Abstract
Purpose
Many Sox proteins play important roles both in mesoderm and ectoderm development. It is reported that Sox2, a member of this family, is essential for the maintenance of the self-renewal of embryonic stem cells (ES) and neural stem cells (NSCs). To investigate whether Sox2 participates in mesoderm development besides ectoderm, Sox2 was introduced into C3H10T1/2 cells.
Methods
We produced recombinant retrovirus expressing Sox2 in GP2-293t cells and infected the virus into C3H10T1/2 cells. Growth property, alkaline phosphatase (ALP) staining, mineralized nodules, osteogenic gene expression and related signal pathways were analysed and compared between Sox2-expressing cells and control cells.
Results
Sox2 over-expression led to increased proliferation of C3H10T1/2 cells, activation of Wnt/β-catenin and p38MAPK pathways. When cultured in osteogenic differentiation medium, ALP and mineralized nodules formation were inhibited in Sox2 over-expressing cells with down-regulation of osteogenic gene expression as well as inhibition of Wnt/β-catenin and p38MAPK pathways.
Conclusions
All these data suggested that over-expression of Sox2 promoted proliferation and inhibited osteoblast differentiation of C3H10T1/2 cells.
Keywords: Medicine & Public Health, Orthopedics
Introduction
The Sox proteins consist of a group of transcription factors characterized by the presence of a SRY box, a 79 amino acid motif that encodes a HMG-type DNA binding domain [1]. Individual members of the Sox family show above 50% identity in their HMG domain to SRY [2], these Sox proteins have critical functions in sex determination, neurogenesis and skeleton formation [3–5].
Some of Sox proteins promoted the mesoderm tissue formation. The transcription factors L-Sox5, Sox6, and Sox9 are major genes of chondrogenic differentiation [6]. Meanwhile, the evidence indicates that these transcription factors also play important roles in nervous system development [7–9]. Sox2 co-operates with transcription factor Oct4 to maintain the pluripotency and self-renewal of ES cells through activating the expression of Oct3/4 and Sox2 itself [10–12]. Sox2 had also been found to maintain the self-renewal and proliferation of NSCs [13]; furthermore, Sox2 is one of the factors in inducing iPS cells (induced pluripotent cells, iPS). By gene expression profiling, the upregulation of Sox2 expression was the most significant in craniofacial skeletal disorders such as Crouzon (CR) and Apert (AP) syndrome [14]. The bone mass of those patients was reduced in the skull. It seems that Sox2 may be correlated with osteoporosis. So we decided to investigate the role of Sox2 in osteoblast differentiation in detail.
In the present study, Sox2 was cloned to the PLEGFP vector and stably over-expressed in C3H10T1/2 cells. C3H10T1/2 is a mesenchymal stem cell line derived from mouse embryos. Due to their multipotent capacity to differentiate into chondrocytes, adipocytes and osteoblasts, this cell line is commonly used as a differentiation model. We investigated the differentiation potential of Sox2 over-expressing C3H10T1/2 cells. Cell proliferation and osteogenic differentiation potential were compared between Sox2 expressing cells and control cells cultured in osteogenic differentiation medium. We further investigated the role of MAPK and Wnt/β-catenin signal pathways in osteoblast differentiation.
Materials and methods
Cell lines and culture
Cell lines C3H10T1/2 and GP2-293t were bought from ATCC (American Type Culture Collection, Manassas, VA, USA). C3H10T1/2 is established from mouse MSCs (mesenchymal stem cells, MSC). C3H10T1/2 and GP2-293t were cultured in Eagle's minimum essential medium (α-MEM) (Gibco, Invitrogen, USA) and DMEM (Biowest, France), respectively, both containing 10% FBS and antibiotics (penicillin–streptomycin). Culture medium was changed every other day.
