doi: 10.1093/aob/mcl111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaw S-M. Pollination, breeding syndromes, and systematics of Trochodendron aralioides Sieb. et Zucc. (Trochodendraceae), a relictual species in Eastern Asia. In: Peng C-I., editor. Phytogeography and botanical inventory of Taiwan. 1992. pp. 63–77. Institute of Botany, Academia Sinica Monograph Series 12. Taipei: ROC. [Google Scholar]
- Chaw S-M, Chan T-H. Simultaneity of synchronized dichogamy and floral odor secretion in Trochodendron aralioides Sieb. & Zucc. (Trochodendraceae) American Journal of Botany. 1988;75(6,2) Abstract 442. [Google Scholar]
- Cruden RW. Temporal dioecism: systematic breadth, associated traits, and temporal patterns. Botanical Gazette (Crawfordsville) 1988;149:1–15. [Google Scholar]
- Darwin C. The different forms of flowers on plants of the same species. London: Murray; 1877. [Google Scholar]
- Diels L. Circaeaster, eine hochgradig reduzierte Ranunculacee. Beihefte zum Botanischen Centralblatt. 1932;49(Ergänzungs-Band):55–60. [Google Scholar]
- Dommée B, Bompar J-L, Dnelle N. Sexual tetramorphism in Thymelaea hirsuta (Thymelaeaceae): evidence of the pathway from heterodichogamy to dioecy at the infraspecific level. American Journal of Botany. 1990;77:1449–1462. [Google Scholar]
- Dommée B, Biascamano A, Dnelle N, Bompar J-L, Thompson JD. Sexual tetramorphism in Thymelaea hirsuta (Thymelaeaceae): morph ratios in open-pollinated progeny. American Journal of Botany. 1995;82:734–740. [Google Scholar]
- Endress PK. The flowering process in the Eupomatiaceae (Magnoliales) Botanische Jahrbücher für Systematik. 1984;104:297–319. [Google Scholar]
- Endress PK. Flower structure and trends of evolution in eudicots and their major subclades. Annals of the Missouri Botanical Garden. 2010a;97:541–583. [Google Scholar]
- Endress PK. The evolution of floral biology in basal angiosperms. Philosophical Transactions of the Royal Society of London B. 2010b;365:411–421. doi: 10.1098/rstb.2009.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress PK, Lorence DH. Heterodichogamy of a novel type in Hernandia (Hernandiaceae) and its structural basis. International Journal of Plant Sciences. 2004;165:753–763. [Google Scholar]
- Foster AS. The morphological and taxonomic significance of dichotomous venation in Kingdonia uniflora Balfour f. et W. W. Smith. Notes from the Royal Botanic Garden Edinburgh. 1959;23:1–2. [Google Scholar]
- Foster AS. The floral morphology and relationships of Kingdonia uniflora. Journal of the Arnold Arboretum. 1961;17:397–411. [Google Scholar]
- Foster AS, Arnott HJ. Morphology and dichotomous vasculature of leaf of Kingdonia uniflora. American Journal of Botany. 1960;47:684–698. [Google Scholar]
- Gabriel WJ. Dichogamy in Acer saccharum. Botanical Gazette (Crawfordsville) 1968;129:334–338. [Google Scholar]
- Galil J, Zeroni M. On the pollination of Zizyphus spina-christi (L.) Willd. in Israel. Israel Journal of Botany. 1967;16:71–77. [Google Scholar]
- Gleeson SK. Heterodichogamy in walnuts: inheritance and stable ratios. Evolution. 1982;36:892–902. doi: 10.1111/j.1558-5646.1982.tb05461.x. [DOI] [PubMed] [Google Scholar]
- Gleiser G, Segara-Moragues JG, Pannell JR, Verdú M. Siring success and paternal effects in heterodichogamous Acer opalus. Annals of Botany. 2008;101:1017–1026. doi: 10.1093/aob/mcn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimmann R. Blütenbiologie von Trochodendron aralioides. 1990 Diploma Thesis, University of Zurich. [Google Scholar]
- He H-X, Zhang X-L, Ren Y. Influence of tepals, sterile and fertile stamens to the pollinators and pollination of Kingdonia uniflora. Acta Botanica Yunnanica. 2006;28:371–377. [Google Scholar]
- Hoot SB, Crane PR. Inter-familial relationships in the Ranunculidae based on molecular systematics. Plant Systematics and Evolution Supplement. 1995;9:119–131. [Google Scholar]
- Hu Z-H, Lee K-M. Morphological studies of Kingdonia uniflora F. Balfour et W. W. Smith II: the anatomy of rhizome. Acta Phytotaxonomica Sinica. 1979;17:23–29. [in Chinese with English abstract] [Google Scholar]
