Abstract
Purpose
The optimal reconstructive method after resection of malignant bone tumours of the proximal ulna is unknown. We report the outcome of endoprosthetic replacement in a young patient population.
Methods
This was a retrospective review of four patients [three males and one female; mean age 17.5 (range 11–31) years] who underwent limb salvage with a proximal ulnar endoprosthetic replacement following excision of malignant bone tumour. Mean follow-up was 85 (range 14–194) months.
Results
All patients were alive at final follow-up and reported an improvement in pain. One patient required transhumeral amputation for intralesional excision complicating a local recurrence at one month. Two patients developed fixed flexion deformities of the elbow, one of whom required radial-head excision. Mean Musculoskeletal Tumour Society (MSTS) score and Toronto Extremity Salvage Score (TESS) were 27 (range 25–28) and 81 (73–88), respectively.
Conclusions
Custom-made proximal ulna endoprosthetic replacement following resection of malignant bone tumours in young patients provides a stable reconstruction option with satisfactory function and without apparent compromise in patient survival.
Keywords: Medicine & Public Health, Orthopedics
Introduction
Primary bone tumours around the elbow represent <1% of all skeletal tumours [1]. The commonest malignant tumour is lymphoma and the commonest benign tumour is osteoid osteoma [1]. Advances in chemotherapy and radiotherapy enable limb salvage to replace amputation as the primary treatment, as although there is a higher rate of local recurrence, patient survival is not compromised [2, 3]. Limb salvage for the lower limb is more cost effective than amputation and results in better physical functioning [4, 5]; however, this has not been proven for the upper limb.
Reconstructive options to address segmental proximal ulna resections include autografts [6, 7], allografts [8, 9] and endoprostheses [10, 11]. Arthrodesis and excision arthroplasty may be used for small bone defects; however, they are not suitable for large defects and may result in poor function and instability, respectively [11]. Autografts are useful for short-segment reconstruction, but graft availability, donor-site morbidity and difficulty in matching the size and shape of the graft to the defect limit their use [12]. Allografts allow accurate matching of graft size to defect, ligament reconstruction and bone formation at the graft–host junction. Complications include instability, fracture, nonunion and infection [9, 13–17]. It has been proposed that their major use is for reconstitution of bone stock to facilitate an arthrodesis or arthroplasty when further reconstruction is likely [9]. Endoprostheses have the advantage of allowing early mobilisation with shorter operative times, do not pose a risk with disease transmission and allow immediate commencement of adjuvant chemotherapy, avoiding the adverse effect on bone healing [18]. Disadvantages include infection, wear, loosening and breakage [10].
There are few studies assessing the functional and oncological outcome, patient survival and prosthesis survivorship for proximal ulna endoprosthetic replacement following bone tumour resections [19–21]. The aim of this case series was to evaluate these outcome measures following custom-made proximal ulna endoprosthetic reconstruction after primary excision of bone tumours in young patients.
Methods and materials
Between October 1994 and October 2009, four patients with tumours of the proximal ulna were treated by segmental excision and endoprosthetic reconstruction with a custom-made proximal ulna replacement. Data were collected from case notes, hospital databases, clinic reviews, imaging studies and functional questionnaires. There were three male and one female patient, with a mean age of 17.5 (range 11–31) years and mean follow-up of 85 (range 14–194) months (Table 1). All patients had been referred to a regional bone tumour unit for multidisciplinary team assessment and underwent pre-operative staging that included plain radiographs, magnetic resonance imaging (MRI) of the limb, technetium (TC99) body scintigraphy and chest computed tomography (CT). Proximal ulna endoprosthetic replacement was not considered in the presence of neurovascular invasion or when tumour resection would leave inadequate muscle to allow function. Isolated neurological involvement was not a contraindication. Neoadjuvant and adjuvant chemotherapy and radiotherapy were administered according to nationally agreed-upon protocols.
Table 1.
