Abstract
Purpose
The aim of this study was to investigate plasma and synovial fluid basic fibroblast growth factor (bFGF) levels in patients with primary knee osteoarthritis (OA) and to evaluate the correlation between bFGF levels and disease severity.
Methods
Thirty-five patients with knee OA and 15 healthy individuals were recruited into this study. Knee OA grading was performed according to the Kellgren–Lawrence classification. bFGF concentrations in both plasma and synovial fluid were determined using enzyme-linked immunosorbent assay.
Results
Plasma and synovial fluid bFGF levels in knee OA patients were significantly higher than in controls (P < 0.001). Moreover, plasma and synovial fluid bFGF concentrations were positively correlated with radiographic severity (r = 0.535, P < 0.001 and r = 0.570, P < 0.001, respectively). Further analysis revealed that there was a positive correlation between plasma and synovial fluid bFGF levels (r = 0.674, P < 0.001).
Conclusions
Plasma and synovial fluid bFGF levels were significantly increased in OA patients, and these elevated levels were positively correlated with radiographic severity. These findings indicate that bFGF levels may be a monitor of disease severity and could play an essential part in the pathophysiology of degenerative process in OA.
Keywords: Medicine & Public Health, Orthopedics
Introduction
Osteoarthritis (OA) is a chronic progressive degenerative joint disorder that ultimately results in the age-related regressive change in articular cartilage, with joint-space narrowing, bony enlargement at joint margins, subchondral sclerosis and synovitis [14]. Clinical characteristics of OA include severe pain, joint stiffness, limited range of motion, swelling, crepitation and disability. A conventional modality for evaluating the affected joint is radiological investigation, which reflects disease severity by grading joint degeneration. Although radiographs are practically used to assess the OA severity, it would be advantageous to have a biochemical parameter that could precisely reflect disease severity and OA progression. Several environmental, mechanical and biochemical factors have been recognised as playing a vital role in OA development; however, its aetiology and pathophysiology remain entirely unclear.
Basic fibroblast growth factor (bFGF), also known as FGF-2, is a soluble heparin-binding polypeptide with pleiotropic effects when applied to diverse tissues, including mitogenesis, cell migration, cell differentiation and a variety of developmental processes [18]. The 18-kD human bFGF sequence shares 97% and 99% amino acid identity with mouse/rat and bovine/ovine bFGF, respectively [1, 16]. It plays a substantial role in tissue repair and angiogenesis [7] and has been implicated in several experimental models of degenerative joint diseases [6, 13, 17]. It can promote wound healing and modulate many cellular functions, including proliferation, differentiation and neovascularisation [5]. In recent years, bFGF expression in osteoarthritic articular cartilage has been explored and implicated in OA development [25, 27]. Previous studies also demonstrated upregulated bFGF expression in chondrocytes in human OA cartilage, which suggests that human chondrocytes can synthesise bFGF [22]. These findings prompted us to speculate that bFGF may be responsible for OA pathogenesis.
Even though plasma and/or synovial fluid levels of several cytokines and growth factors have been studied in patients with knee OA, to our knowledge, there has been no published data regarding the association of circulating and synovial fluid levels of bFGF in various clinical stages of primary knee OA [3, 9, 10, 15, 23]. Accordingly, we hypothesised that bFGF in plasma and synovial fluid could be correlated with the severity of clinical outcomes in knee OA patients. To prove this hypothesis, we analysed bFGF plasma and synovial fluid levels in knee OA patients and healthy controls to determine their possible relationships with radiographic severity of knee OA.
