Abstract
The beneficial effects of probiotics have been demonstrated in many diseases, such as inflammatory bowel disease. The known mechanisms for probiotic action include blocking pathogenic bacterial effects, enhancing the innate immunity and decreasing pathogen-induced inflammation, and promoting intestinal epithelial cell survival, barrier function, and protective responses. We purified and cloned a Lactobacillus rhamnosus GG (LGG)-derived soluble protein, p40. This protein ameliorated cytokine-induced apoptosis in intestinal epithelial cells through activation of the EGF receptor and its down-stream target, Akt. By using special hydrogel beads to protect p40 from degradation, we showed that p40 reduced intestinal epithelial apoptosis and preserved barrier function in the colon epithelium in an EGF receptor-dependent manner, thereby preventing and treating intestinal inflammation in mouse models of colitis. Further works regarding structural analysis of p40, regulation of EGF receptor activation and immunoregulatory effects by p40 are discussed. These results may provide insights into the clinical application of probiotics for intestinal inflammatory disorders.
Keywords: Lactobacillus rhamnosus GG, Apoptosis, barrier, colitis, EGF receptor, inflammatory cytokine, intestinal epithelial cell, intestinal inflammation, probiotics
Introduction
A unique characteristic of the gastrointestinal tract is the continuous contact between the gastrointestinal epithelial cell monolayer and microbial flora, which engage in active cross talk with the host. Selective nonpathogenic living microorganisms, including some present as commensal microbiota, which have beneficial effects on host health and disease prevention and/or treatment were first described as probiotics by Lilly and Stillwell.1 Most commonly used probiotics in humans and animals include Lactobacillus, Bifidobacterium, and Saccharomyces. A significant question regarding clinical use of probiotics is the mechanism underlying the wide range of actions. Three distinct cellular and molecular mechanisms have been demonstrated by both clinical and basic research, blocking pathogenic bacterial effects by production of antibacterial substances and competitive inhibition of pathogen and toxin adherence to the intestinal epithelium, regulating immune responses by upregulation of immune function may improve the ability to fight infections or inhibit tumor formation; downregulation may prevent the onset of allergy or intestinal inflammation, and modulating intestinal epithelial cell homeostasis, including restitution of damaged epithelial barrier, production of anti-bacterial substances and cell-protective proteins, and blockade of cytokine-induced intestinal epithelial cell apoptosis.2
Probiotic-Derived Soluble Proteins
The current clinical applications of probiotics have raised two problems regarding the use of probiotic therapy. First is the difficulty of determining bioavailability of bacteria in the gastrointestinal tract. In addition, use of live probiotic bacteria raises concerns about biosafety of probiotics. Probiotic administration has been reported to be associated with an increased risk of mortality in patients with severe acute pancreatitis.3 This condition may be related to bacteremia since several cases of bacteremia were found in immuno-compromised patients.4 One approach to address these concerns of using probiotics may be through developing probiotic bacteria-derived proteins as innovative therapeutic reagents.
Analyses of current available probiotic genome sequences, including 8 Lactobacillus and 6 Bifidobacterium strains, have predicted a broad group of proteins, including secreted and cell surface proteins with potential regulatory effects on intestinal cells.5 For example, genomic analysis of LGG reveals that a mucus binding pilus on the surface of LGG is a key factor for adhesion of LGG to the host intestinal mucus.6 However, their in vivo effects on host health and disease prevention and/or treatment are not clearly demonstrated.
Thus, we have chosen LGG as a probiotic model to define the mechanisms of probiotic action. Lactobacillus rhamnosus GG (LGG) is a naturally occurring gram-positive bacterium originally isolated from the healthy human intestine.7 LGG is one of the best-studied probiotic bacteria in clinical trials for treating and/or preventing several disorders, including inflammatory bowel disease (IBD), diarrhea and atopic dermatitis.8 LGG treatment is more effective than standard treatment with mesalamine in maintaining remission and prolonging the relapse-free time in patients with ulcerative colitis.9
We have purified and cloned two LGG-derived soluble proteins, p40 and p75, and showed that LGG and LGG-derived soluble proteins prevent cytokine-induced epithelial damage and apoptosis10,11 and hydrogen peroxide disruption of epithelial barrier function.12 Although both of these two proteins regulate intestinal epithelial homeostasis, we were focusing on studying p40 because p40 exerts more potent effects than p75. For example, p40 can stimulate two to 3-fold greater Akt activation and inhibition of apoptosis than p75, even at 5-fold lower molar concentration than p75. In addition, p40 may be a major protein responsible for the cellular effects regulated by probiotic soluble proteins. L. casei-conditioned medium contains the same amount of p40, but very low amount of p75 compared with LGG-conditioned medium. However, L. casei-conditioned medium exerts the same effects of Akt activation and inhibition of apoptosis as LGG-conditioned medium. Other identified functions of LGG and soluble proteins include promoting cytoprotective protein production by intestinal epithelial cells13 and inhibiting lipopolysaccharide-induced tumor necrosis factor production in macrophages.14 These studies indicate that production of soluble proteins by probiotics may be a mechanism of probiotic action for regulating intestinal homeostasis and preventing and/or treating diseases.
