Abstract
Serotonin type 3 receptors (5-HT3) are involved in learning, cognition and emotion, and have been implicated in various psychiatric phenotypes. However, their contribution to the pathomechanism of these disorders remains elusive. Three single nucleotide polymorphisms (SNPs) in the HTR3A and HTR3B genes (rs1062613, rs1176744 and rs3831455) have been associated with bipolar affective disorder (BPAD) in pilot studies, and all of them are of functional relevance. We performed a European multicenter study to confirm previous results and provide further evidence for the relevance of these SNPs to the etiology of neuropsychiatric disorders. This involved analysis of the distribution of the three SNPs among 1804 BPAD cases and 2407 healthy controls. A meta-analysis revealed a pooled odds ratio of 0.881 (P=0.009, 95% confidence intervals=0.802–0.968) for the non-synonymous functional SNP HTR3B p.Y129S (rs1176744), thereby confirming previous findings. In line with this, the three genome-wide association study samples BOMA (Bonn-Mannheim)-BPAD, WTCCC (Wellcome Trust Case Control Consortium)-BPAD and GAIN (Genetic Association Information Network)-BPAD, including >3500 patients and 5200 controls in total, showed an overrepresentation of the p.Y129 in patients. Remarkably, the meta-analysis revealed a P-value of 0.048 (OR=0.934, fixed effect model). We also performed expression analyses to gain further insights into the distribution of HTR3A and HTR3B mRNA in the human brain. HTR3A and HTR3B were detected in all investigated brain tissues with the exception of the cerebellum, and large differences in the A:B subunit ratio were observed. Interestingly, expression of the B subunit was most prominent in the brain stem, amygdalae and frontal cortex, regions of relevance to psychiatric disorders. In conclusion, the present study provides further evidence for the presence of impaired 5-HT3 receptor function in BPAD.
Keywords: association, bipolar, BPAD, HTR3, HTR3B, 5-HT3
Introduction
Serotonin type 3 (5-HT3) receptors are involved in neural processes related to cognition and emotion, and antagonists of these receptors have been shown to be beneficial in the treatment of various psychiatric disorders. In particular, improvement in depressive symptoms, memory and cognition have been described in psychiatric patients.1 Furthermore, distinct 5-HT3 receptor variants have been implicated in the pathophysiology of bipolar affective disorder (BPAD), schizophrenia and eating disorders.2 Functional receptors contain five subunits of diverse composition. In humans, 5-HT3 subunits are encoded by five genes: HTR3A–E. Of these subunits, the 5-HT3A and 5-HT3B have been characterized most extensively. In vitro studies have shown that only the 5-HT3A subunit is capable of forming functional homopentameric receptors whereas the other subunits require 5-HT3A to build heteropentameric complexes.3 Comparative expression analyses of all five HTR3 genes on the mRNA level, using conventional PCR have shown that expression of the 5-HT3E subunit is restricted to certain tissues of the gastrointestinal tract, whereas the other subunits are expressed ubiquitously.4 Early studies in the 1990s using radioligand binding revealed 5-HT3 selective binding in distinct brain regions. Different ligand-binding densities were observed in the forebrain regions analyzed, depending on the radioligand used; for example, zacopride, LY278584 or granisetron.5, 6, 7, 8 However, 5-HT3A was the only known subunit of this type of receptors at that time, the complexity of the human 5-HT3 receptor system first became apparent during the last decade, and the composition of native receptor subtypes remains elusive.2 Consequently, the difference in binding density depending on the applied ligand may reflect the existence of receptor subtypes of differing compositions and affinities.3 Although expression of HTR3A has been reported in the cerebral cortex, amygdala, hippocampus, caudate and thalamus on the mRNA level in humans9, 10 contradictory data exist concerning the expression of HTR3B in the central nervous system.11
In two pilot case–control studies, the variants HTR3A c.−42C>T (rs1062613) and HTR3B p.Y129S (c.386A>C, rs1176744), as well as the small deletion HTR3Bc.−104_−102delAGA (rs3831455) were found to be associated with BPAD.12, 13
All of these variants have been shown to exhibit functional effects in vitro.13, 14, 15, 16, 17, 18, 19 Furthermore, the two single nucleotide exchanges have also been implicated in anorexia nervosa and irritable bowel syndrome, both of which show high comorbidity with anxiety and depression,14, 20 whereas HTR3B p.Y129S appears to have a role in major depression.21 In addition, HTR3A c.−42C>T has been correlated with altered amygdala activity and increased anxiety scores in paradigms testing emotional response in vivo.22, 23
The identification of identical risk variants for different psychiatric phenotypes supports the hypothesis that in spite of discriminable clinical diagnoses, these phenotypes share some aspects of the underlying biology.24 The aim of the present European multicenter study was to confirm the previous association findings and provide further evidence for the hypothesis that functional HTR3 variants are implicated in the etiology of various psychiatric disorders. We successfully replicated the association between the HTR3B variant p.Y129S (rs1176744) and BPAD in a large European case–control cohort consisting of >1800 cases and 2400 controls. Furthermore, we were able to confirm co-expression of both the 5-HT3A and 5-HT3B subunits in brain structures of relevance to anxiety and depression.
