Abstract
Purpose
Controversy still exists as to the best surgical treatment for periprosthetic shoulder infections. The aim of this multi-institutional study was to review a continuous retrospective series of patients treated in four European centres and to assess the respective eradication rate of various treatment approaches.
Methods
Forty-four patients were available for this retrospective follow-up evaluation. Functional and clinical evaluation of treatment for infection was performed using the Constant-Murley score, visual analogue scale and patient satisfaction Neer score. Erythrocyte sedimentation rate, serum leucocyte count and C-reactive protein were measured and shoulder X-ray examination performed prior to surgery and at the latest follow-up.
Results
At a mean follow-up of 41 months (range 24–98), 42 of 44 patients (95.5%) showed no signs of infection recurrence/persistence. Comparable eradication rates were observed after resection arthroplasty (100%; 6/6), two-stage revision (17/17) or permanent antibiotic-loaded spacer implant (93.3%; 14/15). No patient was treated by one-stage revision. On average, both functional and pain scores improved significantly; the worst joint function was observed after resection arthroplasty.
Conclusions
This retrospective analysis conducted on the largest published series of patients to date shows comparable infection eradication rates after two-stage revision, resection arthroplasty or permanent spacer implant for the treatment of septic shoulder prosthesis.
Keywords: Medicine & Public Health, Orthopedics
Introduction
Infection after primary shoulder arthroplasty ranges from 1% 10% after revision surgery [1–7]. For subacute and chronic periprosthetic shoulder infection, there is controversy about the best treatment strategy. Different authors suggest a two-stage revision, which is regarded as the standard method of care by some authors [8, 9]. However, recent reports have shown similar results after one-stage revision [3, 5, 10], whereas both one- and two-stage revisions achieve acceptable pain control and good shoulder function. Proubasta et al proposed the use of a permanent antibiotic-impregnated cement spacer in the septic shoulder after arthroplasty. They stated that this could be a valid treatment option in the elderly, low-demand patient [7]. Themislocleous et al. recently published their series of 11 patients with a permanent cement spacer. They concluded that in the low-demand patient, one can expect infection and pain control, with limited shoulder function [11], and similar results were found in a more recent publication by Coffey and Crosby [12]. On the other hand, Verhelst et al. found no difference in clinical outcome in a mixed retrospective series of patients undergoing either resection arthroplasty or a spacer implant for chronic shoulder infection of different pathogenesis [13].
The aim of this study was to provide a significant contribution to the published case series of septic shoulder prostheses by reviewing pre-operative relevant data, treatment choice and results in four different European centres dedicated to treating bone and joint infections, over a ten year period.
Materials and methods
Local internal review board (IRB) approval was obtained at each participating centre prior to the start of the study. The study was performed under the aegis of the European Bone and Joint Infection Society. Epidemiological and clinical data were gathered using a standard data sheet, distributed electronically to all participating centres. Between January 1999 and November 2009, fifty-three patients were referred for treatment of a periprosthetic shoulder infection to one of the four participating centres. Forty-four patients (28 women and 16 men) were available for this retrospective follow-up. Mean patient age at the time of admission to one of the participating centres was 63 (range 28–80) years. At the time of prosthetic implant, 18 patients had undergone previous surgery at least once (between one and six procedures); reasons for joint replacement were mainly primary osteoarthritis and previous trauma (Table 1). Patients were classified according to the time interval between arthroplasty and the diagnosis of infection [14]: in early (septic complication diagnosed within two months of surgery; nine patients), delayed (diagnosed between two and 12 months of surgery; 21 patients) and late (diagnosed at least 12 months after implant; 14 patients). Diagnosis of infection was attained using the criteria established by Spangehl et al. [15]. The types of implanted prosthesis are reported in Table 1.
Table 1.