Demographics and outcome of the four patients included in the study
| Patient number | Gender | Age (years) | Diagnosis | Previous surgery | Radiotherapy | Chemotherapy | Preop metastases | Patient survival | Complications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 11 | Ewing’s sarcoma | Radical debridement for suspected chronic osteomyelitis | Y | Y | N | Alive | Radial-head excision |
| 15° FFD | |||||||||
| 2 | M | 31 | Osteosarcoma | Needle biopsy | N | Y | N | Alive | |
| 3 | F | 15 | Desmoplastic fibroma | Open biopsy | N | N | N | Alive | 10° FFD |
| 4 | M | 13 | High-grade spindle-cell sarcoma | Recurrence following previous incomplete soft tissue excision | Y | Y | N | Alive | Intralesional excision; transhumeral amputation |
Y yes, N no, FFD Fixed flexion deformity
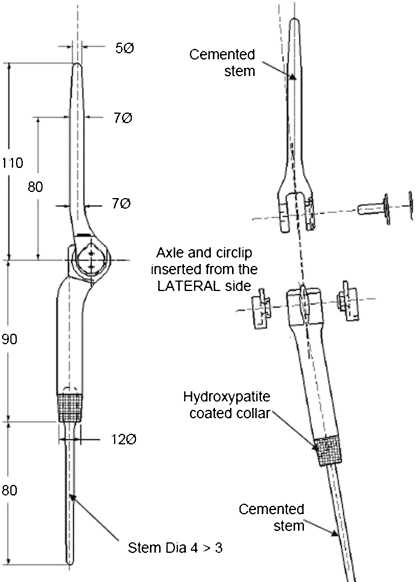
The custom-made prosthesis is a linked, fixed-hinge implant made of titanium alloy (Ti 6Al 4 V) and manufactured using computer-aided design and manufacturing technologies (CAD-CAM) (Stanmore Implants Worldwide Ltd, Stanmore, UK). The humeral and ulna components are connected by a metal pin, which passes through two high-density polyethylene bushes and is fastened with a C-clip (Fig. 1). Each component has an intramedullary stem, which is cemented into the corresponding canal. Both stems are fluted to provide rotational stability, and they contain a hydroxyapatite (HA) collar to allow for osseointegration at the bone–prosthesis junction.
Fig. 1.
a Pre-operative computer-aided designed and computer-aided manufactured (CAD-CAM) proximal ulna replacement for the patient in Fig. 2, demonstrating the sites for bone cuts. The two components are cemented into the bone canals and connected using a pin and bushings
A standard posterior approach taking a skin ellipse with biopsy track was used to expose the tumour. The ulnar and posterior interosseus nerves were indentified and protected. The annular ligament and radial neck may need dividing for better access. Bony transection points on the ulna, as identified on pre-operative MRI, are marked. Tumour resection is carried out according to the principles defined by Enneking et al. [22], endeavouring to achieve en bloc excision with a surrounding cuff of normal tissue without violating the tumour. Proximal and distal tissue samples are taken and the specimen sent to histology. The ulna is prepared with sequential reamers and a notch fashioned in the distal humerus with the aim of part of the trochlear and capitellum. The humerus is prepared and components are trialled. The components are cemented separately and articulated using the pin and bushings. When preserved, a triceps muscle flap is used to cover the prosthesis. The triceps tendon may be reconstructed either by suturing the tendon to the antebrachial fascia or else suturing it to any remaining bone or fascia that remains around the elbow following tumour resection. Postoperative intravenous antibiotics are given for three days. A humeral brace is used for three weeks and physiotherapy commenced day one. Patients were followed up at three monthly intervals for the first two years, then five month intervals for five years and annually thereafter (Fig. 2). Patients were functionally assessed using the Musculoskeletal Tumour Society (MSTS) scoring system [23] and Toronto Extremity Salvage Score (TESS) [24].
