Abstract
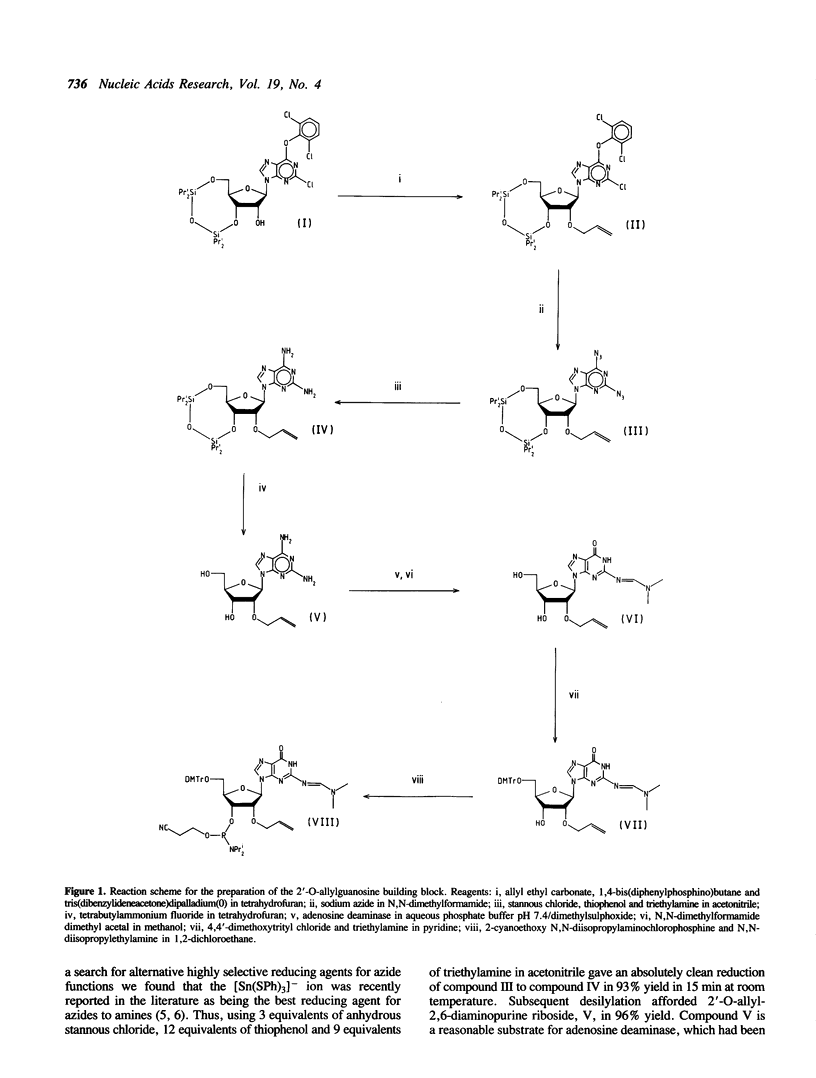
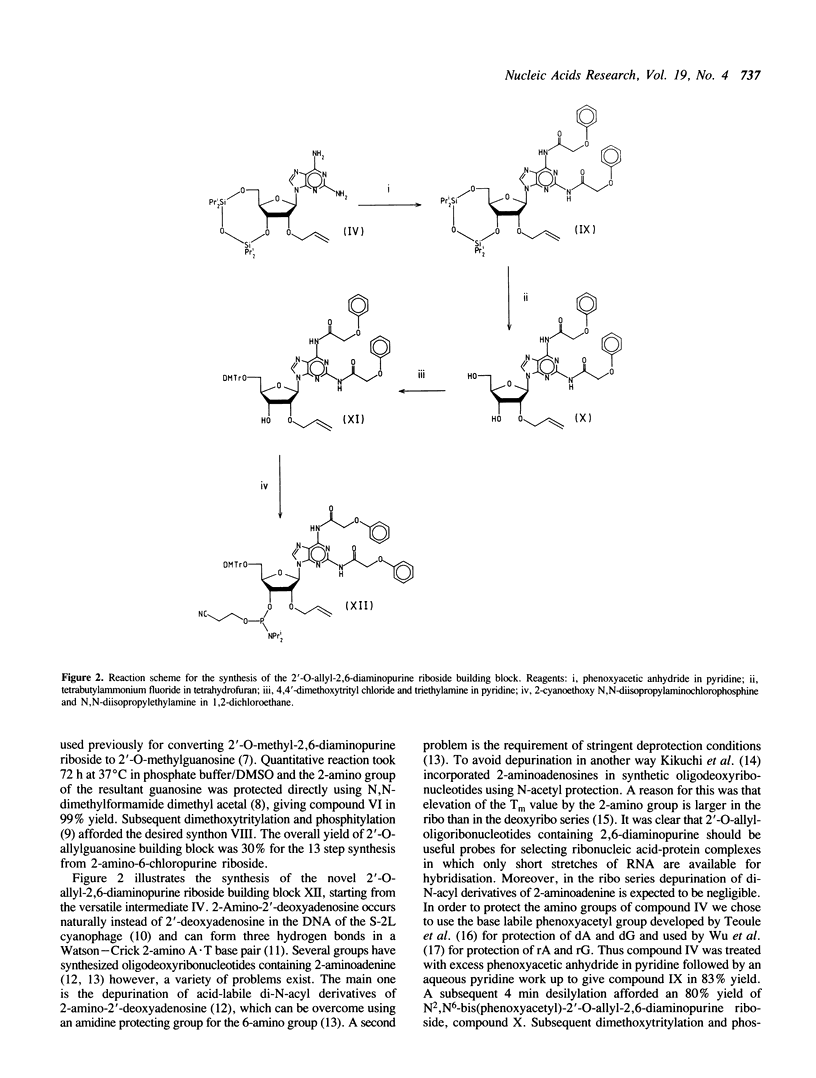
New synthetic routes have been devised for the high yield preparation of protected 2'-O-allylribonucleoside-3'-O-phosphoramidites, exemplified by the ribonucleosides guanosine and 2,6-diaminopurine riboside (2-aminoadenosine). Key features are the use of versatile intermediates and an easy allylation step. The development of a novel synthon based on 2'-O-allyl-2,6-diaminopurine riboside enables short 2'-O-allyl-oligoribonucleotide probes to be synthesized with adenine replaced by 2-aminoadenine. Thus very stable hybrids with complementary RNA target sequences can be formed due to the formation of the three hydrogen bond 2-amino A.U base pairs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerami A., Reich E., Ward D. C., Goldberg I. H. The interaction of actinomycin with DNA: requirement for the 2-amino group of purines. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1036–1042. doi: 10.1073/pnas.57.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y., Kobayashi K., Kanai Y., Honjo M. Synthesis of 2'-O-methyluridine, 2'-O-methylcytidine and their relating compounds. Chem Pharm Bull (Tokyo) 1965 Nov;13(11):1273–1278. doi: 10.1248/cpb.13.1273. [DOI] [PubMed] [Google Scholar]
- Howard F. B., Miles H. T. 2NH2A X T helices in the ribo- and deoxypolynucleotide series. Structural and energetic consequences of 2NH2A substitution. Biochemistry. 1984 Dec 18;23(26):6723–6732. doi: 10.1021/bi00321a068. [DOI] [PubMed] [Google Scholar]
- Iribarren A. M., Sproat B. S., Neuner P., Sulston I., Ryder U., Lamond A. I. 2'-O-alkyl oligoribonucleotides as antisense probes. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7747–7751. doi: 10.1073/pnas.87.19.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnos M. D., Khudyakov I. Y., Alexandrushkina N. I., Vanyushin B. F. 2-aminoadenine is an adenine substituting for a base in S-2L cyanophage DNA. Nature. 1977 Nov 24;270(5635):369–370. doi: 10.1038/270369a0. [DOI] [PubMed] [Google Scholar]