Materials and methods
European patient–control samples
All protocols and procedures were approved by the respective local Ethics Committees. Written informed consent was obtained from all study participants before the study participation. All patients were assigned a lifetime diagnosis of BPAD type I or type II. This was based on Diagnostic and Statistical Manual of Mental Disorders-IV criteria and a consensus best-estimate procedure, including a structured interview-I, review of medical records, the family history method and the Operational Criteria Checklist for Psychotic Illness OPCRIT system. Patients were recruited from consecutive admissions to the psychiatric inpatient units of seven clinical centers: (A) the Central Institute of Mental Health, Mannheim, and the Department of Psychiatry and Psychotherapy of the University of Bonn (n=378 patients/768 controls); (B) Department of Psychiatry, Poznan University of Medical Sciences, Poznan, Poland (n=446 patients/558 controls); (C) Alexandru Obregia Clinical Psychiatric Hospital, Bucharest, Romania (n=237 patients/235 controls); (D) Civil Hospital Carlos Haya, Malaga, Spain (n=297 patients/401 controls); (E) Russian State Medical University, Moscow (n=331 patients/331 controls); (F) Psychiatric Clinic, Clinical Center of the University of Sarajevo, Sarajevo, Bosnia-Herzegovina (n=124 patients/115 controls). Ethnicity was assigned to patients and controls on the basis of self-reported ancestry at each of the seven recruitment sites. The given numbers reflect the sample sizes after quality control (sample call rate >90%). This sample was used before for replication purposes as part of a genome-wide association study study (replication I).25
Independent replication data sets
To further strengthen our association finding, rs1176744 was examined in three independent genome-wide data sets.25, 26, 27 First, this study makes use of data generated for the BOMA (Bonn-Mannheim)-BPAD case–control cohort consisting of 682 cases and 1300 controls.25 In addition, data for two independent, well-known BPAD studies were included. The Wellcome Trust Case–Control Consortium BPAD study26 makes use of a cohort of 1868 patients and 2938 controls. As rs1176744 was not available in the set of genotyped markers for this study, the proxy rs4938058 (r2=0.898) was analysed. Finally, a sample of 987 patients and 1000 controls was derived from the Database of Genotypes and Phenotypes data set28 ‘Whole Genome Association Study of Bipolar Disorder' (phs000017.v3.p1).27 Here, another proxy rs1176743 (r2=0.919) was used.
DNA extraction and genotyping
EDTA anti-coagulated venous blood samples were collected from all individuals. Lymphocyte DNA was isolated by salting-out with saturated sodium chloride solution 4, or by the use of a Chemagic Magnetic Separation Module I (Chemagen, Baesweiler, Germany) according to the manufacturer's recommendations.
Genotyping was performed using the Matrix-Assisted-Laser-Desorption/lonization–Time-Of-Flight (MALDI-TOF)-based MassARRAY system and iPLEX Gold assays (Sequenom, San Diego, CA, USA). Primer sequences and assay conditions are obtainable upon request. Single nucleotide polymorphisms (SNPs) raw genotype data, that is, cluster plots, were visually assessed by two investigators. SNP and sample call rates were >90%.
Statistical analyses
PLINK (v1.07; http://pngu.mgh.harvard.edu/~purcell/plink/) was used for the analysis of statistical association between the single SNPs and affection status (trend test), and to test for deviations from Hardy-Weinberg equilibrium. The SNPassoc package (http://www.creal.cat/jrgonzalez/software.htm) for R was used to calculate P-values, odds ratios (OR) and 95% confidence intervals (CI), assuming a log-additive inheritance model adjusted for sex.29 SPSS Statistics 17.0 (IBM Deutschland GmbH, Munich, Germany) was used in the performance of a Cochran-Maentel-Haentzel (CMH) meta-analysis to obtain pooled estimates of the ORs across nationalities, and Breslow-Day tests for homogeneity of the ORs. Significant P-values were corrected for three tests using the Bonferroni method, but are displayed uncorrected in text and tables. For the replication analysis in genome-wide association study data sets, a fixed effect model was used assuming an additive effect. To assess putative false-negative results, a power calculation was carried out using Genetic Power Calculator (http://pngu.mgh.harvard.edu/~purcell/gpc/).30
Expression analyses on the mRNA level
The total RNA from human adult brain used in the present experiments was either obtained commercially or was isolated from brain tissue obtained from the German Brain Bank Brain-Net (http://www.brain-net.net). The human fetal material was provided by the Joint MRC Wellcome Trust Human Developmental Biology Resource (http://www.hdbr.org). Trizol reagent (Life Technologies, Darmstadt, Germany) was used for the extraction of RNA, as recommended by the manufacturer (Table 1). The experiments were approved by the ethics committee of the University of Heidelberg.