Materials and methods
Study population
This study was approved by the Institutional Review Board on Human Research of the Faculty of Medicine, Chulalongkorn University, and was conducted in conformity with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from patients and healthy volunteers prior to their participation. Thirty-five patients aged 53–91 years with primary knee OA (20 women and 15 men; mean age 68.9 ± 1.6 years) based on criteria of the American College of Rheumatology were recruited. OA severity was determined using weight-bearing anteroposterior radiographs of the affected knee with the participant standing on both legs with fully extended knee and X-ray beam centered on the joint. Radiographic severity was evaluated according to the Kellgren and Lawrence (KL) grading system [12]: grade 0, no radiological changes; grade 1, doubtful narrowing of joint space and possible osteophytic lipping; grade 2, definite osteophytes and possible narrowing of joint space; grade 3, moderate multiple osteophytes, definite narrowing of joint space, some sclerosis and possible deformity of bone contour; grade 4, large osteophytes, marked narrowing of joint space, severe sclerosis and definite deformity of bone contour. The grading scale used for analysis was the higher one upon comparison of both knees. We also recruited 15 gender- and age-matched controls (nine women and six men; mean age 69.7 ± 1.4 years) with normal knee radiographs. None of the participants had underlying diseases such as diabetes, histories of corticosteroid medication, other forms of arthritis, cancer or other chronic inflammatory diseases.
Laboratory methods
Synovial fluid was aspirated from the affected knee using sterile knee puncture just prior to surgery for total knee replacement, centrifuged to remove cells and joint debris and stored immediately at −80°C until the day of measurement. No synovial fluid was extracted from controls due to ethical concerns. Venous blood samples collected from all participants were centrifuged and stored at −80°C until used. Double-blind quantitative measurement of plasma and synovial fluid bFGF was performed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Quantikine, R&D Systems, Minneapolis, MN, USA) according to manufacturer’s instructions. Concisely, standards of recombinant human bFGF, plasma and synovial fluid samples were added to 96-well microtiter plates precoated with mouse monoclonal antibody against human bFGF and incubated for two hours at room temperature. Wells were then washed four times with washing buffer and incubated for two hours at room temperature with a horseradish peroxidase-conjugated monoclonal antibody against bFGF. After four washes, substrate solution was added to each well, and the plate was incubated for 30 minutes at room temperature in the dark. Finally, the reaction was stopped with the stop solution and absorbance was measured at 450 nm using an automated microplate reader. The bFGF level was determined from a standard curve (range 0–320 pg/ml) generated for each set of samples assayed. The manufacturer-reported precision was 3.0–9.7% (intra-assay) and 7.4–9.1% (interassay). Sensitivity was <3.0 pg/ml.
Statistical analysis
Statistical analysis was carried out using the statistical package for social sciences (SPSS) software, version 16.0 for Windows. Demographic data between patients and controls were compared by chi-square tests and unpaired Student’s t tests where appropriate. Comparisons between groups were performed using one-way analysis of variance (ANOVA), with Tukey post hoc test if ANOVA showed significance. Pearson’s correlation coefficient (r) was employed to determine correlation among concentrations of bFGF in plasma and synovial fluid and radiographic severity. Data are expressed as a mean ± standard error of the mean (SEM). P values <0.05 were considered statistically significant for differences and correlations.
Results
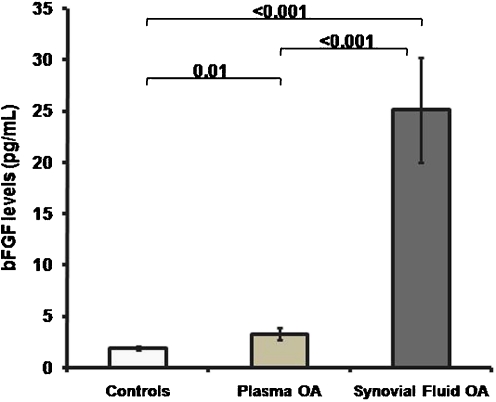
Thirty-five plasma and synovial fluid samples from knee OA patients and 15 plasma samples from healthy controls were enrolled for bFGF concentration measurement. Characteristics of the study population are displayed in Table 1. There was no statistically significant difference in age between OA patients and controls (68.9 ± 1.6 vs. 69.7 ± 1.4 years, P = 0.5). As demonstrated in Fig. 1, OA patients had higher plasma bFGF concentrations compared with controls (3.3 ± 0.6 vs. 1.9 ± 0.2 pg/ml, P = 0.01); bFGF levels in synovial fluid were substantially higher than in paired plasma samples (25.1 ± 5.1 vs. 3.3 ± 0.6 pg/ml, P < 0.001).