Preoperative data from the reviewed patients (N = 44)
| Patients | Characteristics | Raw numbers | Percent | Mean | Standard devation | Maximum | Minimum |
|---|---|---|---|---|---|---|---|
| Sex | Male | 16 | 36.4 | ||||
| Female | 28 | 63.6 | |||||
| Age | 63 | 10.2 | 80 | 28 | |||
| Country of origin | Italy | 24 | 54.5 | ||||
| Belgium/France | 11 | 25 | |||||
| Switzerland | 9 | 20.5 | |||||
| Indication for prosthesis implant | Osteoarthritis | 15 | 34.1 | ||||
| Post-traumatic | 23 | 52.3 | |||||
| Rheumatoid | 2 | 4.5 | |||||
| Other (infection, tumour) | 4 | 9.1 | |||||
| Operations before prosthesis | 0.7 | 1.2 | 6 | 0 | |||
| Type of prosthesis | Hemiarthroplasty | 30 | 68.2 | ||||
| Total standard | 5 | 11.4 | |||||
| Total inverse | 8 | 18.2 | |||||
| Tumour prosthesis | 1 | 2.3 | |||||
| Type of infection | Early (< 2 months) | 9 | 20.5 | ||||
| Delayed (< 1 year) | 21 | 47.7 | |||||
| Late (> 1 year) | 14 | 31.8 |
Functional and clinical evaluation of the treatment received to cure the infection was performed using the Constant-Murley score (CMS) [16, 17] and the visual analogue scale (VAS), whereas patients’ satisfaction was assessed according to the Neer score [18]. Abduction and external rotation were also measured, and all patients received a standard shoulder X-ray examination at the time of surgery and at the latest follow-up (face/profile/scapular Y view). Erythrocyte sedimentation rate (ESR), serum leucocyte count and C-reactive protein (CRP) were measured prior to surgery and at follow-up. Infection eradication was assessed according to the criteria reported by Coste et al. [3], which included the absence of any of the following clinical signs and investigations: draining sinus; positive serum leucocyte count; positive ESR; Positive CRP; positive joint aspiration cultures; loosening of the components on standard radiographs (defined as a complete line greater than one mm around one or both components) and periosteal reaction; positive three-phase bone isotope scanning. Statistical analysis was performed using the paired t test for continuous series of data.
Results
Of the 53 patients originally admitted for a periprosthetic shoulder infection to the four participating centres between January 1999 and November 2009, 44 were available at follow-up and were included in the study (Table 1). At the time of hospital admission, nearly all patients (43/44; 97.7%) complained of pain; approximately half of them presented with a draining fistula (24/44; 54.5%) and/or local redness/warmth (21/44; 47.7%); fever was observed only in nine patients (20.5%). Osteolysis and/or loosening around the infected prosthesis was also a common finding (27/44; 61.4%) (Table 2).
Table 2.
Preoperative assessment of the reviewed patients (N = 44)
| Characteristics | Raw numbers | Percent | Mean | Standard deviation | Maximum | Minimum | |
|---|---|---|---|---|---|---|---|
| Signs and symptoms at presentation | Pain | 43 | 97.7 | ||||
| Draining fistula | 24 | 54.5 | |||||
| Redness/warmth | 21 | 47.7 | |||||
| Swelling | 12 | 27.3 | |||||
| Fever | 9 | 20.5 | 6 | 0 | |||
| Radiology | X-ray: osteolysis/loosening | 27 | 61.4 | ||||
| Leucocyte bone scan | 16 | 36.4 | |||||
| CT scan | 2 | 4.5 | |||||
| Laboratory tests | Serum leucocytes | 7,400 | 2,200 | 13,700 | 4,200 | ||
| CRP (mg/L) | 38.4 | 31.2 | 178 | 2 |
CT computed tomography, CRP C-reactive protein
Causative pathogens could be identified in 34.1% of patients prior to surgery, with a prevalence of methicillin-resistant Staphylococcus aureus (MRSA). Intraoperative examination yielded positive results in 77% of cases, with Gram-positive cocci isolated in approximately 70% of cases (Table 3).
Table 3.