Table 1. Brain tissue used for total RNA extraction followed by nCounter analysis.
| RNA |
Donor information |
|||||
|---|---|---|---|---|---|---|
| Number | Race | Gender | Age | COD | Company | |
| Brain | Pool of 3 | Caucasian | Male | 23 | Cardiac arrest | FirstChoice Human Total RNA Survey Panel |
| Caucasian | Male | 81 | Congestive heart failure | |||
| Caucasian | Female | 78 | Congestive heart failure | |||
| Frontal cortex | Single | Caucasian | Male | 23 | Cardiac Arrest | FirstChoice Human Total RNA |
| Thalamus | Single | n.a. | Female | 71 | Chronic obstructive pulmonary disease | BioChain Total RNA |
| Right cerebellum | Single | n.a. | Male | 82 | Aortic stenosis | BioChain Total RNA |
| Hippocampus | Single | Asian | Male | 27 | Accident | BioChain Total RNA |
| Spinal cord | Single | Asian | Male | 27 | Accident | BioChain Total RNA |
| Striatum | Single | n.a. | Male | 20 | n.a. | Stratagene MVP Total RNA |
| Insula | Pool of 15 | Caucasian | Male/female | 20–68 | Sudden death | Clontech Total RNA |
| Amygdala | Pool of 2 | Caucasian | Female | 21 | Pneumonia | BrainNet Germany |
| Caucasian | Male | 68 | Cardiac arrest | BrainNet Germany | ||
| Brain stem | Pool of 2 | Caucasian | Female | 21 | Pneumonia | BrainNet Germany |
| Caucasian | Male | 68 | Cardiac arrest | BrainNet Germany | ||
| Fetal brain | Single | n.a. | n.a. | F2 | Induced abortion | MRC Wellcome Trust Human Developmental Biology Resource |
| Fetal cerebellum | Single | n.a. | n.a. | F2 | Induced abortion | MRC Wellcome Trust Human Developmental Biology Resource |
| Fetal thalamus | Single | n.a. | n.a. | F2 | Induced abortion | MRC Wellcome Trust Human Developmental Biology Resource |
Abbreviation: COD, cause of death.
Total RNA (100 ng) served as input material in nCounter (Nanostring, Seattle, WA, USA) analysis using a customized codeset, as recommended by the manufacturer. In particular, the used codesets for HTR3A and HTR3B expression profiling among 18 other target genes are included in Table 2 (Supplementary data). Background correction and normalization of data was performed as follows. In brief, for each sample, the average + 2 SDS of background counts (negative controls) was calculated. For each gene for each sample, the average + 2 SDS was subtracted from the counts. Expression of HTR3A (NM_000869.5) and HTR3B (NM_006028.3) was normalized by taking the geometric mean of the expression of the three reference genes ADAM9 (NM_003816.2), PGK1 (NM_000291.3) and SNX17 (NM_014748.2) into account.31
Table 2. Number of genotyped BPAD cases and controls, totals and according to nationality.
| Nationality | N (cases) | N (controls) | N (Cases + controls) |
|---|---|---|---|
| Bosnian-Herzegovinan | 124 | 115 | 239 |
| German | 378 | 768 | 1146 |
| Polish | 446 | 558 | 1004 |
| Romanian | 237 | 233 | 471 |
| Russian | 331 | 333 | 663 |
| Spanish | 298 | 400 | 698 |
| Total | 1814 | 2407 | 4221 |
Abbreviation: BPAD, bipolar affective disorder.
Results
In the current case–control study, >1800 cases and 2400 controls from six European countries were analyzed (Table 2). Genotype call rates were at least 97.6% for each SNP, and none of the tested variants deviated from Hardy-Weinberg equilibrium in patients or controls (data not shown).
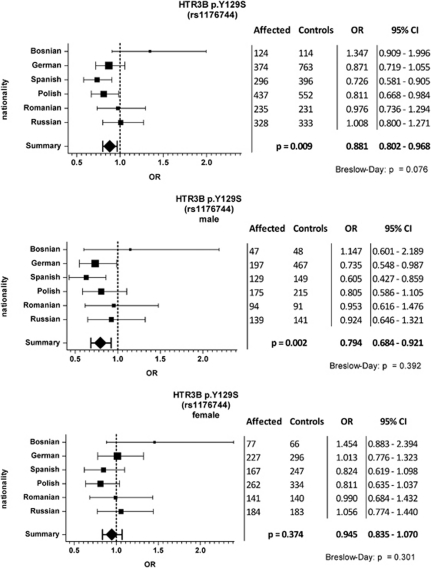
For HTR3B p.Y129S (c.386A>C, rs1176744) a pooled odds ratio (OR) of 0.881 (95% CI=0.802–0.968) was determined in a CMH analysis, yielding a significant P-value of 0.009 (Figure 1). This was because of an overrepresentation of the p.Y129 allele in BPAD patients. A Breslow-Day test for homogeneity of ORs revealed no significant deviation (P=0.076). In the national subgroups, significant P-values were detected for the Polish and Spanish cohorts in a Cochran-Armitage trend test, under the assumption of a log-additive inheritance model (Table 3). The P-values withstanding Bonferroni correction are highlighted in bold in Table 3. In view of statistical power issues, the national subgroups were not separated stratified according to sex. However, CMH analyses showed a clear sex-specific association. Although there was no association with the p.Y129S variant in females, the analysis of male patients vs controls resulted in a P-value of 0.0002 (OR=0.794, CI=0.684–0.921) (Figure 1).
Figure 1.