Construction of recombinant plasmids and retrovirus infection
The Sox2 gene was amplified from mouse ES cells and cloned to the pLEGFP-N1 retroviral expression system (BD Biosciences Clontech). Retrovirus was produced in GP2-293t cells. pLEGFP-Sox2 was cotransfected with a helper plasmid PMD2.0G into GP2-293t cells using Lipofectamine2000 (Cat.No 11668-019, Invitrogen, USA) according to the manufacturer's instructions. The virus-containing supernatant was filtered and added to C3H10T1/2 cells with polybrene (Sigma-Aldrich, Basel, Switzerland) 8 μg/ul for six hours. The medium was then changed to complete medium.
EdU incorporation assay
Cells were incubated with DMEM supplemented with 10 μM EdU (Cat.No.C10310, RiboBio, China) for 24 hours. Cells were then washed with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde fixation and incubation with glycine 2 mg/ml, washed with PBS twice, and permeabilized with PBS containing 0.5% triton X-100. After extensive washing with PBS, cells were incubated with Apollo® staining solution for 30 min, washed with PBS containing 0.5% Triton X-100 three times, followed by 10 min incubation with Hoechst. Photographs of the cells were captured with a fluorescent microscope.
ALP and Alizarin red staining
Cells were fixed in 4% paraformaldehyde at room temperature (RT) for 2 min, then washed in PBS three times, incubated with 1-Step™ NBT/BCIP (Cat.No 34042, Pierce, USA) solution for 30 min at RT and rinsed with water. To detect mineralized nodules, cells were fixed in 95% ethanol, and Alizarin red S (Cat.No A5533, Sigma-Aldrich, Switzerland) was left on the plates overnight and washed with water.
Osteogenic differentiation
Sox2-expressing cells and control cells were seeded at 5 × 105 cells/well in a six-well plate in the maintenance medium. The medium was changed to osteogenic differentiation medium the next day, which was supplemented with 10 mM β-glycerophosphate (Cat.No 35675, Merck, USA), 50 μg/ml of L-ascorbic acid (Cat.No A7506, Sigma-Aldrich, Switzerland) and 10-7 M dexamethasone (Cat.No D1756, Sigma-Aldrich, Switzerland). The differentiation was induced for two weeks and the medium was changed every other day.
Western blotting
Cells were lysed with lysis buffer (Beyotime, P0013B) containing PMSF. Lysates was centrifuged at 12,000 g at 4°C for 10 min, and the supernatant was collected and preserved at −80°C for later use. Protein concentrations were determined using BCA Protein Assay Kit (Cat.No 23227, Pierce, USA). Proteins were separated by 15% SDS-PAGE and transferred to PVDF membrane using a standard protocol. The membrane was incubated with primary antibodies and probed with the respective secondary antibodies. The membrane was exposed and scanned using Odyssey® Infrared Imaging system. The following antibodies were used: p-Erk (#9910, CST, USA), Erk (#9102,CST), p-P38MARK(#9910, CST), PCNA (SC-25280), β-catenin (#9562, CST) and β-actin (#4967,CST).
Immunofluorescence
The culture medium was discarded and the cover slips were rinsed with PBS (pH 7.4) twice. The cells were fixed in 4% paraformaldehyde, then washed in PBS three times, permeabilized with 0.1% Triton X-100 in PBS for 30 min at RT, washed in PBS three times, incubated in PBST with 1% BSA for 60 min to block unspecific binding of the antibodies, incubated with primary antibody Sox2 (SC-17320) in 1% BSA for 60 min at RT, washed with PBS three times, and incubated with secondary antibody in 1% BSA for 30 min at RT. Nuclei were stained with DAPI (Cat.No A1001, Applichem, Germany) at a concentration of 10 ng/ml.
Real-time quantitative PCR (RT-qPCR)
Total RNA was isolated from the cultured cells with Trizol reagent (Cat.No 15596-026, Invitrogen). The first strand cDNA was synthesized with RT Reagent Kit (takara code DRR037A). qPCR was performed with a UPL probe (Roche). Standard curves were determined by running a dilution series on the housekeeping gene and target gene. The amplification efficiency was warranted between 90 and 105%. Then the delta-delta Ct method was used to analyze the result. Primers sequence and probe names are listed in Table 1. The experiments were repeated three times independently.