Isolated pathogens following patient review (N = 44)
| Isolates | Raw numbers | Percent | |
|---|---|---|---|
| Preoperative | CNS | 3 | 6.8 |
| MRSE | 2 | 4.5 | |
| MSSA | 3 | 6.8 | |
| MRSA | 6 | 13.6 | |
| Propionibacterium acnes | 1 | 2.3 | |
| No isolates | 29 | 65.9 | |
| Intraoperative | CNS | 13 | 29.5 |
| MRSE | 2 | 4.5 | |
| MSSA | 9 | 20.5 | |
| MRSA | 7 | 15.9 | |
| Propionibacterium acnes | 6 | 13.6 | |
| Corynebacterium spp. | 2 | 4.5 | |
| Escherichia coli | 1 | 2.3 | |
| Enterococcus spp. | 2 | 4.5 | |
| Pseudomonas aeruginosa | 3 | 6.8 | |
| Peptostreptococcus magnus | 1 | 2.3 | |
| No isolates | 10 | 22.7 |
CNS coagulase-negative staphylococci, MRSE methicillin-resistant Staphylococcus epidermidis, MSSA methicillin-sensitive S. ureus, MRSA methicillin-resistant S. aureus
Preoperative hospital stay ranged from zero to 27 days (mean 4.9 + - 5.8 days), whereas postoperatively, the average hospital stay was 26.1 + - 25.4 (range nine to 110 days). As to the type of treatment; debridement was performed in five patients, and in three of them, mobile parts of the prosthesis were exchanged also; six more patients underwent resection arthroplasty and 15 received an antibiotic-loaded cement spacer as a definitive treatment. In these patients, the spacers were custom-made in 11 cases and preformed in the remaining four. No one-stage shoulder revision was reported, whereas 17 patients (38.6%) underwent a two-stage procedure, in the majority of cases with a reverse prosthesis (Table 4). In one patient, an arthrodesis was performed after failed previous multiple operations. Complications after surgery were observed in seven cases (15.9%). Two patients suffered a humeral fracture during surgery, and five (11.4%) experienced instability/dislocation of the implant.
Table 4.
Postoperative data from reviewed patients after surgery for infected shoulder (N = 44)
| Characteristics | Raw numbers | Percent | Mean | Standard deviation | Maximum | Minimum | |
|---|---|---|---|---|---|---|---|
| Length of hospital stay (days) | Before surgery | 4.9 | 5.8 | 27 | 0 | ||
| After surgery | 26.1 | 25.4 | 110 | 9 | |||
| Type of treatment | Debridement and retention (mobile parts changed) | 5 (3) | 11.4 (6.8) | ||||
| Resection arthroplasty | 6 | 13.6 | |||||
| Permanent spacer | 15 | 34.1 | |||||
| 1-stage exchange | - | - | |||||
| 2-stage exchange | 17 | 38.6 | |||||
| Arthrodesis | 1 | 2.3 | |||||
| Type of prosthesis | Reverse prosthesis | 13 | 76.5 | ||||
| Hemiarthroplasty | 3 | 17.6 | |||||
| Total standard | 1 | 5.9 | |||||
| Antibiotic therapy | Duration < 6 weeks | 19 | 44.2 | ||||
| Duration > 6 weeks | 24 | 55.8 | |||||
| 1 antibiotic | 4 | 9.3 | |||||
| 2 antibiotics | 17 | 39.5 | |||||
| 3 antibiotics | 14 | 32.6 | |||||
| 4 antibiotics | 8 | 18.6 |
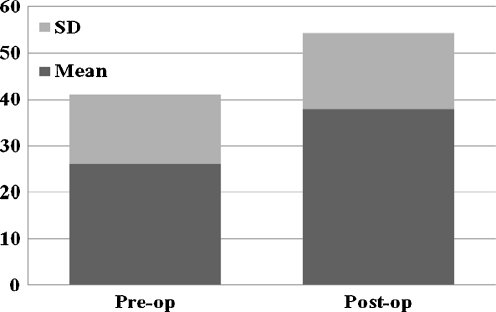
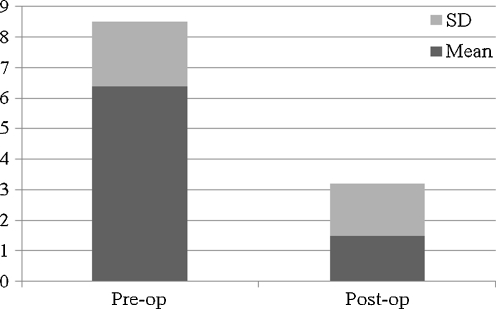
As to antibiotic therapy, treatment duration was around six weeks in 19 patients (44.2%), whereas 24 patients received antibiotics for up to five months. A single antibiotic was rarely administered, and an association and/or subsequent administration of multiple drugs was most commonly preferred, with two to four different antibiotics employed in the same patient (Table 4). At a mean follow-up of 41.1 + - 17.9 (range 24–98) months, 42 of 44 patients (95.5%) showed no signs of infection recurrence/persistence. In particular, an 80% cure rate after debridement (4/5), 93.3% after permanent antibiotic-loaded