Abstract
Human Papillomavirus-16 (HPV-16) associated squamous carcinoma of the oropharynx has a favorable prognosis. Patients with HPV-16 positive cancers have elevated peripheral blood CD8+ T lymphocyte levels that correlate with response to chemotherapy and survival. Tumor infiltrating lymphocyte subpopulations (TIL) were assessed in pretreatment biopsies from a prospective patient cohort to determine if TIL subsets differed by HPV status, clinical factors, patient outcome or correlated with peripheral blood T cell levels.
Methods
Measured were CD8, CD4, CD68 and Treg (FoxP3) lymphocytes by immunohistochemistry in a tissue microarray created from patients (n=46) with advanced oropharynx cancer. Correlations with peripheral blood levels, HPV status, expression of EGFR, clinical tumor and patient characteristics and outcome were determined. Patients were treated with a single course of neoadjuvant chemotherapy (cisplatin, 5-fluorouracil) followed by either surgery (non-responders) or chemoradiation (cisplatin 100 mg/m2 every 3 weeks × 3; 70 Gy, 2 Gy daily × 7 wks) for responders. Median follow up was 6.6 years.
Results
HPV-16 positive patients had improved survival (p=0.016). Degree of T cell infiltration did not differ by HPV status but was significantly related to disease specific (DSS) and overall survival (OS). Higher infiltration by CD8, CD4 and FoxP3 subsets was significantly associated with lower T stage and survival. Even after adjusting for HPV status, CD8, FoxP3 and total T cells were significantly associated with DSS (p=0.0236; 0.0040; 0.0197) and OS (p=0.0137; 0.0158; 0.0115, respectively). Less T cell infiltration (p=0.0130) and CD4 cells in particular (p=0.0792) were associated with higher EGFR expression. FoxP3 infiltration correlated significantly and directly with CD4 and CD8 infiltration but not with peripheral blood levels.
Conclusions
Improved outcomes are associated with increased TILs independent of HPV status and suggest the local immune response to HPV-16 may be related in part to factors such as tumor size, EGFR expression, smoking history, performance status or innate immunity. Assessment of TILs in tissue microarrays is difficult due to small core sample size and variation in tumor representation in tissue cores. Further study of larger numbers of patients and infiltrates in whole tumor sections combined with functional analysis of individual subsets may be necessary to detect differences in local immunity in HPV-16 related cancers.
INTRODUCTION
It is unclear why patients with HPV-16 associated oropharyngeal cancer have a more favorable prognosis than patients with HPV-16 negative cancers (1-3). Patients with HPV-16 associated cancers often are younger in age, frequently have large, cystic regional metastases and often are non-smokers (4,5). The better prognosis of such patients is likely due to differences in tumor biology and differing oncogenesis but may also reflect the role the host immune system and tumor microenvironment play in cancer homeostasis (6,7).
To better characterize potential differences in host cellular immune reactivity among patients with HPV-16 positive or negative cancers, we undertook a systematic analysis of T lymphocyte subpopulations in the peripheral blood and the tumor microenvironment in a cohort of patients with advanced oropharyngeal cancer who were entered in a prospective Phase II clinical trial of induction chemotherapy followed by concurrent chemoradiation (8). We previously identified significantly higher pretreatment levels of CD8 positive T lymphocytes in the peripheral blood of HPV-16 positive patients that correlated with improved survival (9). Higher CD8 levels were also associated with tumor response to induction chemotherapy. Others have noted low CD8 cell infiltrates associated with poor cause specific survival in laryngeal cancer (10) and higher T cell infiltrates in patients with HPV positive cancers which were associated with improved survival only in patients with HPV negative tumors (11). The current investigation extends these findings to an examination of the characteristics of the T cell subpopulations infiltrating primary oropharyngeal cancers by immunohistologic assessment of select T cell subpopulations in a tissue microarray created from pretreatment biopsies.
METHODS
Patient Population
Of 66 patients entered in the prospective clinical trial, adequate tissue for creation of a tissue microarray was available for 50 patients and adequate tissue for immunohistologic analysis of T cell infiltrates on the microarray was present for 46 patients. There were 36 males and 10 female patients. Mean age was 57 years (range 39-77 years). A total of 9 patients were Stage III and 37 were Stage IV. All patients had previously untreated, potentially resectable cancers. Eleven patients were never smokers, 18 were past smokers having quit more than a year prior to diagnosis and 17 were current smokers. Site of primary tumor was base of tongue in 27 and tonsil in 19 patients. Adequate DNA for HPV testing was available for 38 of these cases. A total of 25 patients had HPV-16 positive cancers and 13 were negative for HPV-16 by highly sensitive and specific quantitative method (Sensigen, Ann Arbor, MI) using combined real-time competitive polymerase chain reaction and MALDI-TOF (matrix-assisted laser desorption ionization-time-of-flight) mass spectroscopy testing (12).