Odds ratio (OR) distribution across nationalities for HTR3B p.Y129S (rs1176744). The number of patients and healthy controls for each country is shown along with the respective OR and 95% confidence intervals (CI). The common P-value, OR and 95% CI (bold numbers) were determined using a Cochran-Maentel-Haenszel test. A Breslow-Day test was performed to test for homogeneity of ORs.
Table 3. Genotype counts and association statistics.
| SNP | Study subgroup |
Genotypes (and frequencies) cases |
P-value Trend test, d.f.=1 | P-value (OR, (95% CI)) | ||
|---|---|---|---|---|---|---|
| Genotypes (and frequencies) controls | Log additive, adjusted according to sex | |||||
| rs1062613 | CC (%) | CT (%) | TT (%) | |||
| Bosnia-Herzegovina | 70 (58) | 44 (37) | 6 (5) | |||
| 81 (71) | 30 (27) | 2 (2) | 0.073 | 0.025 (1.72, (1.06–2.80)) | ||
| Germany | 224 (62) | 124 (34) | 15 (4) | |||
| 469 (62) | 260 (34) | 30 (4) | 0.99 | 0.937 (0.99, (0.79–1.24)) | ||
| Poland | 306 (70) | 114 (26) | 16 (4) | |||
| 335 (61) | 180 (33) | 33 (6) | 0.01 | 0.002 (0.71, (0.57–0.89)) | ||
| Romania | 144 (63) | 70 (31) | 13 (6) | |||
| 142 (65) | 69 (31) | 8 (4) | 0.579 | 0.524 (1.11, (0.8–1.53)) | ||
| Russia | 205 (63) | 109 (33) | 11 (3) | |||
| 205 (63) | 105 (32) | 14 (4) | 0.808 | 0.865 (0.98, (0.74–1.28)) | ||
| Spain | 167 (57) | 107 (36) | 20 (7) | |||
| 207 (52) | 157 (39) | 34 (9) | 0.416 | 0.190 (0.85, (0.67–1.08)) | ||
| rs3831455 | ins/ins (%) | ins/del (%) | del/del (%) | |||
| Bosnia-Herzegovina | 97 (81) | 21 (18) | 2 (2) | 0.312 | 0.195 (0.69, (0.40–1.21)) | |
| 82 (73) | 29 (26) | 2 (2) | ||||
| Germany | 278 (77) | 80 (22) | 5 (1) | 0.702 | 0.477 (1.11, (0.84–1.47)) | |
| 594 (78) | 158 (21) | 7 (1) | ||||
| Poland | 328 (75) | 99 (22) | 9 (2) | 0.221 | 0.099 (1.26, (0.96–1.65)) | |
| 430 (79) | 107 (20) | 6 (1) | ||||
| Romania | 174 (77) | 46 (20) | 6 (3) | 0.591 | 0.812 (0.95, (0.65–1.40)) | |
| 162 (75) | 52 (24) | 3 (1) | ||||
| Russia | 252 (78) | 63 (19) | 9 (3) | 0.204 | 0.239 (1.22, (0.87–1.70)) | |
| 261 (81) | 59 (18) | 4 (1) | ||||
| Spain | 245 (83) | 47 (16) | 3 (1) | 0.597 | 0.295 (0.83, (0.58–1.18)) | |
| 318 (80) | 75 (19) | 5 (1) | ||||
| rs1176744 | AA (%) | AC (%) | CC (%) | |||
| Bosnia-Herzegovina | 54 (44) | 57 (46) | 13 (11) | 0.3 | 0.152 (1.34, (0.90–1.99)) | |
| 61 (54) | 44 (39) | 9 (8) | ||||
| Germany | 191 (51) | 152 (41) | 31 (8) | 0.321 | 0.178 (0.87, (0.72–1.06)) | |
| 355 (47) | 335 (44) | 73 (10) | ||||
| Poland | 235 (54) | 157 (36) | 45 (10) | 0.017 | 0.037 (0.82, (0.68–0.99)) | |
| 249 (45) | 245 (44) | 58 (11) | ||||
| Romania | 120 (51) | 93 (40) | 22 (9) | 0.367 | 0.857 (0.97, (0.73–1.30)) | |
| 110 (48) | 105 (46) | 16 (7) | ||||
| Russia | 154 (47) | 140 (43) | 34 (10) | 0.889 | 0.950 (1.01, (0.80–1.27)) | |
| 151 (45) | 154 (46) | 28 (8) | ||||
| Spain | 128 (43) | 136 (46) | 32 (11) | 0.013 | 0.005 (0.73, (0.59–0.91)) | |
| 142 (36) | 181 (46) | 73 (18) | ||||
Abbreviations: CI, confidence interval; d.f., degrees of freedom; OR, odds ratio; SNP, single nucleotide polymorphism.
Frequencies (%) were rounded off to the nearest whole number. P-values are displayed in bold if withstanding Bonferroni correction, nominally significant P-values are shown in italics.
In a further step to strengthen our association finding, rs1176744 (or its proxies) was examined in the three independent genome-wide data sets BOMA-BPAD, WTCCC (Wellcome Trust Case Control Consortium)-BPAD and Genetic Association Information Network-BPAD, including >3500 patients and 5200 controls in total.25, 26, 27 Remarkably, in all three samples, the p.Y129 allele was overrepresented in patients, and a final meta-analysis revealed a P-value of 0.048 (OR=0.934, fixed effects model), thereby confirming the original association with BPAD.