spacer implant (14/15) and 100% after resection arthroplasty (6/6) and after two-stage revision (17/17) was observed; one more successful treatment with shoulder arthrodesis was reported. Both functional and pain scores improved significantly. The overall mean VAS score decreased from 6.4 + - 2.1 preoperatively to 1.5 + - 1.7 after infection treatment (P = 0.0001, paired t test) (Fig. 1); the mean calculated Constant score increased from 26 + - 15 preoperatively to 38.3 + - 16.3 postoperatively (P = 0.007, paired t test) (Fig. 2), with an active abduction and external rotation recorded at follow-up of, respectively, 52.7° + - 25.2° and 12° + - 10.8°. Resection arthroplasty was associated with the poorest functional outcome (Table 5). Notwithstanding the relatively poor final functional outcome, the self-reported global patient satisfaction, scored according to Neer, yielded 14 patients who were very satisfied (31.8%) and 21 satisfied (47.7) with their final result.
Fig. 1.
Overall pre- and postoperative Constant score in the retrospective series of the four participating centres (N = 44)
Fig. 2.
Overall pre- and postoperative visual analogue scale (VAS) score in the retrospective series of the four participating centres (N = 44)
Table 5.
Literature review and comparison of different treatments for peri-prosthetic shoulder infections
| Author/year | Number of patients | Total number of infection free | Percent of infection free | Mean follow-up (years) | Constant score | Neer excellent | Neer satisfied | Neer unsatisfied | Abduction | External rotation |
|---|---|---|---|---|---|---|---|---|---|---|
| ANTIBIOTIC ONLY | ||||||||||
| Coste et al. 2004 [3] | 5 | 2 | 40.0 | 2.8 | 49 | |||||
| OTHER TREATMENTS | ||||||||||
| Coste et al. 2004 [3] | 3 | 2 | 66.7 | 2.8 | 45 | |||||
| This study (arthrodesis) | 1 | 1 | 100.0 | 3.5 | ||||||
| DEBRIDEMENT | ||||||||||
| Coste et al. 2004 [3] | 8 | 7 | 87.5 | 2.8 | 27 | |||||
| Jerosch and Schneppenheim 2003 [6] | 2 | 2 | 100.0 | |||||||
| Sperling et al. 2001 [14] | 6 | 3 | 50.0 | 1 | 2 | |||||
| Weber et al. 2011 [19] | 1 | 1 | 100.0 | 4 | 61 | 1 | 90 | |||
| This study | 5 | 4 | 80.0 | 3.6 | 43 | 3 | 2 | 1 | 74 | 17 |
| RESECTION ARTHROPLASTY | ||||||||||
| Braman et al. 2006 [1] | 7 | 7 | 100.0 | 1.7 | 7 | 28 | 8 | |||
| Coste et al. 2004 [3] | 10 | 7 | 70.0 | 2.8 | 30 | |||||
| Rispoli et al. 2007 [25] | 13 | 13 | 100.0 | 8.3 | 2 | 16 | 70 | 31 | ||
| Sperling et al. 2001 [14] | 21 | 15 | 71.4 | 70 | 31 | |||||
| Weber et al. 2011 [19] | 5 | 5 | 100.0 | 4 | 33 | 31 | ||||
| This study | 6 | 6 | 100.0 | 3.5 | 32 | 1 | 3 | 2 | 33 | 8 |
| PERMANENT SPACE | ||||||||||
| Coffey et al. 2010 [12] | 4 | 4 | 100.0 | 1.8 | 57 | 20 | ||||
| Coste et al. 2004 [3] | 3 | 3 | 100.0 | 2.8 | 38 | |||||
| Jerosch and Schneppenheim 2003 [6] | 2 | 2 | 100.0 | |||||||
| Themistocleous et al. 2008 [11] | 4 | 4 | 100.0 | 75 | 25 | |||||
| This study | 15 | 14 | 93.3 | 3 | 34 | 5 | 7 | 3 | 51 | 13 |
| 1-STAGE | ||||||||||
| Coste et al. 2004 [3] | 3 | 3 | 100.0 | 2.8 | 66 | |||||
| Cuff et al. 2008 [12] | 7 | 7 | 100.0 | 75 | 25 | |||||
| Ince et al. 2005 [10] | 9 | 9 | 100.0 | 5.7 | 33 | |||||
| Sperling et al. 2001 [14] | 2 | 1 | 50.0 | |||||||
| 2-STAGE | ||||||||||
| Coffey et al. 2010 [12] | 12 | 12 | 100.0 | 1.8 | 57 | 20 | ||||
| Coste et al. 2004 [3] | 10 | 6 | 60.0 | 2.8 | 35 | |||||
| Cuff et al. 2008 [4] | 10 | 10 | 100.0 | 75 | 25 | |||||
| Jerosch and Schneppenheim 2003 [6] | 8 | 8 | 100.0 | |||||||