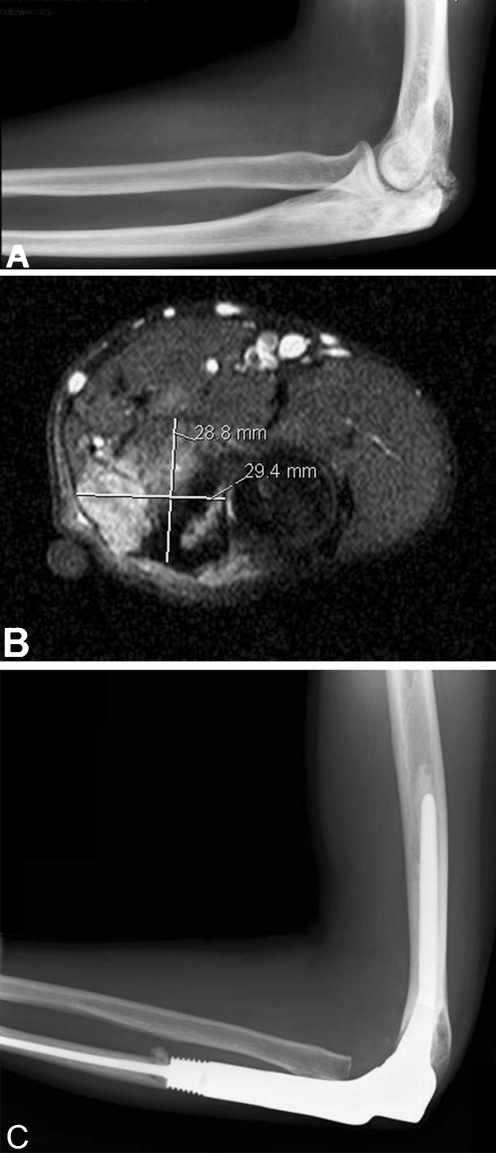
Fig. 2.
a Lateral left elbow radiograph (a) showing dense patchy sclerosis involving the olecranon, with reactive new bone that is in close proximity to the distal humerus. Axial T2-weighted magnetic resonance imaging (MRI) (b) demonstrates the lesion to be displacing but not invading the neurovascular bundle. MRI showed the tumour did not involve the distal humerus. Seven month postoperative lateral radiograph (c) following excision of high-grade osteosarcoma and proximal ulna endoprosthetic replacement
Results
Case 1
An 11-year-old boy presented with systemic disturbance and a painful swollen left elbow. Inflammatory markers were raised and radiographs showed a proximal ulna lytic lesion. The patient underwent drilling of the ulna and debridement. A single microbiology specimen grew coagulase-negative Staphylococcus aureus. The child was treated with six weeks of antibiotics but failed to improve. Two further debridements for suspected chronic osteomyelitis were performed. Histology from the third debridement eight months following initial presentation revealed Ewing’s sarcoma. Staging investigations revealed no metastases. Neoadjuvant chemotherapy was administered, and the child underwent radical excision and limb salvage with proximal-ulna endoprosthetic replacement 14 months following initial presentation. Adjuvant chemotherapy and radiotherapy were administered. Six months following surgery, radial-head excision was required for a 20° fixed flexion deformity (FFD) with restricted supination. This restored full range of movement, but the FFD recurred. The child was alive 81 months following surgery with no metastases, local recurrence or pain, a TESS of 82, MSTS score of 27 and range of movement flexion 15°– 125° and lacking 5° of pronation and supination.
Case 2
A 31-year-old man with Rothmund–Thomson syndrome presented with pain and reduced movement in the elbow. Radiographs showed olecranon sclerosis with reactive new bone that was in close proximity to the distal humerus (Fig. 2). Needle biopsy revealed high grade osteoblastic osteosarcoma. MRI demonstrated the tumour did not involve the distal humerus. Staging investigations showed no metastases. The patient received neoadjuvant and adjuvant chemotherapy and underwent wide local excision and proximal ulnar endoprosthetic replacement with a epicondylar sacrificing prosthesis. The tumour was chemosensitive, and histology demonstrated good tumour necrosis response. The patient was alive 14 months following surgery, with no metastases, local recurrence or pain, a TESS of 88, MSTS score of 28 and range of movement flexion 0°–110°, lacking 10° of terminal pronation and supination.
Case 3
A 15-year-old girl presented with a painful bony lump and restricted movement around the elbow. Open biopsy revealed a desmoplastic fibroma with low-grade malignant features. The patient underwent wide local excision and proximal ulna endoprosthetic replacement. She was alive 194 months after surgery, with a painless functioning elbow, TESS of 73, MSTS score of 25 and range of movement flexion 10°–85°, with full pronation and supination.
