Abstract
The consequence of myocardial ischemia is energetic stress, while reperfusion is accompanied by abrupt ionic shifts and considerable oxidative stress. Cells die by apoptotic and necrotic pathways. After the acute injury, the healing myocardium is subject to biomechanical stress and inflammation, which can trigger a smaller but more sustained wave of cell death, as well as changes in the metabolic and functional characteristics of surviving cells. The goal of cardioprotection is to prevent cell death during the acute injury as well as to modulate the detrimental processes that ensue during remodeling. This review will focus on acute injury, and the central premise is that mitochondria are the key determinant of cardiomyocyte fate.
Keywords: Apoptosis, necrotic cell death, mitochondria, autophagy, cardioprotection
Mitochondria Integrate the Signals of I/R Injury and Cytoprotection
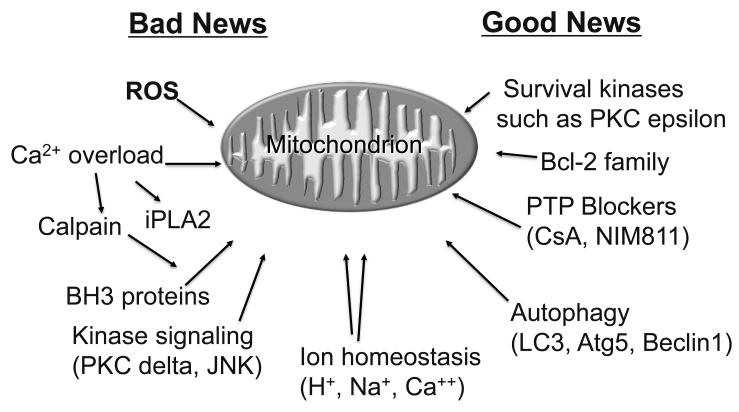
Research over the past 25 years has yielded a still-expanding list of factors that contribute to mitochondrial dysfunction, and an equally long list of factors that protect the mitochondria. Some factors appear on both lists, and the issues may include timing, magnitude, or context. The mitochondria integrate the diverse signals and respond by altering ATP production, calcium homeostasis, ROS production, and in dire cases, membrane permeability. As shown in Figure 1, the signals that destabilize mitochondria include reactive oxygen species (ROS), protein kinases such as PKC delta and JNK, calpain, and pro-apoptotic members of the Bcl-2 family (Bax/Bak and a BH3-only family member such as Bid, Bnip3, Nix, etc.)1, 2. Calcium in combination with ROS can lower the threshold for activation of mitochondrial iPLA2, liberating arachidonic acid and plasmalogens which contribute to further mitochondrial damage3. In contrast, signals that help to stabilize mitochondrial function include protein kinase C epsilon, Akt or its downstream effectors, and pro-survival members of the Bcl-2 family2. This concept of mitochondria as a global signal integrator for cellular homeostasis is based on findings that many different interventions can change the outcome, yet they are not necessarily interdependent. In the following sections, several of these pathways will be discussed in more detail since they have led to therapeutic interventions some of which have gone to clinical trial including the PTP blocker cyclosporine A, a peptide inhibitor of delta PKC (KAI 9803), and cariporide, a regulator of ion homeostasis.
Figure 1.
Mitochondria integrate the signals of I/R injury and cytoprotection. Mitochondria respond to a variety of inputs, both good and bad, including ROS, phospholipases, kinases, phosphatases, Bcl-2 family members (both pro- and anti-apoptotic), Ca++, and the pH of the cytosol and mitochondrial matrix.