No significant results were obtained for the cis-regulatory variants HTR3A c.−42C>T (rs1062613) and HTR3B c.−104 −102delAGA (rs3831455) in the CMH analyses (data not shown). The former was found to be associated in the Polish subgroup (P(log-additive) = 0.002, OR=0.71, 95% CI=0.57–0.89), and nominally associated in the Bosnian-Herzegovinian subgroup (P(log-additive)=0.025, OR=1.72, 95% CI=1.05–2.80). However, the calculated ORs point to different risk alleles.
To address the possibility of false-negative findings, a power calculation was performed assuming the calculated OR of the HTR3B p.Y129S CMH analysis and a population risk of 2%. This yielded a statistical power of 66% for HTR3A c.−42C>T and of 47% for HTR3B c.−104_−102delAGA.
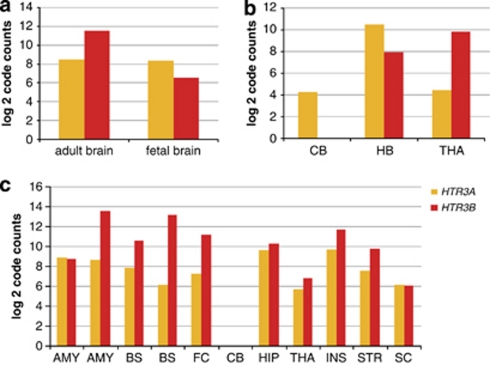
Using the nCounter technology, which measures mRNA expression levels directly without any further enzymatic modification of the sample,32 we detected relative expression of both subunit genes on the transcript level, that is, HTR3A and HTR3B in total RNA samples from different human brain regions (Figure 2). In the tested adult brain tissues, relative code counts of the A and B subunits (code count B/code count A ratio) was found in the following descending order: brain stem (130), amygdala (30), frontal cortex (15), striatum (4.7), insula (4), thalamus (2.2) and hippocampus (1.6). The only tested tissue to show nearly equal code counts in adult tissue was the spinal cord (0.9). No expression was found in adult cerebellum. In the tested fetal tissues, the B:A code count ratio was 41 in the thalamus and 0.16 in hindbrain whereas in the cerebellum B was not detectable at all. Interestingly, the relative expression level of the B subunit was besides the brain stem highest in the amygdala and frontal cortex, that is, regions in which disturbances may lead to the manifestation of psychiatric disorders.
Figure 2.
HTR3A and HTR3B mRNA expression profiling. mRNA levels were directly quantified using the nCounter technology. (a) Comparison of adult and fetal brain overall; (b) fetal brain areas and (c) adult brain regions. Abbreviations: CB, cerebellum; HB, hind brain; HIP, hippocampus; THA, thalamus; INS, insula; STRI, striatum; SC, spinal cord; AMY, amygdala; BS, brain stem; FC, frontal cortex.
Discussion
In the present study, the HTR3B p. Y129S variant was successfully replicated overall, and showed significant associations in the CMH analysis and in the Polish and Spanish subgroups. The p.129Y allele was overrepresented in patients, and this finding is consistent with previous association data for BPAD, anorexia nervosa and major depression.12, 20, 21 On a functional level, homozygous 5-HT3AB p.129Y receptors displayed a decreased 5-HT maximum response, caused by a sevenfold decrease in single channel mean open time, compared with homozygous 5-HT3AB p.129S receptors.16, 19 This striking difference may result in a disease-relevant change in 5-HT3 receptor signaling.
The contribution of 5-HT3 receptors to the etiology of psychiatric disorders is underlined by data from animal studies. Behavioral tests in primates and rodents revealed anxiolytic effects of 5-HT3 antagonists secondary to blockage of the limbic hyperactivity response.33 This is consistent with studies of 5-HT3A knockout mice, which show reduced anxiety-like behavior.34 Further investigation of these knockout mice led to the conclusion that the regulation of depression- and anxiety-related behaviors by the 5-HT3A subunit differs between males and females.35
The effect of 5-HT3A on anxiety control has been reported to be mediated by regulation of hypothalamic-pituitary-adrenal responses to acute stress.36 This suggests a potential interaction between 5-HT3A and corticotropin-releasing hormone in the amygdala. Sex differences in 5-HT3A- receptor-dependent hypothalamic-pituitary-adrenal responses in chronically stressed mice have also been reported.36
The observed association in males in the present study is in line with animal data.35 A feasible hypothesis is that 5-HT3 receptors are involved in the modulation of anxiety-related behaviors, which are important phenotypes in the symptomatic spectrum of BPAD.