Clinical Treatment, Response to Chemotherapy and Survival
Patients received induction chemotherapy consisting of one cycle of cisplatin (100 mg/m2/d for 1 day) and 5-fluorouracil (1,000 mg/m2/d for 5 days). Carboplatin (AUC) was used in place of cisplatin in patients with renal insufficiency or hearing loss. Response to the initial chemotherapy was evaluated by surface measurements at direct laryngoscopy, supplemented by radiographic imaging (CT or MRI) for deeply infiltrative tumors. Patients who demonstrated at least a 50% tumor reduction of the primary tumor were considered responders and underwent definitive concurrent chemotherapy (cisplatin 100 mg/m2 days 1, 22, 43) and radiation therapy (70 Gy; daily 2 Gy fractions × 7 weeks). Following radiation, two cycles of adjuvant paclitaxel (175 mg/m2), every 21 days was administered to complete responders. Non-responders to induction chemotherapy received salvage surgery and radiation. A total of 39 patients responded to induction chemotherapy and 36 were still considered responders three months after completion of concurrent chemotherapy and radiation. One patient was non-evaluable for induction response. Median follow up of the patient cohort is 6.6 years. A total of 26 patients remain alive (56%), 15 have died of their cancer and 5 have died of other causes.
Immunohistology
Pre-treatment biopsies were retrieved from 50/66 patients for construction of a tissue microarray (TMA). Pathology blocks were not available from 15 patients who had outside biopsies. Briefly, TMA slides created from biopsy specimens from the patients in this trial were deparaffinized, rehydrated, and peroxidase quenched (Dako Cytomation, Glostrup, Denmark). For antigen retrieval, slides were incubated with pepsin (EGFR;10 minutes at 37°C) or with citrate buffer (p16 and p53; 30 minutes at 92°C) and were blocked with horse serum (30 minutes at 25°C). Primary antibody, EGFR/31G7 (Zymed Laboratories, South San Francisco, CA), p16/16P04, p53/DO1, (Lab-Vision, Fremont CA) were added for 1 hour and were probed with avidin/biotin peroxidase (ABC Kit; Vector Laboratories, Burlingame, CA). Antibody binding was scored by a pathologist who was blinded to the clinical outcome using a continuous scale (i.e., 10%, 30%, 90%, etc) for the proportion of EGFR-positive tumor cells in each core. For p16, and p53, a scale of 1 to 4 was used: 1 was less than 5%; 2, 5% to 20%; 3, 21% to 50%; and 4, 51% to 100% tumor staining. Intensity was scored as 1 equal to no staining; 2, low intensity; 3, moderate; and 4, high intensity. Scores for multiple cores from each patient were averaged. For p53 mutation, p53 exons 4 to 9 were amplified by using specific primers. Products from two polymerase chain reactions were sequenced in both directions and were analyzed by using Mutation Surveyor version 2.61 (Soft Genetics, State College, PA) and by manual review.
For the immunohistologic evaluation of tumor infiltrating lymphocytes (TIL), all tests were carried out on 5 micron formalin fixed paraffin embedded TMA sections. Sections were baked in hot air oven at 65°C overnight. Each section was dewaxed using a series of xylene, graded alcohol, and buffer immersion steps. Antigen retrieval was performed in a preheated pressure cooker. Immunohistochemical staining was performed on the DAKO Autostainer (DAKO, Carpinteria, CA) using DAKO labeled avidin-biotin-peroxidase method (LSAB+) and 3,3′-diaminobenzidine (DAB) as the chromogen. Deparaffinized sections were stained with five types of Mabs: CD4 1:250 (Abcam ab846), CD8 1:40 (Nova Castra VP-C320), FOXp3 1:200 (Abcam Ab20034), CD104 1:50 (Beta-4 integrin, eBioscience 439-9b), CD68 1:100 (Dako M0814). Appropriate negative (no primary antibody) and positive controls [tonsillar tissue and various carcinomas] were stained in parallel with each set of tumors studied. Digital photomicrographs were obtained at x20 magnification. The number of positively stained TILs for CD4, CD8, FOXP3, and CD68 was assessed quantitatively by two investigators blinded to patient outcome and HPV status. Tissue cores were initially examined after CD104 (beta-4 integrin) staining to confirm the presence and location of tumor nodules in each core and only TILs infiltrating tumor nodules were counted (Figure 1). TILs in each tumor core on the tissue microarray were manually counted using a 20x objective lens. Methods for counting were adapted from published methods used to analyze T cell infiltrates in follicular lymphoma and head and neck TMAs (13, 14). The mean count of replicate cores for each subject was used for analysis.