Table1.
Primers of osteogenic genes for qPCR analysis
| Gene name | GeneBank accession no. | 5′–3′ | Probe name |
|---|---|---|---|
| actin-F | NM_007393.3 | aaggccaaccgtgaaaagat | #64 |
| actin-R | gtggtacgaccagaggcatac | ||
| Sp7-F | NM_130458.3 | ctcctgcaggcagtcctc | #106 |
| SP7-R | gggaagggtgggtagtcatt | ||
| OPN-F | AF515708.1 | cccggtgaaaggactgatt | #82 |
| OPN-R | ttcttcagaggacacagcattc | ||
| Osteocalcin-F | NM_001032298.2 | agactccggcgctacctt | #32 |
| Osteocalcin-R | ctcgtcacaagcagggttaag | ||
| Runx2-F | NM_001146038.1 | gcccaggcgtatttcaga | #34 |
| Runx2-R | tgcctggctcttcttactgag | ||
| ALP-F | NM_007431.2 | cggatcctgaccaaaaacc | #12 |
| ALP-R | tcatgatgtccgtggtcaat |
Results
pLEGFP-Sox2 retrovirus production and infection
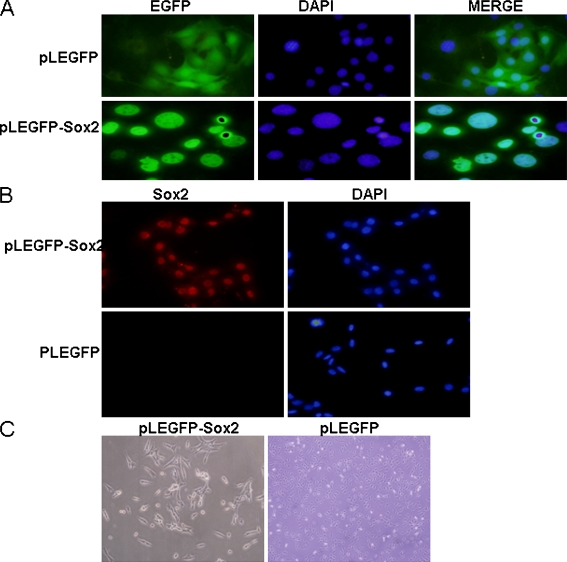
GP2-293t cells were transfected with pLEGFP-Sox2 and PMD2.0G. Virus-containing supernatants from GP2-293t cells were filtered through a 0.45-um cellulose acetate filter and mixed with polybrene at the final concentration of 8 μg/ml. Cells were incubated in the virus/ polybrene containing supernatants for 6 h. EGFP could be detected three days after infection. For Sox2-expressing cells and control cells, EGFP was located in the nucleus and in the whole cells, respectively (Fig. 1a). Immunostaining showed that Sox2 protein was only detected in Sox2-expressing cells (Fig. 1b). About one week later, the morphology of Sox2-expressing cells was different from that of the control cells which were smaller and shorter than control cells (Fig. 1c).
Fig. 1.