| Mileti et al. 2004 [8] | 4 | 4 | 100.0 | 7.4 | 2 | 2 | 80 | 50 | ||
| Seitz and Damacen 2002 [26] | 5 | 5 | 100.0 | 4.8 | 48 | 55 | ||||
| Sperling et al. 2001 [14] | 3 | 3 | 100.0 | 180 | 30 | |||||
| Strickland et al. 2008 [9] | 19 | 12 | 63.2 | 2 | 4 | 13 | 89 | 30 | ||
| Weber et al. 2011 [19] | 4 | 4 | 100.0 | 4 | 40 | 2 | 2 | 62 | ||
| This study | 17 | 17 | 100.0 | 3.8 | 38 | 5 | 9 | 3 | 55 | 12 |
Discussion
Periprosthetic shoulder infection is a serious complication. Although different treatment options have been reported, a consensus about the best therapeutic strategy is still lacking [19]. To our knowledge, this is the largest continuous series of infected shoulder prosthesis ever reported. Also for the first time, our study provides an insight into treatment strategies followed during the last decade in four different European centres dedicated to the treatment of bone and joint infections. As regards the clinical presentation of septic complications in shoulder prosthesis, our data reveal that pain is the most frequently reported symptom, whereas other clinical signs of infection may be lacking and preoperative microbiological examination may often be negative. This finding is not surprising and has been previously described in periprosthetic infections after hip and knee replacement [20, 21]. On the other hand, intraoperative microbiology examination, even if performed without ultrasound, nearly doubled the chances of isolating the causative micro-organism; this finding is different from that reported by Weber et al. [19], which showed a 100% positive intraoperative cultural examination, but is consistent with many other studies, where only a variable percentage of all intraoperative swabs showed positive growth [3, 4, 6, 9, 10]. Our organism spectrum was similar to that reported in most other studies, with Staphylococcus and Propionibacterium spp. being the most commonly isolated bacteria, both pre- and intraoperatively. In particular, Propionibacterium is an agent frequently encountered in infected shoulder arthroplasties but only rarely seen in knee or hip infections [9, 10, 19, 22, 23].
Pre- and postoperative hospital length of stay was assessed for the first time in this study and was extremely variable. This parameter could be useful for further analysis to establish direct costs of treating septic shoulder prosthesis, similar to that done for the septic hip revision surgery [24]. As regards clinical results, our data show a relatively high overall average infection eradication rate; in particular, permanent spacer implant, resection arthroplasty and two-stage revision showed the highest success, whereas debridement yielded less satisfactory results, as previously reported by others (Table 5). The overall high cure rate in our series and the significant improvement in pain may explain the relatively high patient satisfaction observed in this series compared with that previously reported by other authors, despite the relatively poor final functional score.
Limitation of this retrospective study include:
Patient selection bias: It is possible that candidates to different treatments were different in the various centres. The relative proportion of type A, B or C hosts is not considered, and this has been shown to influence the final result [27–30].
Postoperative treatment: Type and duration of antibiotic therapy may influence the eradication rate of infection observed in different surgical treatments.