Table 1.
Plasma and synovial fluid (SF) basic fibroblast growth factor (bFGF) in osteoarthritis (OA) patients. Data are expressed as mean and standard error of the mean (SEM). P values for differences among Kellgren and Lawrence (KL) subgroups
| Total | KL Grade 2 | KL grade 3 | KL grade 4 | P value | |
|---|---|---|---|---|---|
| Number | 35 | 10 | 12 | 13 | |
| SF bFGF (pg/ml) | 25.1 ± 5.1 | 6.3 ± 0.8 | 15.8 ± 1.0 | 48.1 ± 11.0 | <0.001 |
| Plasma bFGF (pg/ml) | 3.3 ± 0.6 | 1.3 ± 0.2 | 2.1 ± 0.2 | 5.9 ± 1.4 | <0.001 |
Fig. 1.
Basic fibroblast growth factor (bFGF) levels in plasma and synovial fluid of patients with osteoarthritis (OA) (n = 35) and healthy controls (n = 15)
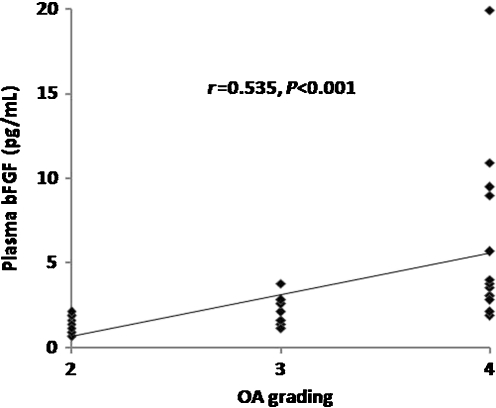
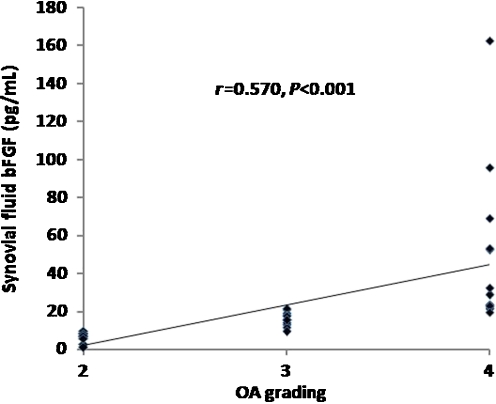
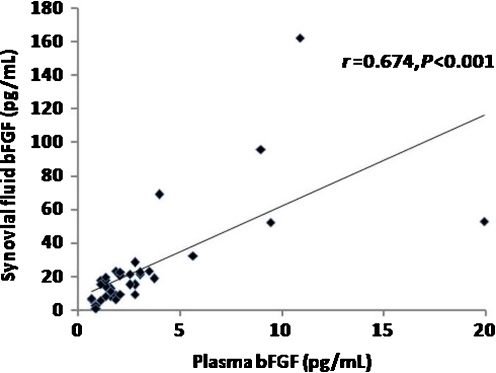
In respect to KL criteria, OA patients were classified into three groups according to OA grading. Ten patients were KL grade 2, 12 were KL grade 3 and 13 were KL grade 4. As illustrated in Table 1, knee OA patients with higher radiographic severity had significantly higher bFGF levels in both plasma and synovial fluid (P < 0.001). Subsequently, we analysed and compared the association between plasma and synovial fluid levels of bFGF and radiographic severity of OA. Plasma bFGF levels were positively correlated with the radiographic grading of knee OA (r = 0.535, P < 0.001) (Fig. 2). Synovial fluid levels of bFGF were positively associated with radiographic severity of disease (r = 0.570, P < 0.001) (Fig. 3). Figure 4 shows that plasma bFGF levels had a positive correlation with synovial fluid bFGF levels (r = 0.674, P < 0.001).
Fig. 2.