Stable expression of Sox2 in MSCs by retroviral vector. a C3H10T1/2 cells were infected with pLEGFP-Sox2 virus and pLEGFP. b Identification of Sox2 protein expression after pLEGFP-Sox2 virus infected C3H10T1/2 cells. c The morphology change of Sox2-expressing and control cells
Effect of Sox2 over-expression on cell proliferation
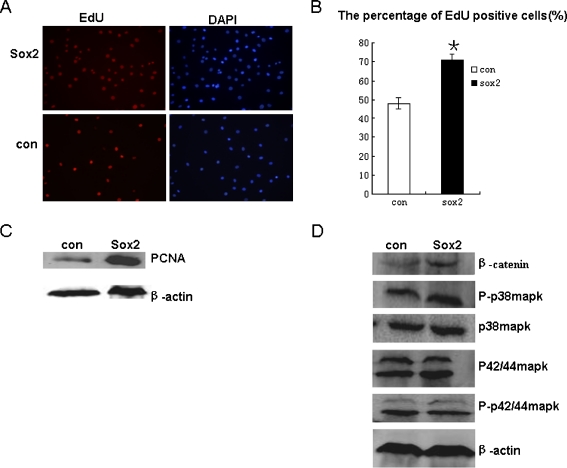
Sox2-expressing cells were cultured for one week and showed significant difference from control cells in growth pattern and morphology. EdU, which is an indicator of DNA synthesis, was used to detect cell proliferation (Fig. 2a). DAPI was used to mark all the nuclei. At least three captured fields were randomly selected and the EdU positive cells were calculated. Results showed that the percentages of EdU positive cells in the Sox2-expressing group and in the control group were 71% and 48%, respectively (Fig. 2b). In addition, we also detected PCNA expression level (Proliferating Cell Nuclear Antigen, commonly known as PCNA). This result was in accordance with the EdU assay (Fig. 2c).
Fig. 2.
Growth properties of Sox2-expressing and control cells. a EdU incorporation assay of two groups. b Statistical analysis of EdU positive cells,**P < 0.01 (n = 3). c Quantitative analysis of PCNA expression between two groups. d Wnt/β-catenin and p38MAPK pathways were activated in Sox2-expressing cells, p-erk was not changed
Wnt and MAPK pathways are regulators of cell proliferation. We tested whether the activation of these two pathways led to the increased proliferation of Sox2 expressing cells relative to control cells. Results indicated that β-catenin was upregulated significantly and there was a slight increase of P-p38MAPK in Sox2-expressing cells. No striking change in P-Erk1/2 was observed between these two groups (Fig. 2d).
Differentiation potential of Sox2 expressing cells
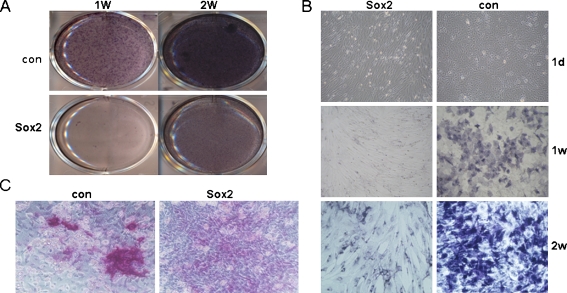
Osteoblast induction was performed after Sox2 had been over-expressed in C3H10T1/2 cells for one week. There was considerable difference in cell morphology between the two groups after being induced for 24 hrs, whereby Sox2-expressing cells became elongated in contrast to control cells (Fig. 3b). The induction lasted for two weeks. The ALP staining was much stronger in the control group than in the Sox2-expressing group at day 7 and day 14, respectively (Fig. 3a). The control group developed more mineralized nodules than the Sox2-expressing group after one month induction (Fig. 3c).
Fig. 3.
Comparison of ALP staining and mineralized nodules between Sox2 expressing cells and control cells. a ALP staining of Sox2-expressing cells and control cells. b The details of ALP staining and cell morphology change after being induced with osteogenic differentiation medium. c Alizarin red staining of Sox2-expressing cells and control cells
Osteoblastic gene expression profile of Sox2 expressing cells and control cells
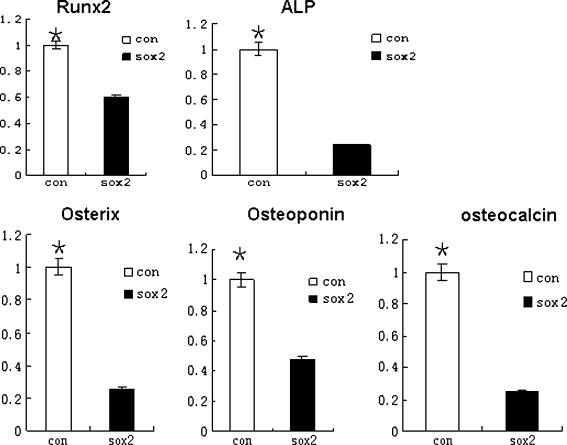
ALP activity increasing and mineralized nodules development are indispensable to osteogenic differentiation. Some specific genes are also expressed in this process. So we examined mRNA expression of osteoblast-related genes by quantitative RT-PCR analysis. Results demonstrated that mRNA expression of those genes were strongly down-regulated in Sox2-expressing cells compared to control cells (Fig. 4). It further indicated that over-expression of Sox2 in C3H10T1/2 cells would lead to the inhibition of osteoblast differentiation.