FIGURE 1.
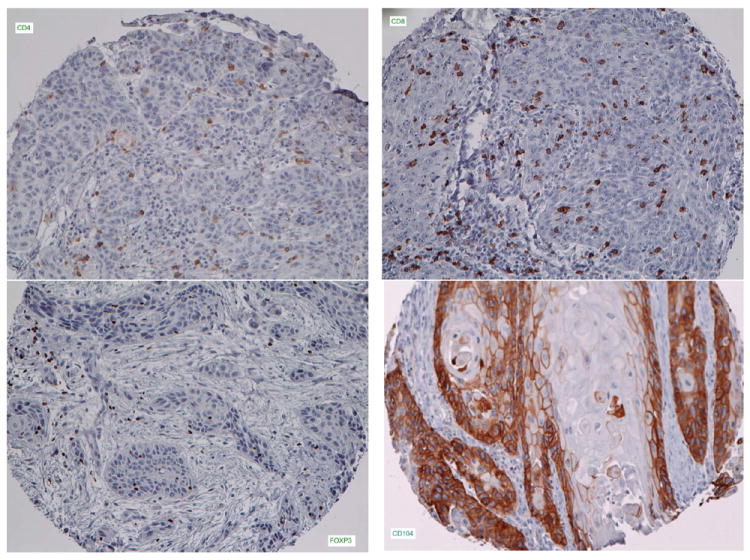
Representative tissue microarray cores stained for (a) CD4, (b) CD8, (c) FoxP3 cells and (d) beta-4 integrin [CD104].
Pretreatment peripheral blood was analyzed by routine automated flow cytometry for T, B, and NK cells and subpopulations of CD3, CD4, and CD8-positive T lymphocytes. Detailed methods have been previously described (9,15). Determinations were made using commercially available monoclonal antibody reagents by an indirect immunofluorescent technique and were performed in the clinical laboratories of the University of Michigan Pathology Department. The results of correlations with survival and HPV-16 status have been previously published (9).
Statistical Methods
Statistical analysis was carried out in two parts. First, all subjects who had measured infiltrate levels (n=46) were analyzed for correlations with clinical outcomes, tumor and patient characteristics. Second, correlations between infiltrate levels and peripheral blood lymphocyte levels (n=34) or HPV-16 status (n=38) were performed on only those with both sets of information.
Infiltrate levels were treated as continuous variables in all analyses. Rank-based statistical methods were used to assess univariate associations between infiltrate levels and covariates of interest. Patient level averages for EGFR score were used in this analysis. Patients were categorized as current, past (quit> 12 months ago), or never smokers. Spearman rank correlation was used to assess the correlation with ordinal variables and the Wilcoxon rank sum test was used to test variables with two groups.
Disease specific survival was defined as the time to death from oropharyngeal cancer. Patients who were alive at last follow-up or who died as a result of reasons other than their cancer were censored. Cox proportional hazards models were used to assess time to event outcomes. For each outcome, three models were constructed: a model with infiltrate level (treated as a continuous variable), a model with clinical variables, and a model with both clinical variables and infiltrate level. Likelihood ratio tests were used to compare models and the maximum likelihood estimate for coefficients in a model was used to test significance after controlling for other variables in the model.
All statistical analyses were done in SAS version 9.2 (SAS Institute, Carey, NC). A two-tailed P value of 0.05 or less was considered statistically significant.
RESULTS
The major finding in this study of immune cell infiltrates in pretreatment biopsies from oropharyngeal cancer patients was the lack of significant differences in types of T cell infiltrates among HPV-16 positive and negative patients. The pattern of T lymphocyte subset infiltration did not differ by HPV-16 status in this group of advanced oropharyngeal cancer patients entered in this prospective, uniform treatment clinical trial. Although the mean CD4/CD8 ratio of tumor infiltrating T lymphocytes tended to be lower in HPV-16 positive patients and the sum of CD4 and CD8 cell infiltrate counts tended to be higher, these differences were not statistically different (Table 1). Likewise, no differences were noted for FoxP3 or CD68 infiltrate counts by HPV status. This was in contrast to the significant differences previously noted in the peripheral blood T lymphocyte subsets in these two groups of patients where CD8 cell levels were higher and CD4 cell levels were lower in HPV-16 positive patients (9).