- Hu Z-H, Tian L-X. Studies on morphology of Kingdonia uniflora F. Balfour et W. W. Smith III. The morphology and anatomy of flowers, fruits and seed. Acta Phytotaxonomica Sinica. 1985;23(170–178 [in Chinese with English abstract]) [Google Scholar]
- Hu Z-H, Li K-M, Lee C-L. Distribution and general morphology in Kingdonia uniflora. Acta Botanica Sinica. 1964;12(351–358 [in Chinese with English abstract]) [Google Scholar]
- Immelman KL. Flowering in Kirkia wilmsii. Engl. Bothalia. 1984;15:151–152. [Google Scholar]
- de Jong PC. Flowering and sex expression in Acer L.: a biosystematic study. Mededelingen Landbouwhogeschool Wageningen. 1976;76–2:1–201. [Google Scholar]
- Kimura M, Seiwa K, Suyama Y, Ueno N. Flowering system of heterodichogamous Juglans ailanthifolia. Plant Species Biology. 2003;18:75–84. [Google Scholar]
- Knuth P. Handbook of flower pollination. 3 vols. London: Clarendon Press; 1906. (English translation by J.R.A. Davis) [Google Scholar]
- Kosuge K, Pu F-D, Tamura M. Floral morphology and relationships of Kingdonia. Acta Phytotaxonomica Geobotanica. 1989;40:61–67. [Google Scholar]
- Kubitzki K. Lauraceae I. Aniba. Flora Neotropica Monograph. 1982;31:1–84. [Google Scholar]
- Kubitzki K, Kurz H. Synchronized dichogamy and dioecy in neotropical Lauraceae. Plant Systematics and Evolution. 1984;147:253–266. [Google Scholar]
- Lei Y-J, Ren Y, Yue M. A survey on the distribution and status of the endangered plant Kingdonia uniflora. Journal of Northwest University Natural Science Edition. 2000a;30(239–243 [in Chinese with English abstract]) [Google Scholar]
- Lei Y-J, Ren L, Li Z-J, Ren Y. Studies on vegetative reproduction pattern of Kingdonia uniflora. Acta Botanica Boreali-Occidentalia Sinica. 2000b;20:432–435. [in Chinese with English abstract] [Google Scholar]
- Li Q-J, Xu Z-F, Kress W, et al. Flexible style that encourages outcrossing. Nature. 2001a;410:432. doi: 10.1038/35068635. [DOI] [PubMed] [Google Scholar]
- Li Q-J, Xu Z-F, Xia Y-M, et al. Study on the flexistyly pollination mechanism in Alpinia plants (Zingiberaceae) Acta Botanica Sinica. 2001b;43:364–369. [in Chinese with English abstract] [Google Scholar]
- Li Z-J, Liu H-X, Huang K-Y, Ren Y, Jia L-Z. Affect of human disturbance on the growth of Kingdonia uniflora. Shaanxi Forest Science and Technology. 2009;2009:17–19. 40 [in Chinese with English abstract] [Google Scholar]
- Li Z-J, Liu H-X, Song R-H, Ren Y. Study on the ratio of flower to leaf in relation to the allocation of nutrition in blossom period of Kingdonia uniflora. Shaanxi Forest Science and Technology. 2010;2010:1–4. [in Chinese with English abstract] [Google Scholar]
- Liu X, Wang Z-C, Ma Y-S, Li Z-J, Ren Y. The primary studies on the macroscopical characteristics variation pattern of leaves of Kingdonia uniflora. Acta Botanica Boreali-Occidentalia Sinica. 2000;20:1076–1081. [in Chinese with English abstract] [Google Scholar]
- Lloyd DG, Webb CJ. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. I. Dichogamy. New Zealand Journal of Botany. 1986;24:135–162. [Google Scholar]
- McCarthy BC, Quinn JA. Reproductive ecology of Carya (Juglandaceae): phenology, pollination and breeding system of two sympatric tree species. American Journal of Botany. 1990;77:261–273. doi: 10.1002/j.1537-2197.1990.tb13551.x. [DOI] [PubMed] [Google Scholar]
- Mu X-J. Ovule, female and male gametophyte and fertilization of Kingdonia uniflora Balfour F. et W. W. Smith. Acta Botanica Sinica. 1983;25:497–504. [in Chinese with English abstract] [Google Scholar]
- Mu X-J. Early development of the endosperm in Kingdonia uniflora. Acta Botanica Sinica. 1984;26:668–671. [in Chinese with English abstract] [Google Scholar]
- Müller H. Flowering of the hazel. Nature. 1875;12:26. [Google Scholar]
- Nowicke JW, Skvarla JJ. Pollen morphology and the relationships of Circaeaster, Kingdonia and Sargentodoxa to the Ranunculales. American Journal of Botany. 1982;69:990–998. [Google Scholar]
- Oxelman B, Lidén M. The position of Circaeaster – evidence from nuclear ribosomal DNA. Plant Systematics and Evolution Supplement. 1995;9:189–193. [Google Scholar]