Unhappy Mitochondria: Intracellular Troublemakers
Functional integrity of the cardiomyocytes requires an abundance of mitochondria capable of producing ATP and participating in calcium homeostasis. However, beyond merely lying down on the job, unhappy mitochondria can actively contribute to the demise of the cell through activation of apoptotic and necrotic pathways. Apoptosis is a form of programmed cell death characterized by activation of caspases, notably caspase 3. Activation of caspase 3—the effector caspase—by caspase 9 requires assembly of a large multimeric complex comprising caspase 9, APAF1, and cytochrome c. Cytochrome c is released from mitochondria after the outer membrane is permeabilized with the help of Bax (or Bak) and a BH3-only protein such as Bid. Also residing in the intracellular space with cytochrome c are other pro-apoptotic factors such as SMAC/DIABLO, a factor that binds to IAPs (inhibitor of apoptosis proteins) that interfere with APAF1 activation. The intricacies of the apoptotic pathway and participation of mitochondria have been covered extensively in other reviews4, 5. Mitochondria may trigger cell death through a second pathway involving the mitochondrial permeability transition pore (MPTP)6. This large-conductance pore is operationally defined, and its molecular identity remains elusive, although genetic studies have clearly demonstrated a requirement for cyclophilin D7. Opening of the pore results in rapid exchange of solutes up to 1.5 kDa in size, with redistribution of NADH to the cytosol and an influx of cytosolic water, causing matrix expansion. In extreme cases, matrix swelling is sufficient to unwrinkle the inner membrane; however, the outer membrane is less distensible and ruptures, releasing pro-apoptotic factors from the intermembrane space. The catastrophic mitochondrial swelling often leads to cell death before apoptosis can proceed, due to energetic collapse and uncontrolled enzymatic processes. Another troublesome mitochondrial phenomenon is ROS-induced ROS release. One unhappy mitochondrion can emit large quantities of ROS which will trigger like-minded behavior on the part of neighbors, propagating a wave of ROS and depolarizations; this unstable electrical behavior within the cell can contribute to electrical conduction defects across a segment of tissue, culminating with cardiac arrhythmias8, 9. This propensity for one or a few damaged mitochondria to trigger an epidemic of mitochondrial mischief-making is the reason they are such an important target for intervention in cardioprotection.
Calcium Overload and Mitochondrial Damage
Based on our understanding of the factors that contribute to catastrophic MPTP opening (summarized in Figure 2), a number of interventions have been explored. During ischemia, intracellular acidosis develops as lactic acid from glycolysis builds up and as protons are released with ATP hydrolysis. At reperfusion, the Na+/H+ exchanger (NHE-1) exchanges intracellular protons for extracellular sodium ions. The increased intracellular sodium ions compete with calcium for extrusion via the Na+/Ca++ exchanger (NCX), resulting in a buildup of intracellular calcium. Interventions which inhibit this process including acidic reperfusion or inhibition of NHE-1 with cariporide limits the calcium overload, and have been shown to protect the heart, presumably through benefits to mitochondria10. Mitochondria acquire calcium more directly through close interaction with specialized regions of sarcoplasmic reticulum (SR)11; it remains to be shown whether disrupting those close interactions which are mediated in part through mitofusin 2 could attenuate mitochondrial calcium overload. However, the significance of mitochondrial calcium overload as a determinant of MPTP opening has been called into question by Kim et al. 12. Calcium uptake and mitochondrial dynamics may also be modulated by other calcium-sensitive regulatory proteins including protein kinases and phosphatases, hexokinase, Drp-1, and Bcl-2 family members 13-15. Beyond direct effects on mitochondria, calcium may activate phospholipases and calpain, which in turn will cause mitochondrial damage. Inhibiting calpain can reduce infarct size 16, 17, presumably without altering calcium overload, suggesting that calcium overload is not nearly as problematic as the downstream events. Calpain has been implicated in the processing and release of the mitochondrial protein AIF which translocates to the nucleus to mediate DNA fragmentation 18.
Figure 2.