TABLE 1.
MEAN (+/-SEM) T CELL SUBSET INFILTRATES IN HPV-16 POSITIVE AND NEGATIVE CANCERS
| HPV Negative (n=13) |
HPV Positive (n=25) |
|
|---|---|---|
| CD4 | 174 +/-31 | 189 +/-27 |
| CD8 | 168 +/-37 | 210 +/-47 |
| FoxP3 | 159 +/-36 | 107 +/-18 |
| CD68 | 241 +/-42 | 250 +/-44 |
| CD4 & CD8 sum | 345 +/-62 | 413 +/-66 |
| CD4/CD8 ratio | 4.16 +/-2.78 | 1.56 +/-0.35 |
Our analysis confirmed that HPV-16 status and EGFR expression were the most significant prognostic factors for overall survival in this study (p=.0455, p=.0083, respectively). When T cell infiltrates were analyzed with respect to overall survival and after including these important prognostic biomarkers in the analysis, the infiltrate CD4/CD8 ratio (p=.0056) and sum of CD4 and CD8 mean infiltrate counts were significant (p=.0154) predictors of overall survival and were still important after adjusting for HPV status (p=.034 and p=.0298, respectively- Cox Regression). After controlling for EGFR, these T cell infiltrate measures remained significant (p=0.008 and p=0.04, respectively – Cox Regression). Overall survival tended to be better in patients with higher tumor infiltrates of CD8 cells (p=.0615) and the mean CD8 infiltrate count added prognostic significance after adjusting for HPV status (p=0.0137 - Likelihood ratio test). Mean infiltrate levels according to whether patients survived or were dead are shown in Table 2. Likewise, higher FoxP3 infiltrates counts were also significantly associated with better overall survival after adjusting for HPV status (p=0.029 Cox Regression).
TABLE 2.
MEAN (+/- SEM) T CELL INFILTRATES BY DISEASE STATUS
| ALIVE | DEAD | |
|---|---|---|
| CD4 | 206 +/-26 | 135 +/-18 |
| CD8 | 236 +/-45 | 122 +/-19 |
| FoxP3 | 145 +/-24 | 90 +/-15 |
| CD68 | 269 +/-37 | 225 +/-29 |
| CD4 & CD8 sum | 471 +/-61 | 254 +/-29 |
| CD4/CD8 ratio | 1.44 +/-0.3 | 3.18 +/-1.4 |
Disease specific survival was significantly associated with EFGR expression (p=.015), HPV status (p=.026), peripheral blood CD4/CD8 ratio (p=.047) and smoking history (p=.003). Of the various infiltrate markers, only the infiltrate CD4/CD8 ratio and sum of CD4 plus CD8 cell infiltrates were significantly associated with disease specific survival (p=.0058 and p=.0244, respectively). However, after adjusting for HPV-16 status, CD8 and FoxP3 infiltrates added significant prognostic information (p=.0236 and p=.004 – Likelihood ratio test), and EGFR intensity remained the most important factor (Table 3).
TABLE 3.
p VALUES FOR THE ASSOCIATION OF INFILTRATE SUBSETS WITH DISEASE SPECIFIC SURVIVAL BY COX REGRESSION
| UNIVARIATE | ADJUSTED FOR HPV-16 STATUS** | |
|---|---|---|
| HPV-16* | .0263 | – |
| EGFR EXPRESION | .0150 | .0470 |
| CD4/CD8 Ratio | .0058 | .0554 |
| CD4 & CD8 Sum | .0244 | .0197 |
| CD8 | .0848 | .0236 |
| FoxP3 | .0824 | .0040 |
log transformed
likelihood ratio test
Smoking status was also a significant prognostic factor for both overall (p=0.01) and disease specific survival (p=0.002) and remained significant for disease specific survival after controlling for HPV-16 status (p=0.008). The sum of mean CD4 and CD8 cell infiltrates was significantly higher in non-smokers (p=.0467) and FoxP3 infiltrates also tended to be higher in non-smokers (p=.08). After controlling for smoking and EGFR intensity, the CD4/CD8 infiltrate ratio and the sum of CD4 and CD8 infiltrates were still prognostically important for overall survival. For disease specific survival, EGFR intensity, HPV status, peripheral blood CD8 levels and the CD4/CD8 infiltrate ratio were significant prognostic factors after controlling for smoking. For overall survival, after controlling for both smoking and HPV status, both CD4/CD8 ratio and infiltrate sum showed a trend for prognostic significance (p=.07 for each).