Spacer implants were all included as a same procedure: However, different types of spacers exist, and this may vary final results of both infection eradication and final functional output.
There is a lack of data on one-stage procedures: This treatment was not performed in any of the centres.
The low number of patients: Even if the one reported in Table 5 is, to our knowledge, the most comprehensive review of available data, including those presented from our four centres, when dividing the results according to the different procedures, the absolute numbers become relatively small and not homogenous for the different procedures, thus introducing a further bias.
In conclusion, our results favour a permanent spacer implant and two-stage revision as the best treatment to eradicate periprosthetic shoulder infection and positively influence functional outcome. However, limitations and bias still warrant the opportunity for prospective, randomised multicentre studies to better confirm the relative efficacy of the different surgical treatment modalities.
Acknowledgments
Competing interests
None of the authors has competing interests concerning the reported study.
Contributor Information
Carlo Luca Romanò, Phone: +39-02-66214907, FAX: +39-02-781657, Email: carlo.romano@grupposandonato.it.
Olivier Borens, Email: Olivier.Borens@chuv.ch.
Lorenzo Monti, Email: lorenzomonti@hotmail.it.
Enzo Meani, Email: enzo.meani@gpini.it.
Jose Stuyck, Email: jose.stuyck@uzleuven.be.
References
- 1.Braman JP, Sprague M, Bishop J, Lo IK, Lee EW, Flatow EL. The outcome of resection shoulder arthroplasty for recalcitrant shoulder infections. J Should Elbow Surg. 2006;15:549–553. doi: 10.1016/j.jse.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Cheung EV, Sperling JW, Cofield RH. Infection associated with hematoma formation after shoulder arthroplasty. Clin Orthop Relat Res. 2008;466:1363–1367. doi: 10.1007/s11999-008-0226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coste JS, Reig S, Trojani C, Berg M, Walch G, Boileau P. The management of infection in arthroplasty of the shoulder. J Bone Joint Surg. 2004;86-B:65–69. [PubMed] [Google Scholar]
- 4.Cuff DJ, Virani NA, Levy J, Frankle MA, Derasari A, Hines B, Pupello DR, Cancio M, Mighell M. The treatment of deep shoulder infection and glenohumeral instability with debridement, reverse shoulder arthroplasty and post-operative antibiotics. J Bone Joint Surg. 2007;90-B:336–342. doi: 10.1302/0301-620X.90B3.19408. [DOI] [PubMed] [Google Scholar]
- 5.Duncan SFM, Sperling JW. Treatment of primary isolated shoulder sepsis in the adult patient. Clin Orthop Relat Res. 2008;466:1392–1396. doi: 10.1007/s11999-008-0213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jerosch J, Schneppenheim M. Management of infected shoulder replacement. Arch Orthop Trauma Surg. 2003;123:209–214. doi: 10.1007/s00402-003-0497-9. [DOI] [PubMed] [Google Scholar]
- 7.Proubasta IR, Itarte JP, Lamas CG, Escribá IU. Permanent articulated antibiotic-impregnated cement spacer in septic shoulder arthroplasty: a case report. J Orthop Trauma. 2005;19:666–668. doi: 10.1097/01.bot.0000153448.80988.dc. [DOI] [PubMed] [Google Scholar]
- 8.Mileti J, Sperling JW, Cofield RH. Reimplantation of a shoulder arthroplasty after a previous infected arthroplasty. J Should Elbow Surg. 2004;13:528–531. doi: 10.1016/j.jse.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Strickland JP, Sperling JW, Cofield RH. The results of two-stage re-implantation for infected shoulder replacement. J Bone Joint Surg. 2008;90-B:460–465. doi: 10.1302/0301-620X.90B4.20002. [DOI] [PubMed] [Google Scholar]
- 10.Ince A, Seemann K, Frommelt L, Katzer A, Loehr JF. One-stage exchange shoulder arthroplasty for peri-prosthetic infection. J Bone Joint Surg. 2005;87-B:814–818. doi: 10.1302/0301-620X.87B6.15920. [DOI] [PubMed] [Google Scholar]
