Abstract
Purpose
The aim of this study was to compare bone marrow-derived mesenchymal stem cells (MSCs) with bone marrow nucleated cells (BNCs) as seed cells in the treatment of cartilage defects.
Methods
Twenty Guizhou minipigs were used to create full-thickness chondral defects of 6.0 mm in diameter in the knee joints and divided between two time points (four and eight weeks) for final assessment. At every time point, animals were separated into four groups: the CON group which underwent no implantation; the collagen type II hydrogel group (COL); the collagen type II hydrogel + bone marrow-derived MSCs group; and the collagen type II hydrogel + BNCs group. The samples were grossly examined, observed through a stereo microscope, histologically analysed and evaluated with the O’Driscoll scoring system, respectively.
Results
The cartilage repair of the two cell-treated groups was improved markedly compared to the CON and the COL groups, while the repair tissues of the two cell-treated groups showed no significant difference eight weeks after surgery.
Conclusions
These data indicate that BNCs contribute to the repair of cartilage with collagen type II hydrogel as scaffolds, which have comparable results with bone marrow-derived MSCs. Moreover, the transplantation of autologous BNCs as seed cells may be a more economical and convenient technique for cartilage repair in clinical applications.
Keywords: Medicine & Public Health, Orthopedics
Introduction
Adult articular cartilage has no blood circulation, lymphatic drainage or innervation, so chondral tissue has poor healing abilities. Although the full-thickness cartilage defect can heal spontaneously sometimes, repair tissue has many of the characteristics of fibrous tissue rather than hyaline cartilage and it will degenerate on physiological loading, which may lead to osteoarthritis [1]. Various surgical options have been proposed to restore cartilage defects, such as autologous chondrocyte transplantation [2, 3] and mosaicplasty transplantation, which have been demonstrated to be effective in enhancing cartilage repair over the past decade. With the development of tissue engineering, more approaches have been adopted [4]. Except for chondrocytes, adult MSCs from various tissues have been used for seed cells [5, 6] and they have many advantages, such as relatively abundant source, easy method of harvesting, no damage to the donor cartilage, strong capacity for proliferation and the potential to differentiate towards the chondrogenic phenotype.
However, there are many difficulties in the application. We can neither control the direction of MSC differentiation precisely nor find a way to obtain completely pure stem cells to date [7]. Recently, some researchers attempted to use uncultured bone marrow-derived nucleated cells to repair articular cartilage defects [8, 9], which proved to be effective. In view of this, it is necessary to compare the effect of BNCs with bone marrow-derived MSCs as seed cells in cartilage repair.
Many materials have been used as scaffolds to serve as temporary supports for cell growth and new tissue development [10]. Among them, hydrogels offer numerous attractive features for tissue engineering [11], including ease of handling where cells are simply mixed in a solution prior to gelation enabling a highly uniform cell seeding, a highly hydrated tissue-like environment and the ability to form in vivo. Many experiments have suggested that collagen type II alone has the potential to induce and maintain MSC chondrogenesis [12]. Using collagen type II hydrogel as a scaffold to encapsulate BNCs or bone marrow-derived MSCs in this study, we observed the results of cartilage repair in vivo with a large animal model of full-thickness articular cartilage defect. In addition, we tried to ascertain whether BNCs can be recommended as seed cells in the treatment of chondral defects in comparison to bone marrow-derived MSCs.
Materials and methods
Experimental design
Twenty Guizhou minipigs (Experimental Animal Centre of the Third Military Medical University, Chongqing, China) weighing 32–41 kg and aged ten to 12 months were used. After surgical intervention, animals were divided between two time points (four and eight weeks) for final assessment. At every time point, animals were separated into four groups: the CON group (n = 5 knees) underwent no implantation; the collagen type II hydrogel group (COL, n = 5 knees); the collagen type II hydrogel + bone marrow-derived MSCs group (BMSCs, n = 5 knees); and the collagen type II hydrogel + bone marrow nucleated cells group (BNCs, n = 5 knees). The experiments were approved by the Third Military Medical University Committee for Animal Experimentation.