When clinical features were analyzed, there were no significant differences in overall T cell infiltrates with respect to tumor stage, response to induction chemotherapy or overall tumor response after concurrent chemoradiation. Mean CD8 infiltrates and total CD4 and CD8 counts were higher in node positive patients (p=.011 Wilcoxon for both) yet CD8 infiltrates declined with increasing T class (p=.003 Spearman rho=-0.424). Because CD8 infiltrates increased with N stage and were associated with improved survival, we analyzed N class with respect to overall and disease specific survival. We found that contrary to what is typical for other tumor sites, increasing N class was not a negative prognostic factor in this cohort of oropharyngeal cancer patients. This was primarily due to better disease specific survival in patients with advanced (N2) neck disease. This is likely related to patients with HPV-16 positive cancers who often have small primary tumors and multiple small lymph nodes or a single large cystic node. Recurrence rates were highest in patients with N0 disease who had larger primary cancers. Interestingly, CD8 infiltrates were also significantly higher in association with better patient Karnofsky performance status (p=.003 Spearman, rho=0.43) and the infiltrates of CD4 and FoxP3 cells also tended to be increased in those patients (p=.10, p=.18, Spearman, respectively) which resulted in the sum of T cell infiltrates (CD4 plus CD8) to be significantly higher when performance status was better (p=.004, Spearman, rho=0.45).
Individual T cell subset infiltrates were not directly associated with either EGFR expression or p53 mutation status. T cell subset levels in the peripheral blood were available in 34 of the 46 patients with cancers analyzed for T cell tumor infiltrates. Type and degree of specific T cell subset infiltration was not related to peripheral blood levels of the various subsets except for CD4 infiltrates which tended to be higher when peripheral blood natural killer cell levels were low (p=.0133, rho=-0.439). Levels of each of the infiltrate subsets except for CD68 cells tended to be directly correlated with each other (p<.0001) and with total T cells. Levels of CD8 and FoxP3 cells were inversely correlated with the CD4/CD8 ratio (p<.0001 and p=.012 respectively).
DISCUSSION
This is the first study to correlate both pretreatment peripheral blood T lymphocyte levels with T cell tumor infiltrates in patients with advanced oropharyngeal cancer and to examine the association with HPV-16 status and other known prognostic factors such as EFGR expression, smoking status, tumor characteristics and performance status. A major finding was the lack of association of T cell infiltrates with HPV status or with EGFR expression. This is particularly interesting since our prior study of systemic T cell levels demonstrated that increased peripheral blood CD8 cell levels were associated with HPV-16 status, lower EGFR expression, response to induction chemotherapy and favorable prognosis (9). This suggests that peripheral blood markers of adaptive immunity are not surrogates for immune parameters in the primary tumor microenvironment. Despite the lack of association with HPV status, the tumor infiltrate CD4/CD8 ratio and the mean sum of CD4 and CD8 infiltrates were predictive of overall and disease specific survival indicating that an immune response in the local tumor microenvironment may be an important factor in overall prognosis. Generally, tumor infiltrating lymphocytes are thought to represent a host immune response. It would be expected that infiltrates would be beneficial (16). However, even in HPV-related cervical cancer, large polyclonal infiltrates of T cells are seen which appear to be functionally inactive (17). Animal model studies suggest that multiple subtypes of CD4 and CD8 are necessary to generate tumor reactive CD8 functional activity and that TILs can be both immune reactive and immunosuppressive (18,19). Relative expansion of infiltrates of FoxP3 regulatory T cells, and cytotoxic T cells have been described in head and neck cancer patients but no associations with outcome were found (14).
The associations of T cell infiltrates with survival remained significant after adjusting for EGFR expression and HPV status. This important observation suggests that the local immune response and its impact on outcome may be independent of these important prognostic markers and not directly related to peripheral blood measures of cellular immunity which previously were shown to differ by HPV status (9). Recently, gene signatures of adaptive immunity have been shown to outperform HPV status in prognostication (20). Increased levels of CD3 positive tumor infiltrating T cells in general have been reported in oropharyngeal cancer patients who were without regional metastases that were related to HPV-16 status (11, 21). Others have reported that higher levels of activated CD4 and FoxP3 cells in tumor stroma are associated with improved locoregional control and survival (22) in a patient cohort consisting of multiple sites of head and neck cancer. CD4+ FoxP3+ regulatory T cells recovered from the peripheral blood of head and neck cancer patients have been associated with local immune suppression mediated by IL-10 and TGF-beta (23). However, higher levels of such suppressive cells in the blood were found in patients with no evidence of disease after therapy (24, 25). Further, when analyzed in human tumor sections, higher Treg cell levels were associated with improved prognosis (26) which was consistent with our findings. Understanding the function of such cells in the local tumor environment and their influence on the inflammatory response will be critical to determining in what situations the local immune response is beneficial or detrimental to outcome.