Case 4
A 13-year-old boy presented with a painful swelling in the elbow. Needle biopsy diagnosed high-grade spindle-cell sarcoma. The child received neoadjuvant chemotherapy and underwent excision of the lesion, which included the biopsy tract and ulna periosteum but not the segmental proximal ulna. Adjuvant chemotherapy and radiotherapy were administered. A repeat MRI was performed at 20 months for pain, which showed signal change in the proximal ulna. Needle biopsy confirmed local recurrence, and the patient underwent segmental proximal ulna excision and endoprosthetic replacement. The postoperative margins showed an intralesional excision, and with high-grade spindle-cell sarcoma confirmed on histology, the patient underwent transhumeral amputation one month following proximal ulna replacement. The patient was with no metastases or local recurrence 52 months after primary surgery.
Discussion
Primary elbow sarcomas account for <1% of all bone tumours [1]. Limb salvage following proximal ulnar tumour resection poses a complex reconstructive challenge. Options include excision arthroplasty, arthrodesis, resection–replantation [25], autografts [6, 7], allografts [9] and endoprostheses. Arthrodesis and excision arthroplasty are not suitable for large defects and may result in poor function and instability. Resection–replantation, which involves cylindrical-segment resection of the tumour and replantation of the distal arm with shortening, allows wide surgical margins to be achieved but at the expense of function and cosmesis [25]. Kimura et al. [6] report good function and graft incorporation at four years in one patient who underwent resection of Ewing’s sarcoma of the proximal ulna and reconstruction with a vascularised fibula graft. Vascularised fibula autografts have the potential to remodel and hypertrophy under mechanical load; however, they do not allow early weight bearing, they result in frequent complications and donor-site morbidity and are not considered suitable for large defects [26, 27]. Allografts provide an alternative biological means of reconstruction; however, instability, fracture, nonunion and infection complicate their use, and the overall complication rate is high (70%) [9]. Allograft prosthetic composites may reduce these complications and help re-establish bone stock.
Endoprostheses generally provide improved functional outcome and enable immediate commencement of adjuvant chemotherapy. The outcome of endoprosthetic reconstruction for proximal ulna tumour resections is largely unknown; however, endoprosthetic reconstruction following distal humerus tumour resection has produced good functional and oncological results. Hanna et al. [10] reported 18 patients who had a distal humeral endoprosthetic replacement following malignant bone tumour resection. At a mean follow-up of 4.4 years, they reported complications in nine patients (50%), including local recurrence in two (11%), infection in two (11%), nerve injury in one (5.5%) and periprosthetic fracture in one (5.5%). In our review, we observed no incidences of nerve injury or infection; however, periprosthetic fracture remains an inherent problem with endoprosthetic reconstruction. The main difference between a distal humeral and proximal ulna endoprosthetic reconstruction is the integrity of the triceps mechanism in the former, which should provide superior function.
We used a fixed-hinge linked prosthesis and had no episodes of instability. Malignant tumour resection requires large soft tissue resection, necessitating use of a linked as opposed to unlinked prosthesis. A stable elbow joint is required for good function and may be one explanation for the good function we observed in the patients in this study. Our observed functional outcome was better than that for distal humeral replacements performed for tumour [10] but not as good as that for total elbow arthroplasty performed for nonneoplastic conditions [28]. Although aseptic loosening is more common in linked prostheses [28], we did not observe this complication, but our follow-up was limited. Fixed flexion deformity following arthroplasty is common and is difficult to treat but, unless severe, rarely affects function and is not an indication for revision [28].
There was no apparent compromise in patient survival following this procedure. All patients were alive at follow-up, and there were no cases of postoperative metastases. Delays in diagnosis, metastases, size, grade, location of primary tumour and response to chemotherapy are the most important factors affecting survival [29]. The one patient who developed local recurrence had intralesional excision of a spindle-cell sarcoma 25 months prior to endoprosthetic replacement. Factors associated with increased recurrence risk include resection margin, poor response to chemotherapy, intravascular tumour extension and pathological fracture [30]. The adverse prognostic factors in this patient, in addition to histological grade progression, necessitated transhumeral amputation to ensure disease control.
There are limitations to this case series. It is a retrospective design, with small patient numbers, a long study period and variable length of follow-up. Interviewer and measurement bias may affect functional outcome and survivorship analyses. Functional outcome scores may be confounded by lifestyle factors, such as the ability to get a job, family circumstances and effects of chemotherapy.