MSC isolation and in vitro proliferation
The bone marrow was harvested from the spongy bone of the iliac crest in a separate procedure three weeks prior to surgery. Under sterile conditions, a 16 gauge marrow needle was used to insert 1.5 cm into the iliac crest, and a total of 15 ml bone marrow was aspirated into a 20-ml plastic syringe containing 0.5 ml of heparin (1,000 U/ml; Jiangsu Wanbang Biochemical Pharmaceutical Co., Ltd., China).
MSCs were isolated and cultured by a method previously described [13]. The cells of the second passages were harvested for seeding in collagen type II hydrogel (Engineering Research Center for Biomaterials, Sichuan University, China). The collagen type II hydrogel used in this study is a natural polymer derived from pig’s joint cartilages, which becomes a gel through physical cross-linking when the temperature rises to 37°C. This polymer was demonstrated to have good cell compatibility, and it is currently going through a patent application process.
Surgical procedure
All surgical procedures were performed by the same team, including two orthopaedic surgeons, one anaesthetist and one laboratory technician in charge of isolating cells and preparing the hydrogel-cell complex. For the BNCs group, the first step was bone marrow aspiration. About 15 ml of bone marrow was harvested by the above-mentioned method and transferred to the cell laboratory immediately. The BNCs were isolated by Percoll (d = 1.073 g/ml; Pharmacia) density gradient centrifugation; 200 μl cells was seeded in 1 ml collagen type II hydrogel to prepare the complex to a cell concentration of 105/ml at 4°C. The hydrogel + bone marrow-derived MSCs complex was prepared using the same concentration and methods.
At the same time, a chondral defect was created (Fig. 1a–c). A lateral parapatellar approach was used to expose the knee. A chondral defect of 6.0 mm in diameter was created in the medial area of the lateral femoral trochlea [14] with a special tube osteotome. The base of the defect was trimmed with a surgical curette. To prevent haemorrhage, the manipulation was carried out carefully in order not to penetrate the subchondral bone. The operating field was douched with physiological saline during the operation process to wash off the chondral debris and prevent dehydration of the cartilage.
Fig. 1.
a–f A chondral defect was created in the medial area of the lateral femoral trochlea (a–c). Hydrogel was injected into the defect and made to set (d–f)
Then a 1-ml plastic syringe was used to suck appropriate hydrogel (the COL, BMSCs or BNCs group), which was injected into the defect (Fig. 1d). The surface of the hydrogel was slightly lower than the surrounding cartilage. A lamp was used to heat the operating field (Fig. 1e); five to ten minutes later, the hydrogel set and adhered firmly to the defect (Fig. 1f). Finally, careful haemostasis, patellar reduction and a layered closure were performed to ensure a watertight seal, and the minipigs were allowed to move freely after the operation with adequate analgesia given. All pigs received ampicillin (Sigma) consecutively for five postoperative days.
Gross and histological analysis
Pigs were sacrificed by an overdose of pentobarbital sodium. During the necropsy, joint cavities were exposed via the original incisions. The defects, adjacent cartilage and synovial membranes were grossly examined. After resection of the distal femurs, osteochondral blocks containing the defects were fixed in 10% neutral buffered formalin for three days, after which the blocks were decalcified with formalin-nitric acid solution (40% formaldehyde 5 ml, concentrated nitric acid 10 ml and distilled water 85 ml) for at least three days. The blocks were cut into slices, 5-mm thick, with a sharp blade and were split perpendicularly from the midportion of the cartilage surface.
In the next stage, the surface and section of the defects were first observed through a stereo microscope (Chongqing Optec Instrument Co., Ltd., China). Secondly, the samples were further dehydrated, cleared and embedded in paraffin and cut into 5-μm thick sections. The sections were stained with Safranin-O/fast green (Sigma), toluidine blue (Sigma) and Sirius red (Sigma), respectively. Immunohistochemical analysis for collagen type II was not performed, as we were worried about the possibility that the collagen type II scaffold would affect the evaluation.
Histological scoring for repaired tissues
The sections in the middle third of the defects were evaluated with the O’Driscoll scoring system [15], which encompassed four major categories (‘nature of the predominant tissue’, ‘structural characteristics’, ‘freedom from cellular changes of degeneration’ and ‘freedom from degenerative changes in adjacent cartilage’) and the total score was 24.