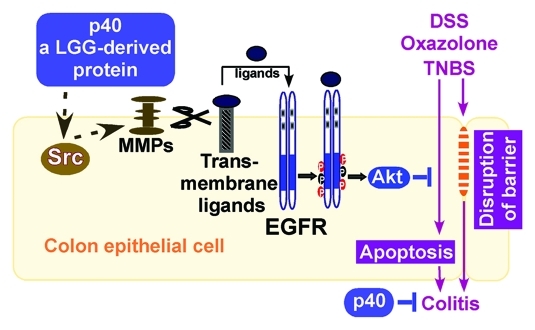
We have recently performed studies to investigate the effects and mechanisms of p40 regulation of cellular responses in intestinal epithelial cells and p40s role in dextran sulfate sodium (DSS)-induced intestinal epithelial injury and acute colitis, oxazolone-induced Th2 cytokine-driven and TNBS-induced Th1 cytokine-driven chronic colitis in mice. We reported that p40 activated EGF receptor and its down-stream target, AKt, leading to amelioration of cytokine-induced apoptosis and disruption of epithelial barrier in intestinal epithelial cells. Furthermore, specific delivery of p40 to the colon using a special hydrogel beads to protect p40 from degradation prevented and treated colon epithelial cell injury and inflammation in these colitis models in an EGF receptor-dependent manner (Fig. 1).15 Our results reveal a previously unrecognized mechanism of probiotic-derived soluble proteins in modulating intestinal inflammation.
Figure 1. Regulation of colitis by p40. p40 stimulates Src activity, which leads to activation of MMPs. MMPs are proteolytic enzymes to induce release of EGFR ligands for transactivation of EGFR and its down-stream target, Akt, in the colon epithelial cells. Increased apoptosis and disruption of barrier function in the intestinal epithelial cells are two pathological factors involved in colitis induced by DSS, oxazolone, and TNBS. Activation of EGFR and Akt by p40 to prevent apoptosis and maintain intestinal integrity serves as a mechanism for p40s preventive and treatment effects on intestinal inflammation in these mouse models of colitis. EGFR, EGF receptor; MMPs, matrix metalloproteinases.
Functional Domain(s) of p40
In order to gain further insights into the structure-functional relationship of p40, we performed sequence analysis of p40, which predicts that the N-terminal portion forms a coiled-coil structure and the C-terminal portion forms a β-sheet structure. Therefore, we expressed the N-terminal 1–180 aa and the C-terminal portion (181–412 aa) of p40 as recombinant peptides
p40 full-length and truncated peptides were used to detect their effects on EGF receptor activation and survival of intestinal epithelial cells. We found that the N-terminal 1–180 aa peptide is as potent as p40 full length for regulating signaling and cellular responses in intestinal epithelial cells. Thus, it may be possible to identify functional domain(s) of probiotic-derived proteins for novel therapeutic application in inflammatory intestinal disorders.
Upstream Regulators for EGF Receptor Activation by p40
One significant finding from this published paper that p40 transactivates EGF receptor in the intestinal epithelial cells leads to the question of what are the upstream regulators for EGF receptor activation by p40. Since several studies have demonstrated that the non-receptor tyrosine kinase, Src, activates EGF receptor directly or indirectly through activation of matrix metalloproteinases, which are proteolytic enzymes to induce release of EGF receptor ligands,16 we sought to determine the involvement of Src and metalloproteinase in p40 regulated signaling in intestinal epithelial cells. We found that p40 stimulated Src activation in intestinal epithelial cells and inhibition of Src or metalloproteinase kinase activity blocked p40 activation of EGF receptor. These data indicate that Src and metalloproteinase may serve as upstream signals to release ligands as the mechanism for p40 transactivation of EGF receptor in intestinal epithelial cells.