It is remarkable and encouraging that we found significant associations of lymphocyte infiltrates with outcome in this study which utilized the small tissue cores present on a tumor microarray created from pretreatment samples. Tumor bearing tissue in the biopsies was specifically selected by the pathologist in creating the arrays, however, there was no effort to specifically select any particular area of the tumor leading to the possibility of sampling errors for areas representative of a local tumor immune response. Also, many of these tumors had associated lymphoid components because of the normal lymphoid tissue present in the tonsil and tongue base sites suggesting the possibility that some of the infiltrates could represent resident normal lymphoid aggregates. To minimize this potentially confounding possibility, the tissue cores were also stained with Beta-4 integrin (CD104) to specifically identify tumor within the cores samples and only T lymphocytes present in the tumor nodules were counted.
This is the first analysis to look at response to induction chemotherapy and local tumor immune parameters in patients with oropharyngeal cancers. We previously found that higher systemic CD8 cell levels were associated with tumor response to chemotherapy. In this further analysis, we found that tumor response was not associated with any of the T lymphocyte infiltrates. This was consistent with other reports of CD4+ lymphocyte infiltrates measured in the stroma of a heterogeneous cohort of head and neck patients (22) and contrasts findings of higher tumor infiltrating CD8+ T cells in metastatic lymph nodes from patients with favorable outcomes (14). It was disappointing that tumor response to chemotherapy was not associated with T lymphocyte infiltrates as has been reported in breast cancer (27).
The consistent correlations of higher T cell infiltrates with favorable outcome are also reflected in the association of higher CD8 infiltrates in patients with lower T classifications and better performance status. Infiltrate levels of CD4, CD8 and Foxp3 cells were also associated with the important prognostic factor of smoking status and a trend for improved survival was present even after adjusting for smoking habit. The potential effects of smoking on tumor initiation, progression, epigenetic gene methylation and inflammation are probably some of the factors influencing T cell levels and outcome in oropharyngeal cancer patients beyond simple HPV status. The relative importance of these various factors related to the attraction and retention of immune cells to the tumor microenvironment remains largely unknown. Our findings extend those of others (7, 16, 28) and suggest that immunologic methods to increase immune reactive, cytotoxic CD8 cell levels in the peripheral blood or in the tumor microenvironment may be beneficial in both HPV+ and HPV – cancer patients. Evidence from studies in head and neck (29) and other cancer types such as breast (30), ovarian (31) or colon (32) support the conjecture that there are differences in numbers and function of T lymphocytes, and Treg cells in particular, in intratumoral versus peritumor stromal locations and that the association of regulatory T cells with outcome differs by tumor type (33). Thus, tumor specific and site specific studies in homogeneous populations of head and neck cancer patients are necessary to better understand how peripheral blood levels and tumor infiltration by immune reactive cells affect prognosis. It remains to be seen if experimental vaccination schemes can modulate levels of both regulatory and cytotoxic T cells in the tumor microenvironment of patients with HPV positive cancers. The lack of differences in infiltrating T cells in patients with oropharyngeal cancer regardless of HPV status suggest that immunotherapy regimens that are effective in HPV positive cancers patients should also be explored in patients with HPV negative cancer.
FIGURE 2.
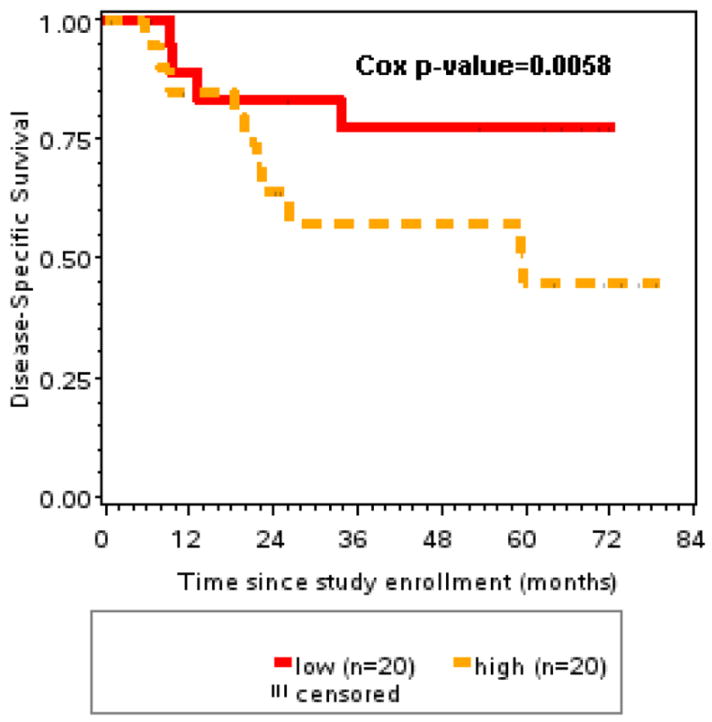
Disease specific survival was significantly better in patients with low CD4/CD8 ratio in tumor infiltrates compared to patients with higher (>median) ratios (p=.0058).