Ion homeostasis protects mitochondria. At reperfusion NHE-1 exchanges extracellular sodium for the excess hydrogen which accumulated during ischemia (cariporide inhibits NHE1); however, the increased intracellular sodium now causes the sodium-calcium exchanger (NCX) to bring in more calcium in order to eliminate the sodium (KB-R7943 inhibits NCX). Calpain proteolytically activates Bid to trigger apoptosis. Calcium uptake via the uniporter may contribute to triggering the MPTP. The mitochondrial ATP-sensitive potassium channel (mitoKATP) can open in response to protective signaling and cause mild depolarization, thereby limiting calcium uptake and opposing MPTP opening. Depolarization via mitoKATP may also trigger mitophagy. Mitochondrial stability is also modulated by fusion and fission, regulated by Mfn1, Mfn2, Drp1, Fis1, and Opa1. Closely appositioned SR is an important mechanism of calcium delivery to mitochondria, and its association is mediated by Mfn2 on SR and mitochondrial outer membrane. Kinases and phosphatases regulate many targets in the outer mitochondrial membrane.
ROS, Complex I, and the MPTP
It is widely accepted that excessive ROS production is a consistent feature of myocardial ischemia/reperfusion injury, and that protective interventions are associated with diminished ROS production. However, the evidence that ROS scavengers are beneficial is less compelling, and a clinical trial with recombinant human superoxide dismutase failed to demonstrate efficacy despite promising results in small pilot studies 19. A number of enzymatic sources of ROS exist, including Complex I, alpha-ketoglutarate dehydrogenase, NADPH oxidase, and a variety of other NAD(P)H-dependent dehydrogenases. Modestly encouraging results have emerged from efforts to inhibit ROS production from selected enzymes using agents such as amytal (complex I inhibitor), allopurinol (xanthine oxidase inhibitor), and apocynin (NADPH oxidase inhibitor). 20. Given the abundance of NAD(P)H-dependent enzymes that can generate ROS, it is surprising that inhibiting just a single enzyme can have a measurable effect on infarct size. A more global approach may be to prevent the buildup of NADH that occurs during ischemia; this may be on reason why pyruvate is beneficial, as it would support NADH oxidation, thus limiting availability of NADH for other ROS-generating dehydrogenases. Administration of a cell-permeable NADH dehydrogenase targeted to mitochondria provided more direct evidence that driving NADH oxidation could attenuate ROS production and limit infarct size in the isolated perfused rat heart 21. Complex I and the ROS derived from it have been implicated in triggering the MPTP; this might be a problem in the setting of high levels of NADH and impaired electron flow through the distal subunits of Complex I, resulting in superoxide production from the FAD site 22. In aggregate, these studies indicate that the MPTP is the central target of calcium overload, calpain, NADH, and ROS.
Strategies to Inhibit the MPTP
Although the molecular identity of the MPTP remains uncertain, a variety of drugs have been shown to modulate its activity, although genetic studies indicate that most of these must work indirectly. However, considerable work has shown that cyclosporine A is cardioprotective through its action to inhibit the MPTP, and genetic deletion of cyclophilin D (the target of cyclosporine A) results in a heart that is resistant to ischemia/reperfusion injury 7, 23, 24. Importantly, cyclosporine A administered at reperfusion was shown to reduce infarct size in some, but not all, large-animal models25-28, providing a compelling rationale for its use in a clinical trial for patients with acute myocardial infarction. In a randomized double-blinded clinical trial reported by Piot et al., cyclosporine A was shown to significantly decrease myocardial enzyme release 29. A subset of patients underwent cardiac magnetic resonance imaging, and the patients receiving cyclosporine A had smaller infarcts 29. Thus strategies aimed at preventing MPTP opening may have value in the clinical setting.