Positive correlation between plasma basic fibroblast growth factor (bFGF) levels in patients with osteoarthritis (OA) and disease severity classified according to Kellgren and Lawrence (KL) grading scale (r = 0.535, P < 0.001)
Fig. 3.
Positive correlation between synovial fluid (SF) basic fibroblast growth factor (bFGF) levels in patients with osteoarthritis (OA) and severity classified according to Kellgren and Lawrence (KL) grading scale (r = 0.570, P < 0.001)
Fig. 4.
Positive correlation between plasma and synovial fluid (SF) basic fibroblast growth factor (bFGF) concentrations in patients with osteoarthritis (OA) (r = 0.674, P < 0.001)
Discussion
FGFs encompass a large cytokine family of structurally related multifunctional polypeptide mitogens of widespread tissue distribution [2]. They exert mitogenic activity towards a variety of cells and play important roles in embryogenesis, cell proliferation and differentiation, angiogenesis and tissue repair. Nine members of the FGF family have been identified. Basic FGF is one of the most well-characterised members of the family and one of the most powerful angiogenic polypeptides [26]. Basic FGF secreted by fibroblasts, platelets or other cells is responsible for connective tissue formation and wound healing [4, 20]. Recently, bFGF was demonstrated to regenerate damaged articular cartilage in the mouse OA model [8, 11].
Previous studies demonstrated that bFGF is present in synovial fluid of the temporomandibular joint with internal derangement and OA [19, 24]. However, neither plasma nor synovial fluid bFGF levels in patients with knee OA have been explored, and their correlations with disease severity have never been specifically determined. To the best of our knowledge, data on the association between plasma and synovial fluid bFGF levels and severity of knee OA have as yet not been documented. This study is the first to show that bFGF was detected in both plasma and synovial fluid obtained from patients with primary knee OA and that bFGF was positively correlated with radiographic grading of knee OA. The study demonstrated a significant elevation of bFGF levels in both plasma and synovial fluid in patients with knee OA compared with plasma levels in controls. Our findings suggest that there is increased local and systemic bFGF production in knee OA. It should be noted that bFGF levels in synovial fluid were a great deal higher than those observed in paired plasma samples. The substantial elevation of bFGF in synovial fluid is presumably attributed to either secretion of bFGF residing in extracellular matrix, increased bFGF synthesis or both. The source of bFGF in the synovial fluid could stem from synovial cells and chondrocytes in local tissues (inflamed synovium, articular cartilage, subchondral bone) and extra-articular tissues. Previous studies have shown that bFGF is expressed in synovial cells, articular cartilage chondrocytes and infrapatellar fat pad in patients with OA [21, 27, 28]. Further studies are required to verify whether concentrations of bFGF in synovial fluid and plasma are related to local expression of bFGF in joint tissues. In addition, plasma and synovial fluid bFGF levels were analysed in a well-defined knee OA population at every stage of disease and were more pronounced in end-stage OA patients compared with early OA patients. This observation indicates a significant elevation in the systemic and local expression of bFGF in patient with advanced knee OA. The mechanisms of bFGF enhancement in the circulation and synovial fluid of patients with OA require further studies.
This study inevitably had some inherent shortcomings. First, investigation was administered as a single-cemtre trial with a relatively small number of participants. Additional study conducted on a random sample of multiple centres with larger sample sizes is warranted to validate our results. Second, only bFGF levels were measured in both plasma and synovial fluid. Further studies analysing bFGF expression in local tissue, in relation to synovial and circulating bFGF levels, could provide a more valuable insight into the pathogenic role of bFGF in OA. Third, synovial fluid samples from healthy controls were not taken for ethical reasons. Last, as this was a cross-sectional study, definite cause and effect relationships might not be possible. However, prospective longitudinal investigations are needed to demonstrate disease progression and determine the exact role of bFGF in knee OA.