Moreover, 5-HT3 antagonist treatment in humans has been shown to be an effective treatment for anxiety and depression.37, 38
A further aim of the present study was to gain insights into 5-HT3 receptor subunit expression in the human brain. As outlined above, the expression of 5-HT3 receptors has mainly been analyzed using 5-HT3-binding site-selective compounds.5, 6, 7, 8 Our data are consistent with these early human brain ligand-binding data. Our results indicate that the expression of both subunits appears to switch during development. This switch in the 5-HT3A and 5-HT3B receptor ratio is interesting, since recent studies have described the relevance of 5-HT3 receptors to neuronal plasticity in the cortex. Application of the 5-HT3 receptor antagonist tropisetron led to a specific blockade of the 5-HT3 response on Cajal-Retzius cells in mice, and 5-HT3 receptors were shown to be involved in postnatal dendritic maturation and neuronal plasticity in the cortex.39 In consequence, changes in the brain organization of variant carriers are conceivable. Disturbed column architecture of the cortex has indeed been reported in the brains of psychiatric patients.40 Whether or not this is related to polymorphic changes in HTR3 genes still awaits investigation.
Future studies are warranted to determine the functional and structural consequences of receptor polymorphisms in vivo. Recent studies by the present authors, using transiently transfected human cell lines, showed that the ratio between the subunits A and B determines receptor composition and consequently the formation of homomeric and heteromeric B subunits.19, 41
We were able to confirm co-expression of both 5-HT3 subunits in brain regions involved in memory, cognition and anxiety control, that is, the frontal cortex, the hippocampus and the amygdala, respectively. Murine studies have shown that 70–80% of 5-HT3 receptors are located presynaptically and are associated with axons and nerve terminals, with the exception of those in the hippocampus, which are mainly postsynaptic and located in somatodendritic regions.42 The high presynaptic expression of 5-HT3 receptors is consistent with their physiological role in neurotransmitter release (dopamine, cholecystokinin, glutamate, acetylcholine and gamma-aminobutyric acid (GABA)).43 Accordingly, both the excitatory and the inhibitory effects of 5-HT3R activation are mediated by activation of excitatory or inhibitory interneurons.44 For example, 5-HT3R activation of GABAergic interneurons innervating the amygdala is thought to exert an inhibitory influence on amygdala activity, whereas the activation of excitatory glutamatergic interneurons is hypothesized to have the opposite effect.44 However, the situation in humans remains unknown. To date, systematic analysis has been hampered by the lack of specific antibodies.
Taken together, our results support the hypothesis that the HTR3B p.Y129S variant is implicated in BPAD, and the association could be replicated in a meta-analysis utilizing large published genome-wide association study data sets. Future studies are warranted to investigate a possible effect of this common genetic variant on further relevant neuropsychiatric phenotypes such as anxiety, stress response and depressive episodes, as well as memory and cognition. In doing so, possible gender-specific effects should also be taken into account.
In contrast to the HTR3B variant p.Y129S, the variants HTR3A c.−42C>T (rs1062613) and HTR3B c.−104 −102delAGA (rs3831455) were not replicated in our study overall. In fact, for the HTR3A variant, association analysis in the national subgroups generated contradictory results. Whereas pilot studies into BPAD, irritable bowel syndrome and eating disorders have implicated the less frequent T allele in the development of the disease phenotypes,14, 20, 45 in the present study the C allele occurred more often in Polish patients. Although the nominally significant association in the subgroup from Bosnia-Herzegovina replicated earlier results (P(log-additive) = 0.025), the sample was small and probably lacked statistical power to detect true positives. Hence, our study does not support the hypothesis that HTR3A c.−42C>T SNP or the small deletion HTR3B c.−104_−102delAGA are involved in the etiology of BPAD. Nevertheless, the retrospective power calculation for the whole sample indicates that our study cohort may have been too small to detect minor effects.
In conclusion, the present study replicated the functional HTR3B variant p.Y129S as a predisposing factor for BPAD. Future studies should investigate the precise role of HTR3B in this disorder. The sex specificity of the association points to a putative function in the hypothalamic-pituitary-adrenal axis, resulting in anxiety-related phenotypes. Interestingly, differing code-count ratios of the A and B subunits were found in various adult and fetal brain regions, suggesting differences in receptor function and composition in the developing, compared with the adult brain.