Statistics
Statistical analysis was performed with SPSS 10.0 (SPSS Inc., Chicago, IL, USA). The analysis of variance and Mann-Whitney test were used for histologically grading the effect of treatment. We considered p values < 0.05 as significant.
Results
Postoperative conditions
Pigs were fully awakened six to eight hours after the operations. One week after the surgery, the animals’ gait had returned to normal. The wounds were healed two weeks after operation.
Four weeks
When the joint capsules were opened, a little bright synovial fluid (about 1 ml) spilled out, and the synovial membranes were mildly hyperaemic. Almost no regenerating tissue was observed in the CON group; the chondral defects were partly repaired with fibrous-like tissues in the COL group; glossy regenerating tissues were generally observed in the two cell-treated groups.
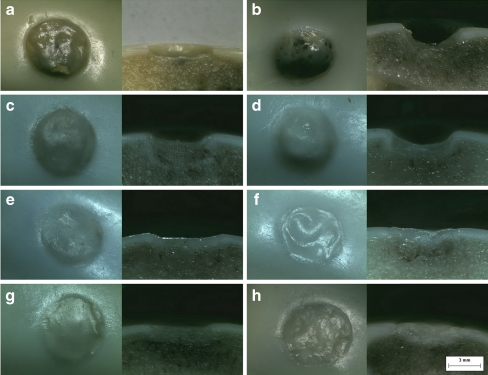
All specimens were observed through the stereo microscope. In the CON group (Fig. 2a), the chondral defects were rarely repaired. Moreover, the subchondral bones had partly subsided. In the COL group (Fig. 2c), the defects had a bowl shape, because various regenerating tissues appeared mainly around the defects while the subchondral bones had moderately subsided. Translucent regenerating tissues with smooth surfaces were widely observed in the two cell-treated groups (Fig. 2e, g). There were no gaps between the regenerating tissues and the circumjacent cartilage, but the interfaces could be identified easily. The subchondral bone showed slight subsidence.
Fig. 2.
a–h The surface and section of the defects observed through a stereo microscope. The chondral defects were rarely repaired and the subchondral bones had partly subsided 4 weeks after the surgery in the CON group (a). The defects had a bowl shape 4 weeks after surgery in the COL group, while the subchondral bones had moderately subsided (c). Repair of the defects showed no notable improvement either in the CON group (b) or the COL group (d) 8 weeks after surgery, and the subsidence of the subchondral bone became more serious. Translucent regenerating tissues were observed in the two cell-treated groups (e and g) 4 weeks after surgery. The subchondral bone showed slight subsidence; there were no gaps between the regenerating tissues and the circumjacent cartilages in the two cell-treated groups (f and h) 8 weeks after surgery, and the subsidence of the subchondral bone was not aggravated (original magnification, ×40)
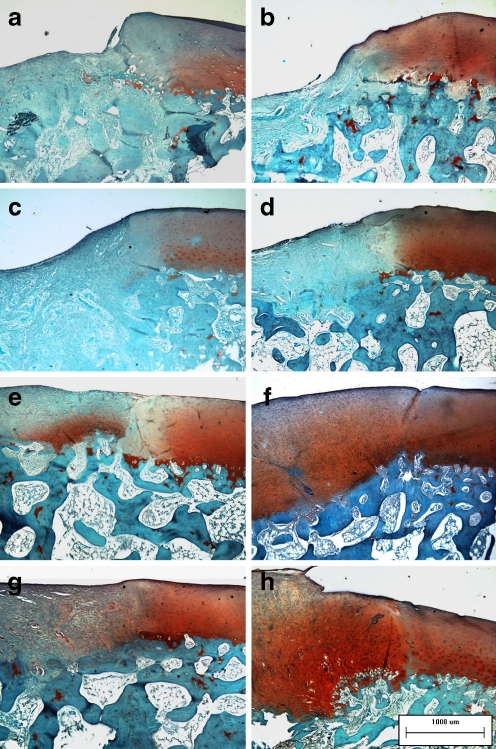
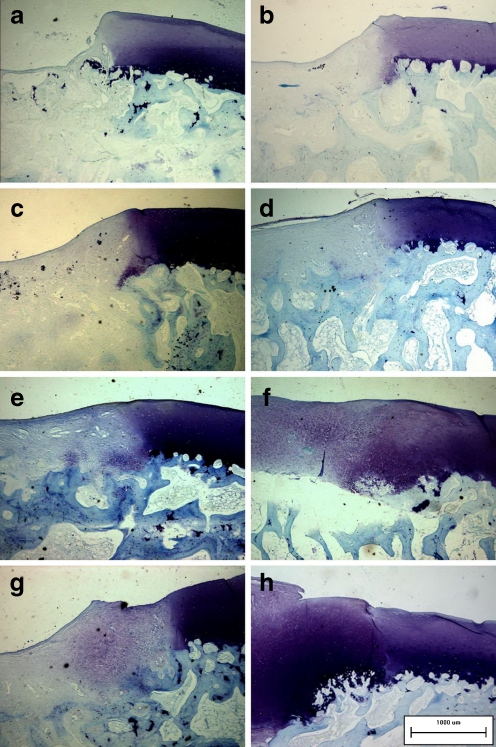
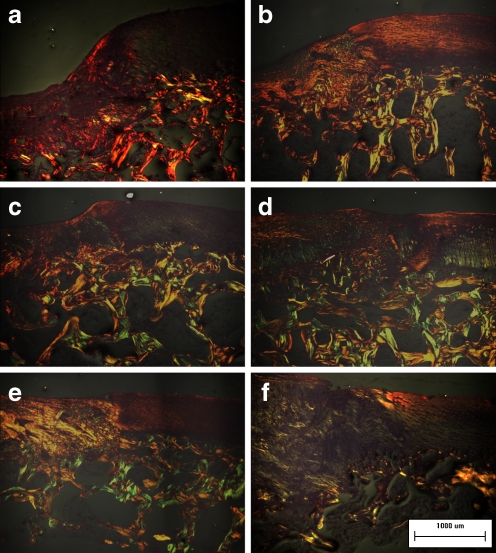