Mitochondrial Fission and Fusion
In all eukaryotic cell types studied to date, mitochondria behave as a reticulum which undergoes dynamic remodeling on a time-scale of minutes. Individual mitochondria undergo fusion and fission events so frequently that photoactivation of GFP in the matrix of a single mitochondrion within a cell will result in redistribution of the GFP to all mitochondria within 40 to 60 minutes30, 31. This phenomenon has been described in a variety of cell lines and primary cells, and has been assumed to be universal. However, the rigid architecture of the cardiomyocytes, including a network of sarcoplasmic reticulum between mitochondria, has raised the possibility that cardiac mitochondria may undergo little to no fusion and fission, although the relevant proteins (Mfn1, Mfn2, Opa1, and Drp1) are all expressed in cardiomyocytes. A recent study32 appears to implicate fusion in cardioprotection, although the evidence that fusion and fission events occur in cardiomyocytes is far from compelling. More work will be required to understand this dynamic process in the cardiomyocytes. However, it is attractive to entertain the notion, as fusion would alter the surface area to volume ratio, resulting in a higher calcium threshold before the MPTP would open. On the other hand, mitochondrial fission could be beneficial, because in other systems it has been shown to be a prerequisite for mitophagy 30, which is a key mechanism to eliminate damaged organelles.
Mitophagy in Cardioprotection
Efforts to understand the role of autophagy in cardiomyocytes have been accelerated by new tools, including several lines of knockout mice that are deficient in key factors required for autophagy (Atg5, Atg7) and transgenic mice that express fluorescent fusion proteins that allow imaging of autophagosomes in situ (GFP-LC3 and mCherry-LC3) 33, 34. Early studies using Beclin1 heterozygous knockout mice may be misleading as Beclin1 plays a role in initiating autophagy but can also interfere with flux 35, 36 . Reduction of Beclin1 expression has been reported to accelerate flux 37, yet most studies have assumed that reduction of Beclin1 decreases the amount of autophagy. Autophagy is a mechanism for bulk removal of intracellular aggregates and organelles, and provides energy-generating substrates under conditions of nutrient limitation. Our own work has demonstrated a requirement for autophagy in cardioprotection by ischemic preconditioning and by various pharmacologic agents including sulfaphenazole and the adenosine A1 agonist CCPA 38-40. Autophagy has been shown to occur with administration of diazoxide, ranolazine, UTP, rapamycin, hydrophobic statins, and chloramphenicol 38, 41-43. The signal transduction pathway that activates autophagy has much in common with ischemic and pharmacologic preconditioning. Thus AMPK, PKC, ROS, and nitric oxide (NO) have been shown to be important to induction of autophagy as well as to cardioprotection 40, 44, 45. Mitochondrial depolarization (as might occur with opening of the mitochondrial ATP-sensitive potassium channel) is a trigger for selective removal of mitochondria, and an important element of cardioprotective autophagy appears to be the sequestration and degradation of damaged mitochondria 46, 47. Mitochondrial turnover, which is a balance of mitophagy and biogenesis, represents an essential aspect of mitochondrial quality control, a homeostatic process that is critical to the integrity of long-lived cells such as neurons and cardiomyocytes. Thus removal of damaged mitochondria will be followed by biogenesis; the replacement mitochondria may differ with respect to protein composition, protein phosphorylation status, and oxidative modifications. At the present time, it is not known whether the differences in protein composition and post-translational modifications observed in mitochondria from preconditioned hearts 48 are due to modifications of pre-existing mitochondria or wholesale replacement of substantial numbers of mitochondria.