Immunoregulatory Effects of LGG p40
Modulation of the immune system is one of the most plausible mechanisms underlying the beneficial effects of probiotics on human health. Probiotics regulate host innate and adaptive immune responses by modulating functions of dendritic cells, macrophages, and T and B lymphocytes.2,17 In addition, since the intestinal epithelium is integral to both discrimination of pathogens and commensal bacteria and is actively involved in immune responses in the intestinal tract, this monolayer is a target of probiotic immunoregulatory effects.18 Therefore, we detected whether p40 exerts immunoregulatory effects on colitis. To address this issue, we detected cytokine levels in the colon and intracellular cytokine protein levels in macrophages and lymphocytes. Our results suggest that p40 plays a role in regulation of innate immunity and Th1 immune response since p40 downregulated proinflammatory cytokines involved in innate immunity, including macrophage-produced TNF, IL-6, and KC TNF and Th1 response, such as TNF and IFN-γ (Supplementary data in ref. 15). It is important to note that it is likely that p40 associates with different aspects of immunity, such as regulation of innate immunity and Th1 immune response. p40 may have effects on macrophages, lymphocytes, intestinal epithelial cells to directly regulate immune responses during inflammation, such as proinflammatory cytokine production. LGG-conditioned cell culture medium decreases TNF production in macrophages, indicating that soluble molecules derived from LGG exert this immunoregulatory role.14 The other possibility is that p40 may function on intestinal epithelial cells to decrease injury, which prevent production of these proinflammatory cytokines.
Conclusions
Our studies clearly demonstrated the preventive and treatment effects of p40 on intestinal inflammation. IBD is characterized by increased production of inflammatory cytokines, epithelial cell apoptosis, and immune cell infiltration, leading to disruption of the intestinal epithelial integrity. The first goal of medical therapy for IBD is to induce and maintain a clinical remission. Remission of these disorders requires both decreased apoptosis and restitution of the damaged epithelium. By using this colon-specific hydrogel delivery system, p40 may exert its protective effects on the intestinal epithelial cells and immunity. Therefore, p40 provides a novel therapeutic approach for preventing and/or treating IBD.
Acknowledgments
This work was supported by NIH grants DK065744, DK081134 and CCFA Senior Research Award (to F.Y.), DK56008 (to D.B.P.), P30DK058404 (Vanderbilt University Digestive Disease Research Center), and CA 68485 (Vanderbilt University Medical Center Imaging Core Research Laboratory).
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/19245
References
- 1.Lilly DM, Stillwell RH. Probiotics: Growth-promoting factors produced by microorganisms. Science. 1965;147:747–8. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 2.Vanderpool C, Yan F, Polk DB. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm Bowel Dis. 2008;14:1585–96. doi: 10.1002/ibd.20525. [DOI] [PubMed] [Google Scholar]
- 3.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Dutch Acute Pancreatitis Study Group Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–9. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 4.Apostolou E, Kirjavainen PV, Saxelin M, Rautelin H, Valtonen V, Salminen SJ, et al. Good adhesion properties of probiotics: a potential risk for bacteremia? FEMS Immunol Med Microbiol. 2001;31:35–9. doi: 10.1111/j.1574-695X.2001.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 5.Ventura M, O’Flaherty S, Claesson MJ, Turroni F, Klaenhammer TR, van Sinderen D, et al. Genome-scale analyses of health-promoting bacteria: probiogenomics. Nat Rev Microbiol. 2009;7:61–71. doi: 10.1038/nrmicro2047. [DOI] [PubMed] [Google Scholar]
- 6.Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human- mucus binding protein. Proc Natl Acad Sci U S A. 2009;106:17193–8. doi: 10.1073/pnas.0908876106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorbach SL. The discovery of Lactobacillus GG. Nutr Today. 1996;31(suppl 1):2S–4S. doi: 10.1097/00017285-199611001-00002. [DOI] [Google Scholar]
- 8.Doron S, Snydman DR, Gorbach SL. Lactobacillus GG: bacteriology and clinical applications. Gastroenterol Clin North Am. 2005;34:483–98, ix. doi: 10.1016/j.gtc.2005.05.011. [ix.] [DOI] [PubMed] [Google Scholar]
- 9.Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567–74. doi: 10.1111/j.1365-2036.2006.02927.x. [DOI] [PubMed] [Google Scholar]
- 10.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–75. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–65. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer C, Kosters A, Sethi G, Kunnumakkara AB, Aggarwal BB, Versalovic J. Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-kappaB and MAPK signalling. Cell Microbiol. 2008;10:1442–52. doi: 10.1111/j.1462-5822.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- 13.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, et al. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–30. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 14.Peña JA, Versalovic J. Lactobacillus rhamnosus GG decreases TNF-α production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell Microbiol. 2003;5:277–85. doi: 10.1046/j.1462-5822.2003.t01-1-00275.x. [DOI] [PubMed] [Google Scholar]
- 15.Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121:2242–53. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene. 2001;20:1594–600. doi: 10.1038/sj.onc.1204192. [DOI] [PubMed] [Google Scholar]
- 17.Yan F, Polk DB. Probiotics as functional food in the treatment of diarrhea. Curr Opin Clin Nutr Metab Care. 2006;9:717–21. doi: 10.1097/01.mco.0000247477.02650.51. [DOI] [PubMed] [Google Scholar]
- 18.Yan F, Polk DB. Probiotics and immune health. Curr Opin Gastroenterol. 2011;27:496–501. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]