The intercellular matrix of the regenerating tissues was stained with Safranin-O (Fig. 3e, g) and toluidine blue (Fig. 4e, g) in the two cell-treated groups, demonstrating its content of proteoglycans and glycosaminoglycans. Cellular distribution was irregular, especially in the BNCs group, in which a few cartilage lacunas could be observed. The collagen networks of the regenerating tissues showed strong double refraction (characteristic for collagen type I, which was detected with Sirius red staining) in the COL group (Fig. 5a). The refraction of the regenerating tissues was still apparent (especially in the BNCs group) in the two cell-treated groups (Fig. 5c, e), which implied that the collagen networks had not formed the truly collagen type II.
Fig. 3.
a–h Safranin-O staining of the regenerating tissues was negative in the CON group (a) and the COL group (c) 4 weeks after surgery, and the intensity of Safranin-O staining at the edge of the cartilage showed various degrees of decrease. The defect was still distinct in the CON group 8 weeks after surgery (b). More regenerating tissues were observed in the COL group 8 weeks after surgery, but the Safranin-O staining was still negative (d). Safranin-O staining of the regenerating tissues was slight or moderate (e and g) 4 weeks after surgery and nearly normal (f and h) 8 weeks after surgery in the two cell-treated groups (original magnification, ×100)
Fig. 4.
a–h Toluidine blue staining of the regenerating tissues and adjacent cartilage. The signs of them were parallel with those in Fig. 3a–h (original magnification, ×100)
Fig. 5.
a–f The collagen network of the regenerating tissues. The regenerating tissues showed strong double refraction in the COL group (a and b). The regenerating tissues showed weak double refraction and a loose network distribution of colours in the two cell-treated groups (d and f) 8 weeks after surgery, although their collagen networks 4 weeks after surgery (c for BMSCs and e for BNCs) were more similar to those of the COL group (original magnification, ×100)
Eight weeks
Hyperaemia and hyperplasia were observed in few synovial membranes when the knee joints were exposed, and nearly no synovial fluid spilled out. In the CON group, the chondral defects were still distinct, but the edges became sleek. In the COL group, more regenerating tissues filled the defects, but the surfaces were not smooth and had no luster of the hyaline-like cartilage. In the two cell-treated groups, the chondral defects were completely filled with hyaline-like regenerating tissues, whose colour was ivory white resembling normal cartilage.