Translational Next Steps
The purpose of these interesting studies is to identify targets and drugs that can reduce the amount of injury associated with myocardial ischemia and reperfusion. Mitochondria are clearly a central target for cardioprotection. Strategies include stabilization of the mitochondria, particularly through inhibition of the MPTP with cyclosporine A, or through elimination of damaged mitochondria through selective mitophagy (Figure 3). Since mitophagy is initiated in part through mitochondrial depolarization49, drugs such as cyclosporine A that inhibit the MPTP may antagonize the therapeutic effects of agents that stimulate mitophagy49; this may be an important consideration in patients receiving statins (which induce autophagy) as well as cyclosporine A. Although it is important to understand the mechanisms by which these various agents elicit cardioprotection, there are other questions that must be addressed in order to select candidates for clinical development from among the panoply of small molecules and interventions that have shown promise in the laboratory. Because positive results in rodents are not always replicated in large animal models (e.g. pigs), it is important to validate efficacy in two or possibly three species, as well as to validate the findings in independent labs. If protection is achieved by inhibiting the MPTP, inducing autophagy, or some other mechanism, how long does the effect need to be sustained? If protection is demonstrated in an acute model, durable benefit must be determined in chronic studies. Are all agents similar with respect to speed of onset, duration of effect, and potency? These questions of pharmacodynamics must be addressed before a drug can be selected for clinical advancement, yet these rather mundane questions are not hypothesis-driven questions that fit easily into the milieu of R01 grant proposals.
Figure 3.
Strategies for mitoprotection. Reperfusion injury is largely due to mitochondrial dysfunction, which may be controlled by inhibiting the MPTP with cyclosporine A or by selective elimination of damaged mitochondria via autophagy.
Reconsidering the Models
A key consideration in planning future studies is the need to use animal models that more closely resemble the human condition. Lean, young, isogenic male mice are a far cry from the studies that must be conducted in the human population. Before an agent can be considered for clinical advancement, it will need to demonstrate reproducible efficacy in both sexes, in aged animals, and in outbred species. Myocardial infarctions are more common and more severe in the presence of co-morbid conditions such as metabolic syndrome, diabetes, and advanced age. For that reason it is reasonable to recommend that a drug that fails to show benefit in animal models of metabolic syndrome, diabetes, or advanced age should not be developed further. Another consideration is the duration of ischemia. Laboratory models have been designed with an optimized duration of ischemia that is quite sensitive, permitting easy detection of a beneficial effect. Unfortunately, many of the drugs which passed this “easy” test subsequently failed to show efficacy in clinical trials. Animal studies involving longer ischemia times (as are typically the case in humans), incomplete reperfusion (which is to be expected in patients with atherosclerosis, microvascular disease, and no-reflow), and presence of co-morbid conditions might be better predictors of which drugs will succeed in the clinical setting. For this reason, efforts should be directed towards developing more clinically relevant models that will be better predictors of which drugs will succeed or fail.
Acknowledgements
This work was supported by NIH R01 HL060590, R01HL034579, R01 AG033283, R43HL095199, and P01 HL085577.
References
- 1.Logue SE, Gustafsson AB, Samali A, Gottlieb RA. Ischemia/reperfusion injury at the intersection with cell death. J Mol Cell Cardiol. 2005;38(1):21–33. doi: 10.1016/j.yjmcc.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Gustafsson AB, Gottlieb RA. Bcl-2 Family Members and Apoptosis, Taken to Heart. Am J Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- 3.Ford DA, Hazen SL, Saffitz JE, Gross RW. The rapid and reversible activation of a calcium-independent plasmalogen-selective phospholipase A2 during myocardial ischemia. J Clin Invest. 1991;88(1):331–335. doi: 10.1172/JCI115296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb RA. Mitochondrial signaling in apoptosis: mitochondrial daggers to the breaking heart. Basic Res Cardiol. 2003;98(4):242–249. doi: 10.1007/s00395-003-0404-0. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson AB, Gottlieb RA. Mechanisms of apoptosis in the heart. J Clin Immunol. 2003;23(6):447–459. doi: 10.1023/b:joci.0000010421.56035.60. [DOI] [PubMed] [Google Scholar]