Acknowledgments
We thank Carolin Wohlfarth for her excellent technical assistance and Claudia Durand and Christine Schmäl for fruitful discussions. We also acknowledge the German Brain Bank Brain-Net (http://www.brain-net.net) for providing amygdala and frontal cortex brain tissue. The ‘Brain-Net' is supported by the German Federal Ministry of Education and Research (BMBF). The human fetal materials were obtained from the Joint MRC Wellcome Trust Human Developmental Biology Resource (http://www.hdbr.org) at the IHG, Newcastle Upon Tyne, UK, with funding from Grants G0700089 and GR082557. The study was funded by the Medical Faculty of the University of Heidelberg. MR, SC and MMN were supported by the German Federal Ministry of Education and Research (BMBF), within the context of the National Genome Research Network 2 (NGFN-2), the National Genome Research Network plus (NGFNplus) and the Integrated Genome Research Network (IG) MooDS (Grant 01GS08144 to SC and MMN, Grant 01GS08147 to MR). JS was supported by the German Research Foundation (GRK 793). This study makes use of data generated by the Wellcome Trust Case–Control Consortium.26 A full list of the investigators who contributed to the generation of the data is available from http://www.wtccc.org.uk. Funding for the project was provided by the Wellcome Trust under award 076113 and 085475.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Rajkumar R, Mahesh R. The auspicious role of the 5-HT3 receptor in depression: a probable neuronal target. J Psychopharmacol. 2010;24:455–469. doi: 10.1177/0269881109348161. [DOI] [PubMed] [Google Scholar]
- Niesler B. 5-HT(3) receptors: potential of individual isoforms for personalised therapy. Curr Opin Pharmacol. 2011;11:81–86. doi: 10.1016/j.coph.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Walstab J, Rappold G, Niesler B. 5-HT(3) receptors: role in disease and target of drugs. Pharmacol Ther. 2010;128:146–169. doi: 10.1016/j.pharmthera.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Niesler B, Frank B, Kapeller J, Rappold GA. Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene. 2003;310:101–111. doi: 10.1016/s0378-1119(03)00503-1. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Laruelle M, Wong DT, Robertson DW, Weinberger DR, Kleinman JE. Pharmacological and regional characterization of [3H]LY278584 binding sites in human brain. J Neurochem. 1993;60:730–737. doi: 10.1111/j.1471-4159.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Costall B, Ironside JW, Naylor RJ. Identification and characterisation of 5-hydroxytryptamine 3 recognition sites in human brain tissue. J Neurochem. 1989;53:1787–1793. doi: 10.1111/j.1471-4159.1989.tb09244.x. [DOI] [PubMed] [Google Scholar]
- Bufton KE, Steward LJ, Barber PC, Barnes NM. Distribution and characterization of the [3H]granisetron-labelled 5-HT3 receptor in the human forebrain. Neuropharmacology. 1993;32:1325–1331. doi: 10.1016/0028-3908(93)90027-z. [DOI] [PubMed] [Google Scholar]
- Parker RM, Barnes JM, Ge J, Barber PC, Barnes NM. Autoradiographic distribution of [3H]-(S)-zacopride-labelled 5-HT3 receptors in human brain. J Neurol Sci. 1996;144:119–127. doi: 10.1016/s0022-510x(96)00211-0. [DOI] [PubMed] [Google Scholar]
- Belelli D, Balcarek JM, Hope AG, Peters JA, Lambert JJ, Blackburn TP. Cloning and functional expression of a human 5-hydroxytryptamine type 3AS receptor subunit. Mol Pharmacol. 1995;48:1054–1062. [PubMed] [Google Scholar]
- Miyake A, Mochizuki S, Takemoto Y, Akuzawa S. Molecular cloning of human 5-hydroxytryptamine3 receptor: heterogeneity in distribution and function among species. Mol Pharmacol. 1995;48:407–416. [PubMed] [Google Scholar]
- Jensen AA, Davies PA, Brauner-Osborne H, Krzywkowski K. 3B but which 3B and that's just one of the questions: the heterogeneity of human 5-HT3 receptors. Trends Pharmacol Sci. 2008;29:437–444. doi: 10.1016/j.tips.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank B, Niesler B, Nothen MM, Neidt H, Propping P, Bondy B, et al. Investigation of the human serotonin receptor gene HTR3B in bipolar affective and schizophrenic patients. Am J Med Genet B Neuropsychiatr Genet. 2004;131B:1–5. doi: 10.1002/ajmg.b.30070. [DOI] [PubMed] [Google Scholar]
- Niesler B, Weiss B, Fischer C, Nothen MM, Propping P, Bondy B, et al. Serotonin receptor gene HTR3A variants in schizophrenic and bipolar affective patients. Pharmacogenetics. 2001;11:21–27. doi: 10.1097/00008571-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Kapeller J, Houghton LA, Monnikes H, Walstab J, Moller D, Bonisch H, et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967–2977. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- Ji X, Takahashi N, Branko A, Ishihara R, Nagai T, Mouri A, et al. An association between serotonin receptor 3B gene (HTR3B) and treatment-resistant schizophrenia (TRS) in a Japanese population. Nagoya J Med Sci. 2008;70:11–17. [PubMed] [Google Scholar]
- Krzywkowski K, Davies PA, Feinberg-Zadek PL, Brauner-Osborne H, Jensen AA. High-frequency HTR3B variant associated with major depression dramatically augments the signaling of the human 5-HT3AB receptor. Proc Natl Acad Sci USA. 2008;105:722–727. doi: 10.1073/pnas.0708454105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywkowski K, Davies PA, Irving AJ, Brauner-Osborne H, Jensen AA. Characterization of the effects of four HTR3B polymorphisms on human 5-HT3AB receptor expression and signalling. Pharmacogenet Genomics. 2008;18:1027–1040. doi: 10.1097/FPC.0b013e328310f950. [DOI] [PubMed] [Google Scholar]