FIGURE 3.
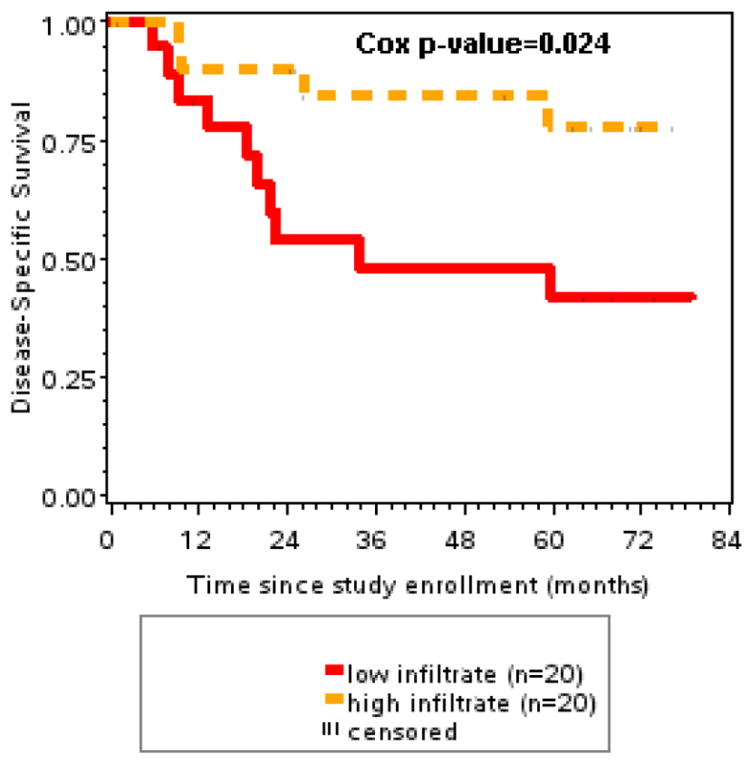
Disease specific survival was significantly better with higher CD4 and CD8 T cell tumor infiltrates compared to patients with lower (<median) T cell infiltrates (p=.024).
Acknowledgments
Supported by: P50 CA097248, R01 DE13346, NIDCD T32 DC005356 and the Sinabaldo and Diane Tozzi Research Fund.
Footnotes
Presented at the American Head and Neck Society 2010 Research Workshop on the Biology, Prevention and Treatment of Head and Neck Cancer, October 30, 2010, Arlington, VA
References
- 1.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. NEJM. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TRPG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24:2606–2611. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer. Its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15:6758–6762. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 5.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–20. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 6.Spanos WC, Nowicki P, Lee DW, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 7.Whiteside TL. Immune responses to malignancies. J Allergy Clin Immunol. 2010;125:S272–83. doi: 10.1016/j.jaci.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Worden FP, Kumar B, Lee JS, et al. Chemoselection as a strategy for organ preservation in advanced oropharyngeal cancer: response and survival positively associated with HPV-16 copy number. J Clin Oncol. 2008;26:3138–3146. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wansom D, Light E, Worden F, et al. Correlation of cellular immunity with human papillomavirus 16 status and outcome in patients with advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2010;136:1267–1273. doi: 10.1001/archoto.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogino T, Shigyo H, Ishii H, et al. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66:9281–9289. doi: 10.1158/0008-5472.CAN-06-0488. [DOI] [PubMed] [Google Scholar]
- 11.Kong CS, Narasimhan B, Cao H, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiation Oncol Biol Phys. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H, Yang K, Khafagi A, et al. Sensitive detection of human papillomavirus in cervical, head/neck, and shistosomiasis-associated bladder malignancies. Proc Natl Acad Sci USA. 2005;102:7683–7688. doi: 10.1073/pnas.0406904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee AM, Clear AJ, Calaminici M, et al. Number of CD4+ cells and location of Forkhead box protein P3=positive cells in diagnostic follicular lymphoma tissue microarrays correlates with outcome. J Clin Oncol. 2006;24:5052–5059. doi: 10.1200/JCO.2006.06.4642. [DOI] [PubMed] [Google Scholar]
- 14.Pretscher D, Distel LV, Grabenbauer GG, et al. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro-and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292–301. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf GT, Schmatlz S, Hudson J, et al. Alterations in T-lymphocyte subpopulations in patients with head and neck squamous carcinoma: correlations with prognosis. Arch Otolaryngol Head Neck Surg. 1987;113:1200–1206. doi: 10.1001/archotol.1987.01860110066010. [DOI] [PubMed] [Google Scholar]
- 16.Vu HL, Sikora AG, Fu S, Kao J. HPV-induced oropharyngeal cancer, immune response and response to therapy. Cancer Letters. 2010;288:149–155. doi: 10.1016/j.canlet.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Van Steenwijk PJDV, Heusinkveld M, Ramwadhdoebe TH, et al. An unexpectedly large polyclonal repertoire of HPV-specific T cells is poised for action in patients with cervical cancer. Cancer Res. 2010;70:2707–17. doi: 10.1158/0008-5472.CAN-09-4299. [DOI] [PubMed] [Google Scholar]