- 6.Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta. 1998;1366(1-2):79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 7.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434(7033):658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 8.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192(7):1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278(45):44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 10.Karmazyn M. Therapeutic potential of Na-H exchange inhibitors for the treatment of heart failure. Expert Opin Investig Drugs. 2001;10(5):835–843. doi: 10.1517/13543784.10.5.835. [DOI] [PubMed] [Google Scholar]
- 11.de Brito OM, Scorrano L. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 29(16):2715–2723. doi: 10.1038/emboj.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;290(5):H2024–2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15(3):521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- 14.Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008;105(41):15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris MH, Thompson CB. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000;7(12):1182–1191. doi: 10.1038/sj.cdd.4400781. [DOI] [PubMed] [Google Scholar]
- 16.Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276(33):30724–30728. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- 18.Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, Prevost MC, Leber B, Andrews D, Penninger J, Kroemer G. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000;14(5):729–739. [PubMed] [Google Scholar]
- 19.Flaherty JT, Pitt B, Gruber JW, Heuser RR, Rothbaum DA, Burwell LR, George BS, Kereiakes DJ, Deitchman D, Gustafson N, et al. Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation. 1994;89(5):1982–1991. doi: 10.1161/01.cir.89.5.1982. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffari MS, Baban B, Liu JY, Abebe W, Sullivan JC, El-Marakby A. Mitochondrial complex I and NAD(P)H oxidase are major sources of exacerbated oxidative stress in pressure-overloaded ischemic-reperfused hearts. Basic Res Cardiol. 106(2):287–297. doi: 10.1007/s00395-011-0150-7. [DOI] [PubMed] [Google Scholar]
- 21.Perry CN, Huang C, Liu W, Magee N, Carreira R Sousa, Gottlieb RA. Xenotransplantation of mitochondrial electron transfer enzyme, ndi1, in myocardial reperfusion injury. PLoS One. 2011;6(2):e16288. doi: 10.1371/journal.pone.0016288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo BB, Marella M, Yagi T, Matsuno-Yagi A. The single subunit NADH dehydrogenase reduces generation of reactive oxygen species from complex I. FEBS Lett. 2006;580(26):6105–6108. doi: 10.1016/j.febslet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the Permeability Transition Pore in Mitochondria Devoid of Cyclophilin D. J. Biol. Chem. 2005;280(19):18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434(7033):652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 25.Skyschally A, Schulz R, Heusch G. Cyclosporine A at reperfusion reduces infarct size in pigs. Cardiovasc Drugs Ther. 24(1):85–87. doi: 10.1007/s10557-010-6219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheu JJ, Chua S, Sun CK, Chang LT, Yen CH, Wu CJ, Fu M, Yip HK. Intra-coronary administration of cyclosporine limits infarct size, attenuates remodeling and preserves left ventricular function in porcine acute anterior infarction. Int J Cardiol. 147(1):79–87. doi: 10.1016/j.ijcard.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Lie RH, Stoettrup N, Sloth E, Hasenkam JM, Kroyer R, Nielsen TT. Post-conditioning with cyclosporine A fails to reduce the infarct size in an in vivo porcine model. Acta Anaesthesiol Scand. 54(7):804–813. doi: 10.1111/j.1399-6576.2010.02241.x. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson LO, Zhou AX, Larsson E, Astrom-Olsson K, Mansson C, Akyurek LM, Grip L. Cyclosporine does not reduce myocardial infarct size in a porcine ischemia-reperfusion model. J Cardiovasc Pharmacol Ther. 15(2):182–189. doi: 10.1177/1074248410362074. [DOI] [PubMed] [Google Scholar]