Again, we observed the defects’ morphology through the stereo microscope. Compared with the images of four weeks, the repair of the defects showed no notable improvement either in the CON group or the COL group (Fig. 2b, d). On the contrary, the subsidence of the subchondral bone became more serious in three specimens. In the BMSCs group and the BNCs group (Fig. 2f, h), the integration of the regenerating tissues and the circumjacent cartilage was fairly good; the interface could hardly be identified in two specimens, and the subsidence of the subchondral bone was not aggravated in most of the specimens.
Regenerating cartilaginous tissues in the two cell-treated groups became more distinctive than those of four weeks after the operation. More intense staining with Safranin-O (Fig. 3f, h) and toluidine blue (Fig. 4f, h) could be detected, while some cartilage lacunas presenting columnar distribution could be observed at the deep layer of the regenerating tissues adjacent to the normal cartilage, although the tidemark still could not be found. The collagen network of the regenerating tissues still showed strong double refraction in the COL group (Fig. 5b). However, the regenerating tissues showed weak double refraction and a loose network distribution of colours in the two cell-treated groups (Fig. 5d, f), resembling normal cartilage.
The O’Driscoll scoring system for regenerating tissue
The BMSCs group had the highest score (expressed as mean ± SD) at four or eight weeks. The scores of the two cell-treated groups were markedly higher than those of the CON group and the COL group (p < 0.01), while the score of the COL group was higher than that of the CON group (p < 0.05 at four weeks, p < 0.01 at eight weeks). Although the score (15.2 ± 0.83666) of the BMSCs group was higher than that (12.6 ± 0.89443) of the BNCs group (p = 0.004) at four weeks, the scores of the two groups had no significant difference (p = 0.984) at eight weeks (Table 1).
Table 1.
The O’Driscoll histological scores of the four groups
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Mean | SD | |
|---|---|---|---|---|---|---|---|
| 4 weeks | |||||||
| CON | 3 | 5 | 3 | 3 | 4 | 3.6 | 0.89443 |
| COL | 5 | 5 | 8 | 6 | 5 | 5.8 | 1.30384 |
| BMSCs | 14 | 16 | 16 | 15 | 15 | 15.2 | 0.83666 |
| BNCs | 13 | 12 | 12 | 14 | 12 | 12.6 | 0.89443 |
| 8 weeks | |||||||
| CON | 5 | 4 | 6 | 4 | 3 | 4.4 | 1.14018 |
| COL | 9 | 11 | 10 | 10 | 9 | 9.8 | 0.83666 |
| BMSCs | 19 | 18 | 17 | 18 | 18 | 18 | 0.70711 |
| BNCs | 17 | 19 | 17 | 18 | 18 | 17.8 | 0.83666 |
SD standard deviation
Discussion
Autologous chondrocytes have been used as seed cells to repair cartilage defects for nearly 30 years since 1984 [2, 3, 16], but this technique has not been widely used around the world to date, especially in the developing countries. The reasons probably are the high cost, the risk of a second operation and anaesthesia, the long-term treatment cycles, the chondrocytes’ instability in monolayer culture [6] together with the additional lesions due to the harvesting of donor cartilage for chondrocyte propagation. Tissue engineering is currently focusing on the use of adult MSCs as an alternative to autologous chondrocytes [17], in which the most common and studied source is bone marrow. Many experiments have confirmed that bone marrow-derived MSCs are the most promising seed cells and have the potential to be employed in clinical applications [18, 19], but the separation and purification of MSCs are still a complex and uncertain processes, which can only be performed in laboratories. So its widespread application is limited. In view of this, researchers have attempted to repair cartilage defects with a single operation. Giannini et al. [9] reported a “one-step technique” for talar osteochondral lesion repair, in which uncultured bone marrow-derived cells were used as seed cells. The clinical results indicated that the patients obtained improved functional scores and that histological evaluation showed regenerated tissue in various degrees of remodelling despite the fact that no entirely hyaline cartilage appeared.