Fig. 4.
qPCR analysis of osteogenic gene expression in Sox2 overexpressing C3H10T1/2 cells cultured in osteogenic differentiation medium, relative to internal control actin and normalized to control cells. **P < 0.01 (n = 3); mRNA levels of osterix, Runx2, ALP, Osteoponin and osteocalcin from control cells were 3.9, 1.7, 4.3, 2.1, and 4-fold higher than Sox2-expressing cells, respectively
Wnt/β-catenin and MAPK pathways are involved in osteoblast differentiation of Sox2-expressing cells
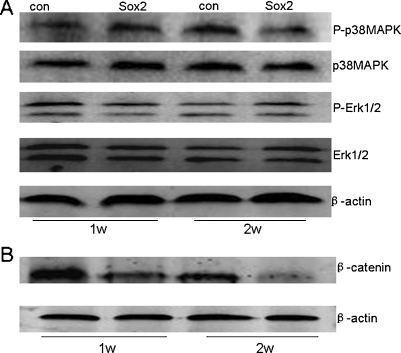
To further understand why Sox2 inhibited osteoblast differentiation, we studied the target effectors of Wnt and MAPK pathways: β-catenin, P-p38mapk and P-Erk1/2. These pathways were analysed after two groups were induced for 7 days and 14 days. Results showed that β-catenin was down-regulated in Sox2-expressing cells in the whole process (Fig. 5b). P-p38mapk was down-regulated only at a later stage and the phosphorylation level of Erk1/2 was not changed obviously between the two groups (Fig. 5a).
Fig. 5.
Western blot of β-catenin, P-p38MAPK and P-Erk1/2 expression in Sox2-expressing cells and control cells cultured under osteogenic differentiation medium. a The phosphorylation level of p38MAPK in Sox2-expressing cells was down-regulated only at a later stage. p-Erk1/2 was not influenced between the two groups at different time points. b β-catenin expression was down-regulated in the whole process in Sox2-expressing cells
Discussion
Mineralized nodule formation and alkaline phosphatase (ALP) activity increases are the most important characteristics of the osteoblast differentiation. Differentiation of MSC to osteoblasts is a complicated process that involves cell expansion and differentiation. Many factors affect the whole process, such as bone morphogenetic proteins (BMPs) and Wnt molecules. BMPs activate the Smad1/5/8 signalling pathway [15], and Wnts activate the β-catenin pathway to promote osteoblast differentiation [16]. In addition, it has been shown that p38MAPKs are often involved in promoting the osteoblast differentiation [17–19]. It is controversial whether Erk1/2 phosphorylation has an effect on differentiation of MSCs to osteogenic lineage. Conclusions in the literature are opposing [20, 21].
In this study, Sox2 was forced expressed in the C3H10T1/2 cell line. In order to achieve high efficiency of infection and stable expression of Sox2 in C3H10T1/2 cells, we chose pLEGFP retrovirus plasmid, which contains the EGFP sequence for an indication of the efficiency of transfection and infection of Sox2. C3H10T1/2 cells were infected by pLEGFP-Sox2. Three days after infection, we found that nearly all the cells were positive for EGFP or Sox2-EGFP. Of note, Sox2-positive cells were smaller than control cells after being cultured for one week. Expression of Sox2 transcript has been reported in MSC isolated from different tissues [22]. In our study, immunostaining results of two groups suggested that Sox2 protein expression was found only in Sox2-expressing cells.
