Abstract
The current global outbreak of Clostridium difficile infection exemplifies the major public health threat posed by clostridial glucosylating toxins. In the western world, C. difficile infection is one of the most prolific causes of bacterial-induced diarrhea and potentially fatal colitis. Two pathogenic enterotoxins, TcdA and TcdB, cause the disease. Vancomycin and metronidazole remain readily available treatment options for C. difficile infection, but neither is fully effective as is evident by high clinical relapse and fatality rates. Thus, there is an urgent need to find an alternative therapy that preferentially targets the toxins and not the drug-resistant pathogen. Recently, we addressed these critical issues in a Nature Medicine letter, describing a novel host defense mechanism for subverting toxin virulence that we translated into prototypic allosteric therapy for C. difficile infection. In this addendum article, we provide a continued perspective of this antitoxin mechanism and consider the broader implications of therapeutic allostery in combating gut microbial pathogenesis.
Keywords: C. difficile, allostery, dietary supplement, inositol phosphate, S-nitrosylation, toxin
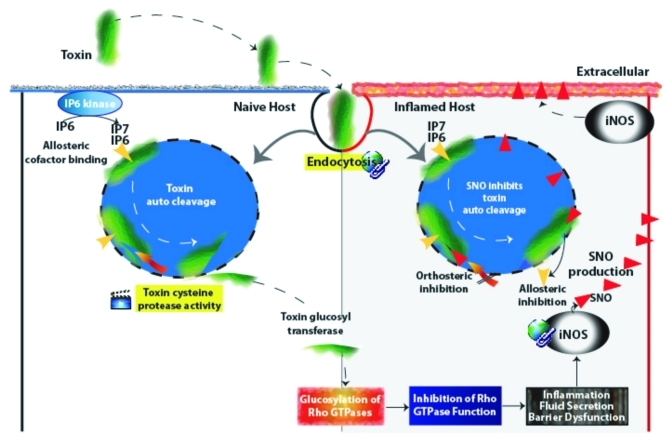
Cysteine proteases degrade polypeptides via a mechanism that normally involves a reactive cysteine thiol in a catalytic motif.1 This prevalent enzyme class regulates many important cellular activities in gut host cells and their associated microflora.1,2 It has been recently appreciated that several gut pathogens produce toxins that also depend upon a conserved cysteine protease for virulence.3-6 The autocatalytic cysteine protease of these large, secreted bacterial proteins is required for intoxication of target host cells and includes the disease-inducing clostridium glucosylating toxins, as well as RTX, α-hemolysin, FrpC, and adenylate cyclase pore-forming toxins from Vibrio cholerae, Escherichia coli, Neisseria meningiditides and Bordetella pertussis, respectively.3-6 Although not yet demonstrated for all of the above bacterial exoproteins, cellular intoxication by the clostridium glucosylating toxins and Vibrio cholerae RTXVC toxin also depend on host-derived inositol phosphate cofactors.7-14 These regulatory cofactors bind to an allosteric site on the toxin after its insertion into the plasma membrane, activating the autocatalytic cysteine protease to facilitate toxin self-cleavage (Fig. 1). A smaller toxin effector domain is then injected into the cytosol where it inactivates Rho GTPases in target cells.5-7 The Rho family members act as molecular switches in a number of important cell signaling pathways associated with actin polymerization, inflammation and cell death.
Figure 1. Summary figure with video links of the toxin allosteric mechanism.
The dramatic increase in severity of C. difficile-associated disease in North America and Europe over the last decade highlights the clinical prominence of C. difficile’s glucosylating toxins, and is partially due to the spread of new epidemic-associated strains, for example BI/NAP1/027 that produce high amounts of these toxins.15 Accompanying this surge in disease severity is a rise in recurrent clinical episodes in up to 35% of patients with symptomatic C. difficile infection (CDI).15 These unmet clinical issues represent a significant medical and financial challenge to health care systems, and have rekindled interest in improving therapy against this increasingly prevalent pathogen. Fidaxomicin has shown promise in reducing CDI relapse, but this new antibiotic appears less effective against the epidemic strain BI//NAP1/027.16 Adjuvant antitoxin immunotherapy has also shown promise in preventing CDI relapse, but the economic costs are potentially high.17 These issues highlight the complexity of CDI management, and emphasize the need to identify susceptible patients and alternative approaches to therapy.