- Pendleton RL, McArthur ED, Freeman DC, Blauer AC. Heterodichogamy in Grayia brandegei (Chenopodiaceae): report from a new family. American Journal of Botany. 1988;75:267–274. [Google Scholar]
- Pendleton RL, Freeman DC, McArthur ED, Sanderson SC. Gender specialization in heterodichogamous Grayia brandegeei (Chenopodiaceae): evidence for an alternative pathway to dioecy. American Journal of Botany. 2000;87:508–516. [PubMed] [Google Scholar]
- Polito VS, Pinney K. The relationship between phenology of pistillate flower organogenesis and mode of heterodichogamy in Juglans regia L. (Juglandaceae) Sexual Plant Reproduction. 1997;10:36–39. [Google Scholar]
- Ren Y, Hu Z-H. Morphological studies on anastomoses and blind veins in dichotomous venation of the leaf in Kingdonia uniflora. Acta Phytotaxonomica Sinica. 1996;34:569–576. [in Chinese with English abstract] [Google Scholar]
- Ren Y, Hu Z-H. Anatomical studies on root, node and leaf of Kingdonia uniflora. 1998;18:72–77. Acta Botanica Boreali-Occidentalia Sinica [in Chinese with English Summary] [Google Scholar]
- Ren Y, Wang M-L, Hu Z-H. Kingdonia, embryology and its systematic significance. Acta Phytotaxonomica Sinica. 1998a;36:423–427. [Google Scholar]
- Ren Y, Xiao Y-P, Hu Z-H. The morphological nature of the open dichotomous leaf venation of Kingdonia and Circaeaster and its systematic implication. Journal of Plant Research. 1998b;111:225–230. [Google Scholar]
- Ren Y, Li Z-J, Lei Y-J. Achene and seed abortion contribute to the rarity of Kingdonia uniflora. Israel Journal of Plant Sciences. 2003;51:39–44. [Google Scholar]
- Ren Y, Li Z-J, Chang H-L, Lei Y-J, Lu A-M. Floral development of Kingdonia (Ranunculaceae s.l., Ranunculales) Plant Systematics and Evolution. 2004;247:145–153. [Google Scholar]
- Ren Y, Liu X, Ge S. Low genetic diversity among populations of the rare Chinese endemic Kingdonia uniflora revealed by RAPD-analysis. Israel Journal of Plant Sciences. 2005;53:65–73. [Google Scholar]
- Renner SS. How common is heterodichogamy? Trends in Ecology and Evolution. 2001;16:595–597. [Google Scholar]
- Renner SS, Beenken L, Grimm GW, Kocyan A, Ricklefs RE. The evolution of dioecy, heterodichogamy, and labile sex expression in Acer. Evolution. 2007;61:2701–2719. doi: 10.1111/j.1558-5646.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- Sato T. Phenology of sex expression and gender variation in a heterodichogamous maple. Acer japonicum. Ecology. 2002;83:1226–1238. [Google Scholar]
- Skutch AF. The behavior of the flowers of the aguacatillo (Persea caerulea) Torreya. 1945;45:110–116. [Google Scholar]
- Stout AB. The flower behavior of avocados. Memoirs of the New York Botanical Garden. 1927;7:145–203. [Google Scholar]
- Tal O. Acer pseudoplatanus (Sapindaceae): heterodichogamy and thrips pollination. Plant Systematics and Evolution. 2009;278:211–221. [Google Scholar]
- Tamura M. Ranunculaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 17 a IV (ed. 2) Berlin: Duncker & Humblot; 1995. pp. 1–555. [Google Scholar]
- Tang Z–Y, Fang JY. Altitudinal pattern of temperature along the northern and southern slope on Mt. Taibai. In: Ren Y, Liu M–S, Tian L–H, Tian X–H, Li Z–J, editors. Biodiversity, conservation and management of Taibaishan Nature Reserve. China Forest Publishing House; 2006. pp. 17–22. [Google Scholar]
- Thompson TE, Romberg LD. Inheritance of heterodichogamy in pecan. Journal of Heredity. 1985;76:456–458. [Google Scholar]
- Wang W, Lu A-M, Ren Y, Endress ME, Chen Z-D. Phylogeny and classification of Ranunculales: evidence from four molecular loci and morphological data. Perspectives in Plant Ecology, Evolution and Systematics. 2009;11:81–110. [Google Scholar]
- Wester PJ. Pollination experiments with anonas. Bulletin of the Torrey Botanical Club. 1910;37:529–539. [Google Scholar]
- Wood MN. Pollination and blooming habits of the Persian walnut in California. US Department of Agriculture, Technical Bulletin. 1934 No. 387. [Google Scholar]
- Zhang Y-L. Pollen morphology of Kingdonia uniflora and its taxonomic significance. Acta Phytotaxonomica Sinica. 1983;21:441–444. [in Chinese with English summary] [Google Scholar]