In conclusion, the study revealed that plasma bFGF in patients with OA was significantly elevated compared with that of healthy controls and that bFGF concentrations in synovial fluid were remarkably higher with regard to paired plasma bFGF. Both plasma and synovial fluid bFGF levels were positively correlated with the degree of radiographic severity in patients with primary knee OA. These findings suggest that bFGF could be a useful biochemical parameter to reflect disease severity in knee OA. Additionally, bFGF levels in plasma were directly correlated with those in synovial fluid. This study was the first to investigate such a correlation, and further research is necessary to delineate the mechanisms underlying this association. More longitudinal studies are necessary to gain insight into the precise role of bFGF in the pathogenesis of chronic degenerative processes in patients with OA.
Acknowledgments
This investigation was supported by the Ratchadaphiseksomphot, Faculty of Medicine, Chulalongkorn University, the Thailand Research Fund, the Commission on Higher Education, the National Research Council of Thailand, and the National Research University Project of CHE and the Ratchadaphiseksomphot Endowment Fund (HR1155A). The authors also thank Dr. Saran Tantavisut and Ms. Tanyawan Suantawee for technical assistance and Research Core facility of the Department of Biochemistry, Chulalongkorn Medical Research Center (ChulaMRC), for generously providing facilities.
References
- 1.Abraham JA, Whang JL, Tumolo A, Mergia A, Friedman J, Gospodarowicz D, Fiddes JC. Human basic fibroblast growth factor: nucleotide sequence and genomic organization. EMBO J. 1986;5:2523–2528. doi: 10.1002/j.1460-2075.1986.tb04530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer C, Werner S. Fibroblast growth factors and neuroprotection. Adv Exp Med Biol. 2002;513:335–351. doi: 10.1007/978-1-4615-0123-7_12. [DOI] [PubMed] [Google Scholar]
- 3.Anitua E, Sánchez M, Fuente M, Azofra J, Zalduendo M, Aguirre JJ, Andía I. Relationship between investigative biomarkers and radiographic grading in patients with knee osteoarthritis. Int J Rheumatol. 2009;2009:747432. doi: 10.1155/2009/747432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 5.Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18:26–45. doi: 10.1210/er.18.1.26. [DOI] [PubMed] [Google Scholar]
- 6.Chia SL, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, Vincent T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009;60:2019–2027. doi: 10.1002/art.24654. [DOI] [PubMed] [Google Scholar]
- 7.Friesel RE, Maciag T. Molecular mechanisms of angiogenesis: fibroblast growth factor signal transduction. FASEB J. 1995;9:919–925. doi: 10.1096/fasebj.9.10.7542215. [DOI] [PubMed] [Google Scholar]
- 8.Fujisato T, Sajiki T, Liu Q, Ikada Y. Effect of basic fibroblast growth factor on cartilage regeneration in chondrocyte-seeded collagen sponge scaffold. Biomaterials. 1996;17:155–162. doi: 10.1016/0142-9612(96)85760-7. [DOI] [PubMed] [Google Scholar]
- 9.Honsawek S, Chayanupatkul M, Tanavalee A, Sakdinakiattikoon M, Deepaisarnsakul B, Yuktanandana P, Ngarmukos S. Relationship of plasma and synovial fluid BMP-7 with disease severity in knee osteoarthritis patients: a pilot study. Int Orthop. 2009;33:1171–1175. doi: 10.1007/s00264-009-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honsawek S, Tanavalee A, Sakdinakiattikoon M, Chayanupatkul M, Yuktanandana P. Correlation of plasma and synovial fluid osteopontin with disease severity in knee osteoarthritis. Clin Biochem. 2009;42:808–812. doi: 10.1016/j.clinbiochem.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Jingushi S, Shida J, Iwamoto Y, Kinoshita T, Hiyama Y, Tamura M, Izumi T. Transient exposure of fibroblast growth factor-2 induced proliferative but not destructive changes in mouse knee joints. Connect Tissue Res. 2006;47:242–248. doi: 10.1080/03008200600883146. [DOI] [PubMed] [Google Scholar]