A Novel Toxin Sensor for Evading Dietary Antitoxins
A majority of the C. difficile bacterial strains that cause disease in humans secrete two large toxins, TcdA (308 kDa) and TcdB (270 kDa). There is little ambiguity that these pathogenic toxins are the major cause of CDI since toxin-deficient clinical isolates are avirulent and may form a new line of clinical therapy by competing with pathogenic strains.15 Microbial genetic manipulation studies have highlighted the disease-inducing potential of both toxins but implicate TcdB as the primary virulence factor in CDI,18,19 supporting earlier unequivocal reports that TcdB is the major enterotoxin in the human colon.20,21 This notion is supported by a recent clinical study reporting that antibodies against TcdB (but not TcdA) are associated with asymptomatic hospital acquired C. difficile colonization.22 Nevertheless, novel antitoxin-based therapy should neutralize both toxins since each has the capacity to induce disease.
TcdA and TcdB are structurally similar, with functional domains that are now reasonably well defined.5-7 The C-terminus receptor binding domain is involved in toxin attachment to the host cell membrane. The transmembrane and cysteine protease domains are involved in toxin entry into target cells, and the N-terminus is a catalytic glucosyltransferase domain. Interactions between the C-terminus binding domain and host cell receptors initiate receptor-mediated endocytosis (Fig. 1). Although the precise intracellular mode of action remains unclear, the toxins undergo a conformational change in the endosome, leading to membrane insertion. A cytosolic virulence cofactor, myo-inositol hexakisphosphate (InsP6), is then required to trigger an allosteric structural change that activates the cysteine protease domain (CPD) to induce toxin self-cleavage and release of the glucosyltransferase domain into the cytosol (Fig. 1).7-14 In this instance, allosteric regulation or allostery refers to a change in the shape and activity of the toxin cysteine protease that results from molecular binding with an inositol phosphate regulatory factor at a site other than the enzymatically active one (orthosteric site). Once in the cytosol, this effector domain mono-O-glucosylates and inactivates small Rho GTPases, leading to alterations in the actin cytoskeleton, diarrhea, inflammation, and necrosis of the colonic mucosa.
Cysteine-dependent cleavage is a key regulatory mechanism for C. difficile virulence since it facilitates entry of the glucosyltransferase domain into target cell cytosol. Allosteric coupling by InsP6 activates the toxin cysteine protease catalytic reaction to facilitate toxin self-cleavage. Specific inhibition of this cleavage reaction by mutagenesis or alkylation of the active site cysteine,5,7,12,13 or by competitive peptide inhibition,23 significantly attenuates cytotoxicity. Although irreversible chemical modifiers of cysteine thiol and peptide inhibitors of the cysteine protease active site are known to inhibit toxin virulence with great sensitivity, poor selectivity for microbial over human cysteine proteases remains a potential concern. Also, it is desirable to design non-peptide-based reversible inhibitors for oral therapy so as to minimize the potential toxicity that can be observed with irreversible inhibitors.