- Nyilas A., Chattopadhyaya J. Synthesis of O2'-methyluridine, O2'-methylcytidine, N4,O2'-dimethylcytidine and N4,N4,O2'-trimethylcytidine from a common intermediate. Acta Chem Scand B. 1986 Nov;40(10):826–830. doi: 10.3891/acta.chem.scand.40b-0826. [DOI] [PubMed] [Google Scholar]
- Pieles U., Sproat B. S., Lamm G. M. A protected biotin containing deoxycytidine building block for solid phase synthesis of biotinylated oligonucleotides. Nucleic Acids Res. 1990 Aug 11;18(15):4355–4360. doi: 10.1093/nar/18.15.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese C. B., Stewart J. C., van Boom J. H., de Leeuw H. P., Nagel J., de Rooy J. F. The synthesis of oligoribonucleotides. Part XI. Preparation of ribonucleoside 2'-acetal 3'-esters by selective deacylation. J Chem Soc Perkin 1. 1975;(10):934–942. doi: 10.1039/p19750000934. [DOI] [PubMed] [Google Scholar]
- Schulhof J. C., Molko D., Teoule R. The final deprotection step in oligonucleotide synthesis is reduced to a mild and rapid ammonia treatment by using labile base-protecting groups. Nucleic Acids Res. 1987 Jan 26;15(2):397–416. doi: 10.1093/nar/15.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Beijer B., Iribarren A. New synthetic routes to protected purine 2'-O-methylriboside-3'-O-phosphoramidites using a novel alkylation procedure. Nucleic Acids Res. 1990 Jan 11;18(1):41–49. doi: 10.1093/nar/18.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Lamond A. I., Beijer B., Neuner P., Ryder U. Highly efficient chemical synthesis of 2'-O-methyloligoribonucleotides and tetrabiotinylated derivatives; novel probes that are resistant to degradation by RNA or DNA specific nucleases. Nucleic Acids Res. 1989 May 11;17(9):3373–3386. doi: 10.1093/nar/17.9.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Ogilvie K. K., Pon R. T. Prevention of chain cleavage in the chemical synthesis of 2'-silylated oligoribonucleotides. Nucleic Acids Res. 1989 May 11;17(9):3501–3517. doi: 10.1093/nar/17.9.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]