Although the one-step technique with uncultured BNCs has achieved encouraging results, the question still remains whether the repair tissues have a better or similar effect compared with culture-expanded MSCs. In this study, we established a full-thickness cartilage defect model in minipigs whose joint size, weight-bearing requirements and cartilage thickness were closer to those of humans than smaller animal models [20]. Furthermore, we used a convenient method to deliver autologous culture-expanded bone marrow-derived MSCs or autologous uncultured BNCs to the cartilage defect area, which demonstrated that the cartilage repair was improved markedly in comparison to the CON and COL groups. Meanwhile, we also observed that the repair tissues of the two cell-treated groups showed no significant difference. This appears to be good news for joint surgeons and patients suffering from articular cartilage lesions being very simple, economical and safe method by using autologous uncultured BNCs.
In this study, no growth factors [21, 22] were added into the implants, but the cartilage repair of the cell-treated groups was still improved markedly. The reason probably was that in vivo culture in the joint cavity environment may provide better conditions for MSC differentiation than in vitro culture, involving mechanical stimulation caused by the motions of the joint [23], the lower oxygen tension in the knee joint capsule [24] and many nutritive materials of the synovial fluid. Four weeks after the operations, the BMSCs group represented the highest O’Driscoll histological score because of its highest cell density of MSCs. However, four weeks later, the regenerated tissues of the BNCs group were as good as those of the BMSCs group. We speculated that BNCs including T cells, B cells, monocytes and macrophages might secrete abundant cytokines (or growth factors) [8], which provide more consummate and constant microenvironment support, consequently contributing to the proliferation and chondrogenic differentiation of MSCs. Another source of cytokines was platelets, because there were always some platelets mixed with the BNCs during the cell collection with the pipette, which would finally be transplanted to the cartilage defects as a whole.
Moreover, the choice of the scaffold for cell transplantation is very important in cartilage regeneration. Collagen type II hydrogel was a 3-D culture system offering good interactions for cell-cell and cell-matrix, which had the characteristic of gelation at 37°C and the convenience in surgical handling [25]. Being one of the most predominant components in articular cartilage, collagen type II demonstrated that it can initiate and maintain MSC chondrogenesis [12]. In this study, we observed gratifyingly that all collagen type II hydrogel implants did not shed from the defects, and the regenerating tissues closely integrated with the circumjacent cartilage, especially in the cell-treated groups. We eliminated the possibility that the highly hydrated 3-D environment benefited cell migration and new tissue growth, since the lower implant surface avoided direct friction from adjacent tissues.
We found that the cartilage repair of the COL group was superior to that of the CON group. The cartilage defects we created did not penetrate the subchondral bone, so bone marrow blood that contains glycoproteins, platelets, growth factors and MSCs had no chance to participate in the cartilage regeneration [3]. However, when the chondral defects were occupied by the collagen type II hydrogel, some chondrocytes existing in the interface between host and implant would migrate into the hydrogel and proliferate under the induction of collagen type II, though the effect was minor.
In conclusion, with collagen type II hydrogels as scaffolds, the transplantation of autologous uncultured BNCs contributes to articular cartilage repair in large animal models, in which the regenerated cartilaginous tissues closely integrated with the circumjacent cartilage. Considering postoperative recovery of the animals, the gross and histological observation of repair tissues, and the O’Driscoll scoring system for repaired tissue, there were no significant differences in the repair results between the BNCs group and the BMSCs group. Therefore, the transplantation of autologous uncultured BNCs as seed cells may be an effective, economical, convenient and safe technique for cartilage repair in clinical applications.
Acknowledgments
We thank Professor Yujiang Fan for his provision of collagen type II hydrogel and Li Yin M.D. for statistical analysis. This work was supported by National Natural Science Foundation of China (No. 30870639), Clinical project of National 863 program (No. 2006AA02A125) and the Key Project of Medical Science in the Military “11th 5-year Plan”, China (06 G079).
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Yi Zhang, Email: ghostknife7801@gmail.com.
Fuyou Wang, Email: wfy731023@163.com.
Jiarong Chen, Email: chjr1983@163.com.
Zhigang Ning, Email: nizhga619@163.com.
Liu Yang, Phone: +86-23-68765280, FAX: +86-23-65464006, Email: jointsurgery@163.com.