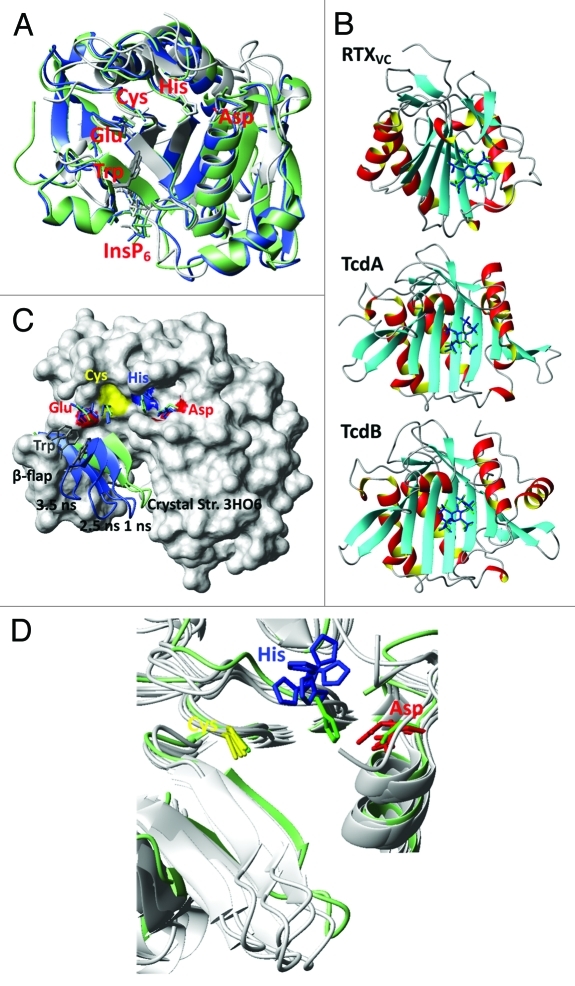
High resolution CPD crystal structures for TcdA and TcdB closely align with the CPD crystal structure for RTXVC (Fig. 2A), and show a well-defined catalytic cleft that is structurally distinct from a positively charged InsP6 binding pocket abutting a flexible β-hairpin fold (β-flap).12,22,24 A potential deficiency of these TcdA and TcdB CPD crystal structures is the lack of their native N-terminus cleavage fragment that may significantly alter the ordering of the substrate cleft and catalytic triad. To gain further structural insight into the toxin allosteric mechanism, we used the coordinates of the CPD crystal structures for TcdA and TcdB, and the N-terminus from RTXVC11 to generate native structural models for TcdA and TcdB that included the uncut N-terminus substrate within the catalytic cleft. These toxin CPD homology models spanned CPD residues V534-S801 in TcdA and K535-T799 in TcdB (Fig. 3). Because it has not yet been possible to generate crystal structures of the native InsP6 unbound toxin CPD configuration, we performed molecular dynamics (MD) structural simulations25,26 to better understand the structural basis of the InsP6 allosteric mechanism. These simulation models consistently demonstrated two distinct allosteric mechanisms that were highly dependent on whether the uncut N-terminus substrate is absent (short model simulation) or present (long model simulation) in the catalytic cleft. In the short model simulations (essentially using CPD crystal structures lacking the N-terminus substrate), in silico docking of InsP6 bound accurately to the allosteric pocket (Fig. 2B; Supplemental Material), and induced conformational changes that facilitated substrate access to the active site cysteine by leveraging the flexible β-flap away from catalytic cleft (Fig. 2C; Vids. 1 and 3). Furthermore, InsP6 induced allostery may bolster cysteine thiol reactivity toward the substrate by promoting spatial proximity and alignment of catalytic residues (Fig. 2D). Nevertheless, even in the InsP6 bound crystal configuration, the catalytic cysteine thiolate and the histidine imidazolium remain > 6Å apart, raising the question as to whether the toxin CPD functions as a conventional cysteine protease. In the related clan CD cysteine proteases, which includes the caspases, the histidine normally lies within a 5Å radius of its catalytic dyad partner to impart significant cysteine thiol nucleophilicity.1,2 Thus, our short model simulations show that InsP6 allostery promotes both accessibility and catalytic reactivity toward substrate, and in general this is in agreement with a series of elegant experiments that have recently defined the allosteric circuit in the short CPD domain of TcdB.24
Figure 2. (A) The β-strand core region, the active site and the InsP6 binding site together with the β-flap are conserved and align well for CPD’s of RTXVC (gray), TcdA (green) and TcdB (blue). Shown are the crystal structures with the PDB-codes 3EEB (RTXVC), 3OH6 (TcdA) and 3PA8 (TcdB). They all have InsP6 bound and the substrate is cleaved. Therefore, there are no peptides bound in the active site (we refer to these structures as short). In TcdB the shown residues represent: C698, H653, D587, E743 and W761. (B) Re-docking of InsP6 to the crystal structures of RTXVC (3EEB), TcdA (3HO6) and TcdB (3PA8) with Autodock (blue) shows excellent agreement with the original position of InsP6 (green). (C) β-flap movement of the InsP6 free TcdA CPD (short form 3HO6) during a MD-simulation. Shown residues are C700, H655, D589, E745 and W763. (D) Along with the β-flap the Asp (D589) and His (H655) in the catalytic triad move drastically during an InsP6 free MD-simulation of TcdA CPD (3HO6). Different snapshots at 3.5, 4.0, 5.0, 5.5 and 7.0 ns are compared with the crystal structure positions (green).