- Meineke C, Tzvetkov MV, Bokelmann K, Oetjen E, Hirsch-Ernst K, Kaiser R, et al. Functional characterization of a -100_-102delAAG deletion-insertion polymorphism in the promoter region of the HTR3B gene. Pharmacogenet Genomics. 2008;18:219–230. doi: 10.1097/FPC.0b013e3282f51092. [DOI] [PubMed] [Google Scholar]
- Walstab J, Hammer C, Bonisch H, Rappold G, Niesler B. Naturally occurring variants in the HTR3B gene significantly alter properties of human heteromeric 5-hydroxytryptamine-3A/B receptors. Pharmacogenet Genomics. 2008;18:793–802. doi: 10.1097/FPC.0b013e3283050117. [DOI] [PubMed] [Google Scholar]
- Hammer C, Kapeller J, Endele M, Fischer C, Hebebrand J, Hinney A, et al. Functional variants of the serotonin receptor type 3A and B gene are associated with eating disorders. Pharmacogenet Genomics. 2009;19:790–799. doi: 10.1097/FPC.0b013e32833132b3. [DOI] [PubMed] [Google Scholar]
- Yamada K, Hattori E, Iwayama Y, Ohnishi T, Ohba H, Toyota T, et al. Distinguishable haplotype blocks in the HTR3A and HTR3B region in the Japanese reveal evidence of association of HTR3B with female major depression. Biol Psychiatry. 2006;60:192–201. doi: 10.1016/j.biopsych.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Ozaki N, Matsumoto A, Nogawa J, Kinoshita Y, Suzuki T, et al. A variant C178T in the regulatory region of the serotonin receptor gene HTR3A modulates neural activation in the human amygdala. J Neurosci. 2005;25:6460–6466. doi: 10.1523/JNEUROSCI.5261-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Labus JS, Coveleskie K, Hammer C, Rappold G, Tillisch K, et al. The HTR3A polymorphism c. -42C>T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology. 2011;140:1943–1951. doi: 10.1053/j.gastro.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, O'Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32:9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon S, Muhleisen TW, Degenhardt FA, Mattheisen M, Miro X, Strohmaier J, et al. Genome-wide association study identifies genetic variation in neurocan as a susceptibility factor for bipolar disorder. Am J Hum Genet. 2011;88:372–381. doi: 10.1016/j.ajhg.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EN, Bloss CS, Badner JA, Barrett T, Belmonte PL, Berrettini W, et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14:755–763. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman MD, Feolo M, Jin Y, Kimura M, Tryka K, Bagoutdinov R, et al. The NCBI dbGaP database of genotypes and phenotypes. Nat Genet. 2007;39:1181–1186. doi: 10.1038/ng1007-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JR, Armengol L, Sole X, Guino E, Mercader JM, Estivill X, et al. SNPassoc: an R package to perform whole genome association studies. Bioinformatics. 2007;23:644–645. doi: 10.1093/bioinformatics/btm025. [DOI] [PubMed] [Google Scholar]
- Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ, Tyers MB. The psychopharmacology of 5-HT3 receptors. Pharmacol Ther. 1990;47:181–202. doi: 10.1016/0163-7258(90)90086-h. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Bratt AM, Hodge CW. Targeted gene deletion of the 5-HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol. 2003;461:19–25. doi: 10.1016/s0014-2999(02)02960-6. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Nowak N, Babich L, Bok L. Deletion of the 5-HT3 receptor differentially affects behavior of males and females in the Porsolt forced swim and defensive withdrawal tests. Behav Brain Res. 2004;153:527–535. doi: 10.1016/j.bbr.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Pituitary-adrenal activity in acute and chronically stressed male and female mice lacking the 5-HT-3A receptor. Stress. 2004;7:251–256. doi: 10.1080/10253890500044422. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Puech AJ, Azcona A, Bailey PE, Lataste X. A randomized double-blind placebo-controlled study of tropisetron in the treatment of outpatients with generalized anxiety disorder. Psychopharmacology (Berl) 1993;112:129–133. doi: 10.1007/BF02247373. [DOI] [PubMed] [Google Scholar]
- Hewlett WA, Schmid SP, Salomon RM. Pilot trial of ondansetron in the treatment of 8 patients with obsessive-compulsive disorder. J Clin Psychiatry. 2003;64:1025–1030. doi: 10.4088/jcp.v64n0907. [DOI] [PubMed] [Google Scholar]
- Chameau P, Inta D, Vitalis T, Monyer H, Wadman WJ, van Hooft JA. The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc Natl Acad Sci USA. 2009;106:7227–7232. doi: 10.1073/pnas.0810764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxhoeveden DP, Casanova MF. The minicolumn hypothesis in neuroscience. Brain. 2002;125 (Part 5:935–951. doi: 10.1093/brain/awf110. [DOI] [PubMed] [Google Scholar]
- Walstab J, Hammer C, Lasitschka F, Moller D, Connolly CN, Rappold G, et al. RIC-3 exclusively enhances the surface expression of human homomeric 5-hydroxytryptamine type 3A (5-HT3A) receptors despite direct interactions with 5-HT3A, -C, -D, and -E subunits. J Biol Chem. 2010;285:26956–26965. doi: 10.1074/jbc.M110.122838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel MC, Emerit MB, Nosjean A, Simon A, Rumajogee P, Brisorgueil MJ, et al. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur J Neurosci. 2002;15:449–457. doi: 10.1046/j.0953-816x.2001.01872.x. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesler B, Flohr T, Nothen MM, Fischer C, Rietschel M, Franzek E, et al. Association between the 5′ UTR variant C178T of the serotonin receptor gene HTR3A and bipolar affective disorder. Pharmacogenetics. 2001;11:471–475. doi: 10.1097/00008571-200108000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.