Transcription factor Sox-2 is known to regulate the self-renewal of ES cells and NSCs. So we also investigated whether its over-expression may affect the proliferative and differentiative properties of C3H10T1/2 cells. Two different methods were applied to compare cell proliferation between the two groups. The percentage of EdU positive cells in the Sox2-expressing group was much higher than that of the control group. Also, the expression level of PCNA was upregulated significantly in Sox2-expressing cells. This result confirmed that Sox2-expressing cells grew faster than control cells again. Wnt/β-catenin and MAPK signal pathways are not only involved in cell differentiation but also in cell proliferation. Our data showed that the Wnt/β-catenin pathway was activated obviously in Sox2-expressing cells. In contrast to β-catenin, the MAPK pathway was activated partly for the slight increase in the phosphorylation of p38MAPK with no change in the phosphorylation level of Erk1/2.
We next examined osteoblast differentiation potential of Sox2-expressing cells and control cells. The appearance of ALP staining was delayed in the Sox2-expressing group and most of the cells still maintained the phenotype of undifferentiated cells. We also found that osteogenic potential of Sox2-expressing cells was blocked in terms of calcium deposition experiment. Comparison of osteogenic gene expression by qPCR indicated that RNA levels of ALP, Runx2, Osteoponin, Osterix and Osteocalcin in control cells were evidently higher than in Sox2-expressing cells suggesting that constitutive expression of Sox2 in C3H10T1/2 cells impedes osteoblast differentiation.
It is interesting to note that osteoblast differentiation conditions have adverse effects on Wnt signalling in our study. β-catenin was obviously down-regulated in the Sox2-expressing group in the whole inducing process. P-p38MAPK was down-regulated in contrast to control cells only at two weeks. It seemed that P-Erk1/2 was not affected by osteogenic inducing conditions in C3H10T1/2 cells.
A previous report had uncovered that Sox2 expression was induced by FGF in the osteoblastic cell line and subsequently inhibited osteoblast differentiation [14]. Another study indicated that Sox2 overexpression in human MSCs still maintained the differentiation capability towards osteoblasts [23]. In in vivo experiments, transgenic mice expressing Sox2 exhibited decreased bone mass and mineralization [24]; Sox2 expression is also essential for the self-renewal of osteoprogenitor cells [25]. In our experiment, Sox2 over-expression in C3H10T1/2 cells inhibited osteogenic differentiation. Our finding was also different from others in that P-p38MAPK was downregulated and led to osteogenic differentiation inhibition. Different cell types and different stimuli may be the major reason to explain these seemingly contradictory results regarding osteogenesis. Another possible reason is that C3H10T1/2 is a stem cell line, and more stemness are endowed with Sox2 over-expression. The stems will enhance the self-renewal of C3H10T1/2 cells and block the osteogenic differentiation.
Acknowledgments
This work were supported by the National Basic Research Program of China (973 Program, 2010CB530400), the Key Project of National Natural Science Foundation of China (30930111), the Changjiang Scholar Chair Professor project (Teach people [2009] 17), the Shanghai Education Innovation Project (08YZ56), the “Shu Guang” project supported by the Shanghai Municipal Education Commission and the Shanghai Education Development Foundation (10GG20), the Shanghai University Innovation Team Programmer (Shanghai Education Commission, Division 6 [2009]), and the China Postdoctoral Science Foundation (20090450725).