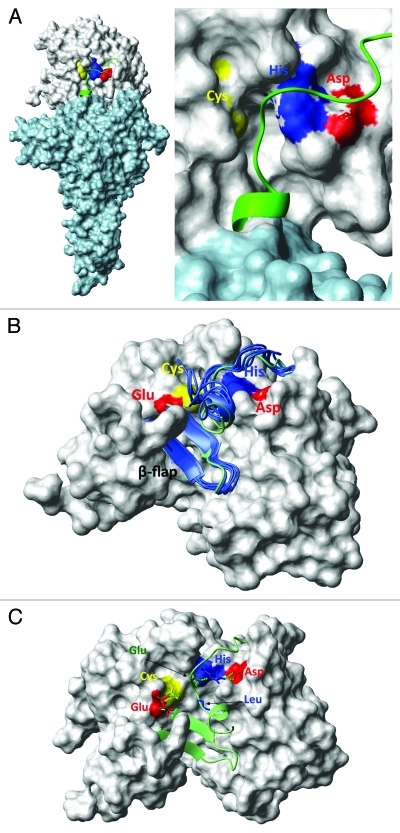
Figure 3. (A) Model of the TcdB glucosyltransferase (light blue) and cysteine protease domains (gray). Residues C698, H653 and D743 are shown in yellow, blue and red, respectively. (B) β-flap does not move during a MD-simulation of the InsP6 free longer TcdB CPD (long form based on the crystal structures 3PA8 and 3FYZ). Starting conformation is shown in green and snapshots at 1, 2, 3, 4 and 5 ns are shown in blue. Residues E761 (red), C698(yellow), H653 (blue) and D587 (red) are highlighted at the surface. (C) Active site of the InsP6 free longer TcdB CPD. Asparagine (D587 in red) and histidine (H653 in blue) stabilize the substrate via hydrogen bonds (dotted green). The cysteine (C698 in yellow) is inhibited by another hydrogen bond to the glutamic acid (E743 in red).
In our Nature Medicine letter we propose an alternate mechanism for the InsP6 induced allostery based on our CPD structural homology models that contain the N-terminus glucosyltransfease domain (Fig. 3A). It appears clear from these studies that the toxin catalytic dyad does not function via a conventional enzymatic mechanism. Our long model simulations show comparatively little evidence of leveraging of the flexible β-flap away from catalytic cleft as is evident in the short CPD simulations (Fig. 3B; Vid. 2). Furthermore, following conformational coupling by InsP6, no interaction between the catalytic cysteine and histidine is evident because the N-terminus cleavage substrate is positioned between these dyad residues. Alternatively, the catalytic histidine appears to play a major role in guiding and orienting the cleavage substrate within the catalytic cleft (Fig. 3C). Our long models also show that hydrogen bonding exists between the catalytic cysteine and a juxtaposed glutamic acid in the simulated InsP6 unbound CPD structure, and this interaction likely inhibits the cysteine protease activity in the inactive state. Thus, conformational coupling by InsP6 regulates CPD hydrogen bond interactions that on the one hand suppress catalytic cysteine thiol reactivity, while on the other align the N-terminus substrate within the catalytic cleft (Fig. 3; Vids. 2 and 4). Experimental site-directed mutagenesis studies using TcdB confirmed that toxin mutants lacking the catalytic cysteine (residue 698) or histidine (residue 653) become enzymatically dead, whereas toxin mutants lacking the juxtaposed glutamic acid (residue 743) exhibit greatly enhanced catalytic activity in response to allosteric coupling by InsP6.27 This toxin mutant is also prone to spontaneous autocatalytic cleavage, indicating that the regulatory glutamic acid governs the equilibrium between catalytically active and inactive states.