References
- 1.Mierisch CM, Wilson HA, Turner MA, Milbrandt TA, Berthoux L, Hammarskjöld ML, Rekosh D, Balian G, Diduch DR. Chondrocyte transplantation into articular cartilage defects with use of calcium alginate: the fate of the cells. J Bone Joint Surg Am. 2003;85-A(9):1757–1767. doi: 10.2106/00004623-200309000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett W, Skinner JA, Gooding CR, Carrington RWJ, Flanagan AM, Briggs TWR, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 3.Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A(3):455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Ivkovic A, Marijanovic I, Hudetz D, Porter RM, Pecina M, Evans CH. Regenerative medicine and tissue engineering in orthopaedic surgery. Front Biosci (Elite Ed) 2011;3:923–944. doi: 10.2741/e299. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Kim YH, Kim SH, Han SH, Hahn SB. Chondrogenic differentiation of mesenchymal stem cells and its clinical applications. Yonsei Med J. 2004;45(Suppl):41–47. doi: 10.3349/ymj.2004.45.Suppl.41. [DOI] [PubMed] [Google Scholar]
- 6.Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noël D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27(5):307–314. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 8.Chang F, Ishii T, Yanai T, Mishima H, Akaogi H, Ogawa T, Ochiai N. Repair of large full-thickness articular cartilage defects by transplantation of autologous uncultured bone-marrow-derived mononuclear cells. J Orthop Res. 2008;26(1):18–26. doi: 10.1002/jor.20470. [DOI] [PubMed] [Google Scholar]
- 9.Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467(12):3307–3320. doi: 10.1007/s11999-009-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoddart MJ, Grad S, Eglin D, Alini M. Cells and biomaterials in cartilage tissue engineering. Regen Med. 2009;4(1):81–98. doi: 10.2217/17460751.4.1.81. [DOI] [PubMed] [Google Scholar]
- 11.Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14(2):149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93(6):1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 13.Jing XH, Yang L, Duan XJ, Xie B, Chen W, Li Z, Tan HB. In vivo MR imaging tracking of magnetic iron oxide nanoparticle labeled, engineered, autologous bone marrow mesenchymal stem cells following intra-articular injection. Joint Bone Spine. 2008;75(4):432–438. doi: 10.1016/j.jbspin.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Gotterbarm T, Breusch SJ, Schneider U, Jung M. The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: retrospective analysis of 180 defects. Lab Anim. 2008;42(1):71–82. doi: 10.1258/la.2007.06029e. [DOI] [PubMed] [Google Scholar]
- 15.Rutgers M, Pelt MJ, Dhert WJ, Creemers LB, Saris DB. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. 2010;18(1):12–23. doi: 10.1016/j.joca.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Giannini S, Buda R, Vannini F, Caprio F, Grigolo B. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36(5):873–880. doi: 10.1177/0363546507312644. [DOI] [PubMed] [Google Scholar]
- 17.Raghunath J, Salacinski HJ, Sales KM, Butler PE, Seifalian AM. Advancing cartilage tissue engineering: the application of stem cell technology. Curr Opin Biotechnol. 2005;16(5):503–509. doi: 10.1016/j.copbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Lee KB, Hui JH, Song IC, Ardany L, Lee EH. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cells. 2007;25(11):2964–2971. doi: 10.1634/stemcells.2006-0311. [DOI] [PubMed] [Google Scholar]
- 19.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10(3):199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 20.Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16(1):105–115. doi: 10.1089/ten.teb.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pecina M, Vukicevic S. Biological aspects of bone, cartilage and tendon regeneration. Int Orthop. 2007;31(6):719–720. doi: 10.1007/s00264-007-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pecina M, Jelic M, Martinovic S, Haspl M, Vukicevic S. Articular cartilage repair: the role of bone morphogenetic proteins. Int Orthop. 2002;26(3):131–136. doi: 10.1007/s00264-002-0338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang PY, Chow HH, Lai JY, Liu HL, Tsai WB. Dynamic compression modulates chondrocyte proliferation and matrix biosynthesis in chitosan/gelatin scaffolds. J Biomed Mater Res B Appl Biomater. 2009;91(1):143–152. doi: 10.1002/jbm.b.31384. [DOI] [PubMed] [Google Scholar]
- 24.Buckley CT, Vinardell T, Kelly DJ. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthritis Cartilage. 2010;18(10):1345–1354. doi: 10.1016/j.joca.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21(32–33):3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]