- 29.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359(5):473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 30.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27(2):433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wikstrom JD, Katzman SM, Mohamed H, Twig G, Graf SA, Heart E, Molina AJA, Corkey BE, de Vargas LM, Danial NN, Collins S, Shirihai OS. {beta}-Cell Mitochondria Exhibit Membrane Potential Heterogeneity That Can Be Altered by Stimulatory or Toxic Fuel Levels. Diabetes. 2007;56(10):2569–2578. doi: 10.2337/db06-0757. [DOI] [PubMed] [Google Scholar]
- 32.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121(18):2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 33.Iwai-Kanai E, Yuan H, Huang C, Sayen MR, Perry-Garza CN, Kim L, Gottlieb RA. A method to measure cardiac autophagic flux in vivo. Autophagy. 2008;4(3):322–329. doi: 10.4161/auto.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizushima N, Kuma A. Autophagosomes in GFP-LC3 Transgenic Mice. Methods Mol Biol. 2008;445:119–124. doi: 10.1007/978-1-59745-157-4_7. [DOI] [PubMed] [Google Scholar]
- 35.Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, Maejima I, Shirahama-Noda K, Ichimura T, Isobe T, Akira S, Noda T, Yoshimori T. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11(4):385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 36.Zhong Y, Wang QJ, Yue Z. Atg14L and Rubicon: Yin and yang of Beclin 1-mediated autophagy control. Autophagy. 2009;5(6):890–891. doi: 10.4161/auto.9162. [DOI] [PubMed] [Google Scholar]
- 37.Foyil SA, Ma X, Hill JA, Dorn GW, 2nd, Diwan A. BNIP3 Induced Autophagy Contributes to Adverse Ventricular Remodeling. J Cardiac Failure. 2010;16(8, Supplement):S35. [Google Scholar]
- 38.Huang C, Yitzhaki S, Perry CN, Liu W, Giricz Z, Mentzer RM, Jr., Gottlieb RA. Autophagy Induced by Ischemic Preconditioning is Essential for Cardioprotection. J Cardiovasc Transl Res. 2010 doi: 10.1007/s12265-010-9189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yitzhaki S, Huang C, Liu W, Lee Y, Gustafsson AB, Mentzer RM, Jr., Gottlieb RA. Autophagy is required for preconditioning by the adenosine A1 receptor-selective agonist CCPA. Basic Res Cardiol. 2009;104(2):157–167. doi: 10.1007/s00395-009-0006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C, Liu W, Perry CN, Yitzhaki S, Lee Y, Yuan H, Tsukada YT, Hamacher-Brady A, Mentzer RM, Jr., Gottlieb RA. Autophagy and Protein Kinase C Are Required for Cardioprotection by Sulfaphenazole. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.00716.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sala-Mercado JA, Wider J, Undyala VV, Jahania S, Yoo W, Mentzer RM, Jr., Gottlieb RA, Przyklenk K. Profound cardioprotection with chloramphenicol succinate in the swine model of ischemia-reperfusion injury. Circulation. 2010;122 doi: 10.1161/CIRCULATIONAHA.109.928242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Araki M, Motojima K. Hydrophobic statins induce autophagy in cultured human rhabdomyosarcoma cells. Biochem Biophys Res Commun. 2008;367(2):462–467. doi: 10.1016/j.bbrc.2007.12.166. [DOI] [PubMed] [Google Scholar]
- 43.Khan S, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol. 2006;41(2):256–264. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Yuan H, Perry CN, Huang C, Iwai-Kanai E, Carreira RS, Glembotski CC, Gottlieb RA. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol. 2009;296(2):H470–479. doi: 10.1152/ajpheart.01051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gottlieb RA, Finley KD, Mentzer RM., Jr Cardioprotection requires taking out the trash. Basic Res Cardiol. 2009;104(2):169–180. doi: 10.1007/s00395-009-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8(1):3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 47.Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol. 2010;299(2):C203–210. doi: 10.1152/ajpcell.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arrell DK, Elliott ST, Kane LA, Guo Y, Ko YH, Pedersen PL, Robinson J, Murata M, Murphy AM, Marban E, Van Eyk JE. Proteomic analysis of pharmacological preconditioning: novel protein targets converge to mitochondrial metabolism pathways. Circ Res. 2006;99(7):706–714. doi: 10.1161/01.RES.0000243995.74395.f8. [DOI] [PubMed] [Google Scholar]
- 49.Carreira RS, Lee Y, Ghochani M, Gustafsson AB, Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy. 2010;6(4) doi: 10.4161/auto.6.4.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]