- 18.Anichini A, Molla A, Vegetti C, et al. Tumor-reactive CD8+ early effector T cells indentified at tumor site in primary and metastatic melanoma. Cancer Res. 2010;70:8378–87. doi: 10.1158/0008-5472.CAN-10-2028. [DOI] [PubMed] [Google Scholar]
- 19.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70:83-68–77. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurlow JK, Murillo CLP, Hunter KD, et al. Spectral clustering of microarray data elucidates the roles of microenvironment remodeling and immune responses in survival of head and neck squamous cell carcinoma. J Clin Oncol. 2010;28:2881–2888. doi: 10.1200/JCO.2009.24.8724. [DOI] [PubMed] [Google Scholar]
- 21.Rajjoub S, Basha SR, Einhorn E, et al. Prognostic significance of tumor-infiltrating lymphocytes in oropharyngeal cancer. ENT-Ear Nos Throat J. 2007;86:506–511. [PubMed] [Google Scholar]
- 22.Badoual C, Hans S, Rodriquez J, et al. Prognostic value of tumor-intiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–471. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 23.Strauss L, Bergmann C, Szczepanski M, et al. A unique subset of CD4+CD25high Fozp3+ T cells secreting Interleukin-10 and transforming growth factor-Beta1 mediates suppression in the tumor microenvironment. Clin Cancer res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 24.Strauss L, Bergmann C, Gooding W, et al. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–6311. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- 25.Chikamatsu K, Sarakura K, Whiteside TL, Furuya N. relationships between regulatory T cells and CD8+ effector populations in patients with squamous cell carcinoma of the head and neck. Head Neck. 2007;29:120–127. doi: 10.1002/hed.20490. [DOI] [PubMed] [Google Scholar]
- 26.Loose D, Signore A, Bonanno E, et al. Prognostic value of CD25 expression on lymphocytes and tumor cells in squamous-cell carcinoma of the head and neck. Cancer Biothera Radiopharma. 2008;23:25–33. doi: 10.1089/cbr.2007.0373. [DOI] [PubMed] [Google Scholar]
- 27.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent poredictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2009;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 28.Badoual C, Sandoval F, Pere H, et al. Better understanding tumor-host interaction in head and neck cancer to improve the design and development of immunotherapeutic strategies. Head Neck. 2010;32:946–958. doi: 10.1002/hed.21346. [DOI] [PubMed] [Google Scholar]
- 29.Wolf GT, Hudson JL, Peterson KA, et al. Lymphocyte subpopulations infiltrating squamous carcinomas of the head and neck: correlations with extent of tumor and prognosis. Otolaryngol Head Neck Surg. 1986;95:142–152. doi: 10.1177/019459988609500203. [DOI] [PubMed] [Google Scholar]
- 30.An T, Sood U, Pietruk T, et al. In situ quantitation of inflammatory mononuclear cells in ductal infiltrating breast carcinoma. Relation to prognostic parameters. Am J Pathol. 1987;128:52–60. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Conejo-Garcia JR, Kasaros D, et al. Intratumor T cells, recurrence and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 32.Galon J, Costges A, Sancez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 33.Menetrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009;69:7895–8. doi: 10.1158/0008-5472.CAN-09-1642. [DOI] [PubMed] [Google Scholar]