Because this cysteine protease catalytic motif is structurally conserved among microbial CPDs, we have proposed that the above structural features function as a general allosteric switch mechanism to prevent premature toxin self-cleavage by InsP6 in the extracellular gut environment. Extracellular InsP6 concentrations in blood and plasma are generally too low (< 1 nM) to facilitate toxin self-cleavage.28 However, InsP6 can reach much higher concentrations in the gut lumen from dietary sources (micromolar range),29 where it may play a protective role in some patients by prematurely inactivating the toxin. Dietary InsP6 (phytate or phytic acid) is the principle storage form of phosphorous in many plants, especially in bran and seeds.29 It is also available as a dietary nutritional supplement. Thus, the Achilles heel of the C. difficile toxins may be their reliance on allosteric InsP6 as a virulence sensor. Indeed, if this is the case, then dietary InsP6 supplementation may confer clinical benefits to symptomatic CDI patients. This notion is supported by a recent metabolomics approach that we have used to demonstrate that dietary InsP6 bioavailablity is markedly dimininished in stool specimens from symptomatic CDI patients. As a result, we are currently in the process of initiating a clinical trial to test the efficay of dietary InsP6 supplementation in symptomatic CDI patients (http://www.its.utmb.edu/mtts/clostridial_difficile_infection.html).
Host S-Nitrosylation: A Gut Defense Mechanism for Subverting Toxin Virulence
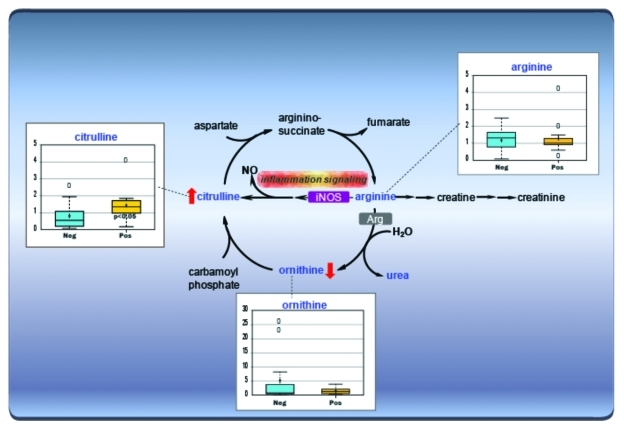
Other gut defense mechanisms that might be employed to protect against CDI are not well defined, although the toxins induce potent mucosal antibody and nitric oxide responses that may explain why this disease is self-limited in some patients. However, the precise protective mechanisms are only just being defined. Elevated nitric oxide production has been shown to induce anti-inflammatory transcription factor activity and inhibits leukocyte homing in murine toxigenic ileal loop models of CDI.30,31 Using a metabolomics approach, we have confirmed in patient stool specimens that biochemical molecules associated with nitric oxide synthesis are significantly elevated in symptomatic CDI (Fig. 4). In our Nature Medicine letter we additionally report that foreign microbial protein, notably toxin itself, is subject to functional regulation by elevated nitric oxide production in the infected host.27
Figure 4. Arginine Metabolism. C. difficile is known to be the primary causative agent for pseudomembranous colitis and indicators of inflammation including significantly elevated levels of citrulline in stool of infected patients compared with non-infected patients with antibiotic-associated diarrhea.