Benign tumours of the proximal ulna can be generally managed by curettage with bone grafting or cementoma [31]; however, malignant tumours require radical or wide excision with proximal ulna and elbow reconstruction [32]. The optimal reconstructive method is not known. Autografts are an attractive biological option in the younger patient, but there are few reports of their use [6], and concerns regarding subsequent limb growth and elbow stability remain problems. The complication rate following allograft reconstruction is high. There are few reports on the use of endoprosthetic reconstruction following proximal ulna tumour excision [19–21]. We have studied their use in a young patient population and conclude this method alleviates pain, provides good function, does not appear to compromise patient survival and provides a stable means of reconstruction.
Acknowledgements
The authors acknowledge the contribution made by Stanmore Implants Worldwide Ltd, and particularly by Dr. Paul Unwin. No competing interests declared
Contributor Information
Mathew D. Sewell, Email: matbuzz1@hotmail.com
Sammy A. Hanna, Email: sammyhanna@hotmail.com
References
- 1.Pritchard DJ, Dahlin DC (1985) Neoplasms of the elbow. In: Morrey BF, Sanchez-Sotelo J (eds) The elbow and its disorders. WB Saunders, Philadelphia, pp 713–735
- 2.Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb salvage treatment versus amputation for osteosarcoma of the distal end of the femur. JBJS [Am] 1986;68:1331–1337. [PubMed] [Google Scholar]
- 3.Rougraff BT, Simon MA, Kneisel JS, Greenberg DB, Mankin HJ. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur: a long-term oncological, functional, and quality-of-life-study. JBJS [Am] 1994;76:649–656. doi: 10.2106/00004623-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Grimer RJ, Carter SR, Pynsent PB. The cost effectiveness of limb salvage surgery for bone tumours. JBJS [Br] 1997;79:558–561. doi: 10.1302/0301-620X.79B4.7687. [DOI] [PubMed] [Google Scholar]
- 5.Aksnes LH, Bauer HCF, Jebsen NL, Folleras G, Allert C, Haugen GS, Hall KS. Limb-sparing surgery preserves more function than amputation. JBJS [Br] 2008;90:786–794. doi: 10.1302/0301-620X.90B6.19805. [DOI] [PubMed] [Google Scholar]
- 6.Kimura K, Tatezaki S, Ishii T, et al. Hemiarthroplasty of the elbow with a vascularised fibular graft after excision of Ewing’s Sarcoma of the proximal Ulna : a case report. Jpn J Clin Oncol. 2002;32:430–434. doi: 10.1093/jjco/hyf088. [DOI] [PubMed] [Google Scholar]
- 7.Gianoutsos MP, Marsden FW, McCarthy SW, Lee KK. Ulnar adamantinoma: en bloc excision and fibular osteoseptocutaneous free flap reconstruction. J Hand Surg [Am] 1994;19:495–499. doi: 10.1016/0363-5023(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 8.Urbaniak JR, Black KE. Cadaveric elbow allografts: A six-year experience (1985) Clin Orthop. 1985;197:131–140. [PubMed] [Google Scholar]
- 9.Dean GS, Holliger EH, Urbaniak JR. Elbow allograft for reconstruction of the elbow with massive bone loss: long term results. Clin Orthop. 1997;341:12–22. [PubMed] [Google Scholar]
- 10.Hanna SA, David LA, Aston WJS, et al. Endoprosthetic replacement of the distal humerus following resection of bone tumours. JBJS [Br] 2007;89:1498–1503. doi: 10.1302/0301-620X.89B11.19577. [DOI] [PubMed] [Google Scholar]
- 11.Sperling JW, Pritchard DJ, Morrey BF. Total elbow arthroplasty after resection of tumors at the elbow. Clin Orthop. 1999;367:256–261. [PubMed] [Google Scholar]
- 12.Chang DW, Weber KL. Use of a vascularized fibula bone flap and intercalary allograft for diaphyseal reconstruction after resection of primary extremity bone sarcomas. Plast Reconstr Surg. 2005;116:1918–1925. doi: 10.1097/01.prs.0000189203.38204.d5. [DOI] [PubMed] [Google Scholar]