- 12.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan IM, Palmer EA, Archer CW. Fibroblast growth factor-2 induced chondrocyte cluster formation in experimentally wounded articular cartilage is blocked by soluble Jagged-1. Osteoarthr Cartil. 2010;18:208–219. doi: 10.1016/j.joca.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Kraus VB. Pathogenesis and treatment of osteoarthritis. Med Clin North Am. 1997;81:85–112. doi: 10.1016/S0025-7125(05)70506-X. [DOI] [PubMed] [Google Scholar]
- 15.Ku JH, Lee CK, Joo BS, An BM, Choi SH, Wang TH, Cho HL. Correlation of synovial fluid leptin concentrations with the severity of osteoarthritis. Clin Rheumatol. 2009;28:1431–1435. doi: 10.1007/s10067-009-1242-8. [DOI] [PubMed] [Google Scholar]
- 16.Kurokawa T, Sasada R, Iwane M, Igarashi K. Cloning and expression of cDNA encoding human basic fibroblast growth factor. FEBS Lett. 1987;213:189–194. doi: 10.1016/0014-5793(87)81489-8. [DOI] [PubMed] [Google Scholar]
- 17.Muddasani P, Norman JC, Ellman M, Wijnen AJ, Im HJ. Basic fibroblast growth factor activates the MAPK and NFkappaB pathways that converge on Elk-1 to control production of matrix metalloproteinase-13 by human adult articular chondrocytes. J Biol Chem. 2007;282:31409–31421. doi: 10.1074/jbc.M706508200. [DOI] [PubMed] [Google Scholar]
- 18.Okada-Ban M, Thiery JP, Jouanneau J. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32:263–267. doi: 10.1016/S1357-2725(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 19.Orito K, Koshino T, Saito T. Fibroblast growth factor 2 in synovial fluid from an osteoarthritic knee with cartilageregeneration. J Orthop Sci. 2003;8:294–300. doi: 10.1007/s10776-003-0647-6. [DOI] [PubMed] [Google Scholar]
- 20.Przybylski M. A review of the current research on the role of bFGF and VEGF in angiogenesis. J Wound Care. 2009;18:516–519. doi: 10.12968/jowc.2009.18.12.45609. [DOI] [PubMed] [Google Scholar]
- 21.Qu Z, Huang XN, Ahmadi P, Andresevic J, Planck SR, Hart CE, Rosenbaum JT. Expression of basic fibroblast growth factor in synovial tissue from patients with rheumatoid arthritis anddegenerative joint disease. Lab Invest. 1995;73:339–346. [PubMed] [Google Scholar]
- 22.Quintavalla J, Kumar C, Daouti S, Slosberg E, Uziel-Fusi S. Chondrocyte cluster formation in agarose cultures as a functional assay to identify genes expressed in osteoarthritis. J Cell Physiol. 2005;204:560–566. doi: 10.1002/jcp.20345. [DOI] [PubMed] [Google Scholar]
- 23.Saetan N, Honsawek S, Tanavalee A, Tantavisut S, Yuktanandana P, Parkpian V. Association of plasma and synovial fluid interferon-γ inducible protein-10 with radiographic severity in knee osteoarthritis. Clin Biochem. 2011;44:1218–1222. doi: 10.1016/j.clinbiochem.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Sato J, Segami N, Kaneyama K, Konishi H, Yoshitake Y, Nishikawa K. Levels of fibroblast growth factor 2 in synovial fluids in human patients with internal derangement of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:673–679. doi: 10.1016/j.tripleo.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Tchetina EV, Squires G, Poole AR. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early humanarticular cartilage lesions. J Rheumatol. 2005;32:876–886. [PubMed] [Google Scholar]
- 26.Thomas KA. Fibroblast growth factors. FASEB J. 1987;1:434–440. doi: 10.1096/fasebj.1.6.3315806. [DOI] [PubMed] [Google Scholar]
- 27.Uchino M, Izumi T, Tominaga T, Wakita R, Minehara H, Sekiguchi M, Itoman M. Growth factor expression in the osteophytes of the human femoral head in osteoarthritis. Clin Orthop Relat Res. 2003;377:119–125. doi: 10.1097/00003086-200008000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Ushiyama T, Chano T, Inoue K, Matsusue Y. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis. 2003;62:108–112. doi: 10.1136/ard.62.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]