- 11.Themislocleous G, Zalavras C, Stine I, Zachos V, Itamura J. Prolonged implantation of an antibiotic cement spacer for management of shoulder sepsis in compromised patients. J Should Elbow Surg. 2007;16:701–705. doi: 10.1016/j.jse.2007.02.118. [DOI] [PubMed] [Google Scholar]
- 12.Coffey MJ, Ely EE, Crosby LA. Treatment of glenohumeral sepsis with a commercially produced antibiotic-impregnated cement spacer. J Shoulder Elbow Surg. 2010;19(6):868–873. doi: 10.1016/j.jse.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Verhelst L, Stuyck J, Bellemans J, Debeer P. Resection arthroplasty of the shoulder as a salvage procedure for deep shoulder infection: does the use of a cement spacer improve outcome? J Shoulder Elbow Surg. 2011;20(8):1224–1233. doi: 10.1016/j.jse.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop. 2001;382:206–216. doi: 10.1097/00003086-200101000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg. 1999;81-A:672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. J Bone Joint Surg. 2004;86-B:65–69. [Google Scholar]
- 17.Katolik LI, Romeo AA, Cole BJ, Verma NN, Hayden JK, Bach BR. Normalization of the Constant score. J Shoulder Elbow Surg. 2005;14:279–285. doi: 10.1016/j.jse.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Neer CS., 2nd Displaced proximal humeral fractures. II. Treatment of three-part and four-part displacement. J Bone Joint Surg Am. 1970;52(6):1090–1103. [PubMed] [Google Scholar]
- 19.Weber P, Utzschneider S, Sadoghi P, Andress HJ, Jansson V, Müller PE. Management of the infected shoulder prosthesis: a retrospective analysis and review of the literature. Intern Orthop. 2011;35:365–373. doi: 10.1007/s00264-010-1019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrack RL, Jennings RW, Wolfe MW, Bertot AJ. The Coventry Award. The value of preoperative aspiration before total knee revision. Clin Orthop Relat Res. 1997;345:8–16. doi: 10.1097/00003086-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Romanò CL, Romanò D, Bonora C, Degrate A, Mineo G. Combined Diagnostic Tool” for joint prosthesis infection. Infez Med. 2009;17(3):141–150. [PubMed] [Google Scholar]
- 22.Wirth MA, Rockwood CA., Jr Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603–616. doi: 10.2106/00004623-199604000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2005;55(2):119–124. doi: 10.1016/j.jinf.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Romanò CL, Romanò D, Logoluso N, Meani E. Septic versus aseptic hip revision: how different ? J Orthop Traumatol. 2010;11(3):167–174. doi: 10.1007/s10195-010-0106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rispoli DM, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Pain relief and functional results after resection arthroplasty of the shoulder. J Bone Joint Surg Br. 2007;89(9):1184–1187. doi: 10.1302/0301-620X.89B9.19464. [DOI] [PubMed] [Google Scholar]
- 26.Seitz WH, Jr, Damacen H. Staged exchange arthroplasty for shoulder sepsis. J Arthroplasty. 2002;17(4 Suppl 1):36–40. doi: 10.1054/arth.2002.32445. [DOI] [PubMed] [Google Scholar]
- 27.Rolf O, Stehle J, Gohlke F. Treatment of septic arthritis of the shoulder and periprosthetic shoulder infections. Special problems in rheumatoid arthritis. Orthopade. 2007;36(8):700–707. doi: 10.1007/s00132-007-1116-1. [DOI] [PubMed] [Google Scholar]
- 28.Cierny G, 3rd, DiPasquale D. Periprosthetic total joint infections. Staging, treatment, and outcomes. Clin Orthop Relat Res. 2002;403:23–28. doi: 10.1097/00003086-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 29.McPherson EJ, Woodson C, Holtom P, Roidis N, Shufelt C, Patzakis M. Periprosthetic total hip infection. Outcomes using a staging system. Clin Orthop Relat Res. 2002;403:8–15. doi: 10.1097/00003086-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Romanò CL, Logoluso N, F. Dell’Oro F, Elia A, Drago L (2011) Bone and joint infections in adults: a comprehensive classification proposal. European Orthopaedics and Traumatology 1/6: 207–217 [DOI] [PMC free article] [PubMed]