- 13.Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop. 1996;324:86–97. doi: 10.1097/00003086-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Thompson RC, Garg A, Clohisy DR, Cheng EY. Fractures in large-segment allografts. Clin Orthop. 2000;370:227–235. doi: 10.1097/00003086-200001000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Capanna R, Campanacci DA, Belot N, Beltrami G, Manfrini M, Innocenti M, Ceruso M. A new reconstructive technique for intercalary defects of long bones: the association of massive allograft with vascularized fibular autograft. Long-term results and comparison with alternative techniques. Orthop Clin North Am. 2007;38:51–60. doi: 10.1016/j.ocl.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Donati D, Capanna R, Campanacci D, et al. The use of massive bone allografts for intercalary reconstruction and arthrodeses after tumour resection. Chir Organi Mov. 1993;78:81–94. [PubMed] [Google Scholar]
- 17.Gebhardt MC, Flugstad DI, Springfield DS, Mankin HJ. The use of bone allografts for limb salvage in high grade osteosarcoma. Clin Orthop. 1991;270:181–196. [PubMed] [Google Scholar]
- 18.Muscolo DL, Ayerza MA, Aponte-Tinao L, Ranalletta M, Abalo E. Intercalary femur and tibia segmental allografts provide an acceptable alternative in reconstructing tumor resections. Clin Orthop. 2004;426:97–102. doi: 10.1097/01.blo.0000141652.93178.10. [DOI] [PubMed] [Google Scholar]
- 19.Tang X, Guo W, Yang R, Tang S, Yang Y. Custom-made prosthesis replacement for reconstruction of the elbow after tumor resection. JSES. 2009;18:796–803. doi: 10.1016/j.jse.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Guo W, Tang S, Yang RL, Ji T. Total elbow arthroplasty after resection of tumors at the elbow. Zhonghua Wai Ke Za Zhi. 2008;46:1734–1737. [PubMed] [Google Scholar]
- 21.Weber KL, Lin PP, Yasko AW. Complex segmental elbow reconstruction after tumor resection. Clin Orthop. 2003;415:31–44. doi: 10.1097/01.blo.0000093894.12372.53. [DOI] [PubMed] [Google Scholar]
- 22.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop. 1980;153:106–120. [PubMed] [Google Scholar]
- 23.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop. 1993;286:241–246. [PubMed] [Google Scholar]
- 24.Davis AM, Wright JG, Williams JI, et al. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5:508–516. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 25.Windhager R, Millesi H, Kotz R. Resection-replantation for primary malignant tumours of the arm. An alternative to fore-quarter amputation. J Bone Joint Surg Br. 1995;77:176–184. [PubMed] [Google Scholar]
- 26.Chen CM, Disa JJ, Lee HY, Mehrara BJ, Hu QY, Nathan S, Boland P, Healey J, Cordeiro PG. Reconstruction of extremity long bone defects after sarcoma resection with vascularized fibula flaps: a 10-year review. Plast Reconstr Surg. 2007;119:915–924. doi: 10.1097/01.prs.0000252306.72483.9b. [DOI] [PubMed] [Google Scholar]
- 27.Zaretski A, Amir A, Meller I, et al. Free fibula long bone reconstruction in orthopedic oncology: a surgical algorithm for reconstructive options. Plast Reconstr Surg. 2004;113(7):1989–2000. doi: 10.1097/01.PRS.0000122213.82011.C5. [DOI] [PubMed] [Google Scholar]
- 28.Little CP, Graham AJ, Carr AJ. Total elbow arthroplasty. JBJS [Br] 2005;87:437–444. doi: 10.1302/0301-620X.87B4.15692. [DOI] [PubMed] [Google Scholar]
- 29.Skubitz KM, D’Adamo DR. Review article. Sarcoma. Mayo Clin Proc. 2007;82:1409–1432. doi: 10.4065/82.11.1409. [DOI] [PubMed] [Google Scholar]
- 30.Ward WG, Eckardt JJ, Dorey F, et al. Local recurrence following surgical treatment of 242 primary malignant bone tumors: an analysis of 39 cases. Orthop Trans. 1994;18:27. [Google Scholar]
- 31.Giffen N, Smet L. Osteoblastoma of the proximal ulna, an unusual cause of ulnar wrist pain. Acta Orthop Belg. 2005;71:736–739. [PubMed] [Google Scholar]
- 32.Rydholm A. Reconstruction after resection of the proximal ulna. Report of a case of chondrosarcoma. Acta Orthop Scand. 1987;58:671–672. doi: 10.3109/17453678709146513. [DOI] [PubMed] [Google Scholar]