References
- 1.Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227(2):239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- 2.Wright EM, Snopek B, Koopman P. Seven new members of the Sox gene family expressed during mouse development. Nucleic Acids Res. 1993;21(3):744. doi: 10.1093/nar/21.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright E, Hargrave MR, Christiansen J, et al. The Sryrelated gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- 4.Laudet V, Stehelin D, Clevers H. Ancestry and diversity of the HMG box superfamily. Nucleic Acids Res. 1993;21:2493–2501. doi: 10.1093/nar/21.10.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pevny LH, Lovell-Badge R. Sox genes find their feet. Curr Opin Genet Dev. 1997;7:338–344. doi: 10.1016/S0959-437X(97)80147-5. [DOI] [PubMed] [Google Scholar]
- 6.Akiyama H, Chaboissier MC, Martin JF, Schedl A, Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stolt CC, Wegner M. SoxE function in vertebrate nervous system development. Int J Biochem Cell Biol. 2010;42:437–440. doi: 10.1016/j.biocel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- 9.Scott CE, Wynn SL, Sesay A, et al. SOX9 induces and maintains neural stem cells. Nat Neurosci. 2010;13:1181–1189. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- 10.Okumura-Nakanishi S, Saito M, Niwa H, Ishikawa F. Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. J Biol Chem. 2005;280:5307–5317. doi: 10.1074/jbc.M410015200. [DOI] [PubMed] [Google Scholar]
- 11.Tomioka M, Nishimoto M, Miyagi S, et al. Identification of Sox-2 regulatory region which is under the control of Oct-3/4-Sox-2 complex. Nucleic Acids Res. 2002;30:3202–3213. doi: 10.1093/nar/gkf435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew JL, Loh YH, Zhang W, et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/ Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/S0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 14.Mansukhani A, Ambrosetti D, Holmes G, Cornivelli L, Basilico C. Sox2 induction by FGF and FGFR2 activating mutations inhibits Wnt signaling and osteoblast differentiation. J Cell Biol. 2005;168:1065–1076. doi: 10.1083/jcb.200409182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celil AB, Hollinger JO, Campbell PG. Osx transcriptional regulation is mediated by additional pathways to BMP2/Smad signaling. J Cell Biochem. 2005;95:518–528. doi: 10.1002/jcb.20429. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann C. A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol. 2006;16:151–158. doi: 10.1016/j.tcb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Udagawa N, Takami M, Sato N, Kobayashi Y, Takahashi N. p38 mitogen-activated protein kinase is crucially involved in osteoclast differentiation but not in cytokine production, phagocytosis, or dendritic cell differentiation of bone marrow macrophages. Endocrinology. 2003;144:4999–5005. doi: 10.1210/en.2003-0166. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Chan E, Wang SX, Li B. Activation of p38 mitogen-activated protein kinase is required for osteoblast differentiation. Endocrinology. 2003;144:2068–2074. doi: 10.1210/en.2002-220863. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki A, Guicheux J, Palmer G, Miura Y, Oiso Y, Bonjour JP, Caverzasio J. Evidence for a role of p38 MAP kinase in expression of alkaline phosphatase during osteoblastic cell differentiation. Bone. 2002;30:91–98. doi: 10.1016/S8756-3282(01)00660-3. [DOI] [PubMed] [Google Scholar]
- 20.Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi C, Myoui A, Hashimoto N, Kuriyama K, Yoshioka K, Yoshikawa H, Itoh K. Continuous inhibition of MAPK signaling promotes the early osteoblastic differentiation and mineralization of the extracellular matrix. J Bone Miner Res. 2002;17:1785–1794. doi: 10.1359/jbmr.2002.17.10.1785. [DOI] [PubMed] [Google Scholar]
- 22.Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G, Muiznieks I, Muceniece R, Ancans J. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 2009;5:378–386. doi: 10.1007/s12015-009-9094-9. [DOI] [PubMed] [Google Scholar]
- 23.Go MJ, Takenaka C, Ohgushi H. Forced expression of Sox2 or Nanog in human bone marrow derived mesenchymal stem cells maintains their expansion and differentiation capabilities. Exp Cell Res. 2008;314:1147–1154. doi: 10.1016/j.yexcr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Holmes G, Bromage TG, Basilico C (2011) The Sox2 high mobility group transcription factor inhibits mature osteoblast function in transgenic mice. Bone 49:653–661. doi:10.1016/j.bone.2011.06.008 [DOI] [PMC free article] [PubMed]
- 25.Basu-Roy U, Ambrosetti D, Favaro R, Nicolis SK, Mansukhani A, Basilico C. The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ. 2010;17:1345–1353. doi: 10.1038/cdd.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]