It is increasingly appreciated that many diverse signaling cascades associated with nitric oxide production are attributed to S-nitrosothiol species that act via covalent modification of cysteine thiol groups in target molecules (S-nitrosylation), and that aberrant S-nitrosylation plays a major role in disease-etiology.32 Because cysteine residues are often key regulators of protein function, S-nitrosylation represents a physiologically important signaling mechanism that regulates virtually all known cellular signaling pathways. The emerging mechanism for regulation of protein function by S-nitrosylation is that it is governed by structural motifs that are targeted by nascent nitrosylases.33 The recent discovery of specific nitrosylases that transduce nitric oxide action places S-nitrosylation firmly on the path of being the nitric oxide signaling equivalent of phosphorylation and ubiquitylation (regulated by protein kinases and ubiquitin E3 ligases, respectively).33,34
We have reported that S-nitrosylation may also function as a gut defense mechanism for subverting microbial pathogenesis in CDI.27 Small peptide S-nitrosothiols (the most significant being S-nitrosoglutathione or GSNO) are endogenous inhibitors of C. difficile toxin action, acting in significant part by S-nitrosylation of the cysteine protease active site thiol. This active site cysteine forms part of a novel microbial S-nitrosylation-catalytic motif that co-serves as a regulator of InsP6 induced toxin self-cleavage (Fig. 3). Physiological context is provided by showing that InsP6 and inositol pyrophosphate (InsP7) are specificity-determinants of toxin S-nitrosylation. Further, because plasma membrane-associated InsP7 is the more cogent allosteric activator of the toxin cysteine protease, this phylogenically ancient family of inositol phosphates may constitute the preferred specificity-determinant for toxin virulence. Thus, GSNO attenuates the C. difficile toxins by a novel, dual orthosteric and allosteric mechanism of action: InsP6 enables S-nitrosylation of the toxin cysteine protease active site, which then displaces the allosteric activator (Fig. 1). S-nitrosylation of the active site cysteine may itself alter the allosteric transition of the toxin cysteine protease by masking existing (or revealing new) binding sites, or by changing surface charge distributions in the catalytic cleft.
Perspectives: New Therapeutic Concepts for Gut Microbial Pathogenesis
Host S-nitrosylation is not a random event, but is most often governed by consensus motifs that encompass the cysteine residue targeted for posttranslational modification.32,33 Protein S-nitrosylation is often also subject to regulation by host allosteric cofactors. The novelty of our work may be viewed in the broader context in which hypo- and hyper-nitrosylation of specific bacterial proteins represent disease-modifying events. We demonstrate that a structurally conserved microbial catalytic motif is targeted for inactivation by host nitrosylases, and that endogenous inositol phosphate cofactors act as specificity determinants in the S-nitrosylation action. A physiological correlate may be drawn from the structurally related caspase family of allosterically regulated cysteine proteases, as these may be maintained in a constitutively S-nitrosylated and inactive state in the inner mitochondrial membrane.32 Our studies show that under certain pathophysiological conditions, the plasma membrane compartment may also facilitate privileged access of host nitrosylases and allosteric cofactors to exogenous microbial proteins in order to maintain these in an avirulent state. Because the structurally conserved bacterial catalytic-S-nitrosylation motif is found in abundantly diverse disease-associated cysteine proteases, we have proposed that host S-nitrosylation may play a universal role in subverting gut microbial pathogenesis.27
As a direct counter measure to evade host S-nitrosylation defenses, bacterial nitric oxide detoxification strategies appear to have evolved in several gut pathogens to inhibit cellular S-nitrosothiol formation.35 Therapeutic strategies that elevate S-nitrosothiol bioavailability may therefore enhance the clearance of certain gut bacterial infections. GSNO is well tolerated in humans, and is already known to be a multifaceted protective agent that exhibits broad-spectrum anti-microbial activity.32 Studies by our group and other investigators have demonstrated that exogenous GSNO provides potent disease-attenuating signals in the gastrointestinal tract.36-38 A primary goal in nitric oxide therapeutics is to identify the nitrosylation state of proteins that are identified with pathophysiology, and to selectively and specifically control this modification. We have shown that GSNO can function as the physiological corollary of therapeutic inhibitors currently being developed to treat CDI. Furthermore, allosteric regulation of S-nitrosylation by inositol phosphate cofactors (which has not been previously demonstrated), suggests new therapeutic approaches to regulate the S-nitrosylation state of specific disease-related targets. Therapeutic context for the principle that allosteric modulation of S-nitrosylation can be employed to treat CDI is demonstrated by the efficacy of exogenous GSNO and InsP6–separately or in a combined form — in treatment of experimental CDI.
Supplementary Material
Supplementary video file supplied by authors.
Supplementary video file supplied by authors.
Supplementary video file supplied by authors.
Supplementary video file supplied by authors.
Supplementary PDF file supplied by authors.
Acknowledgments
This work was supported by the John S. Dunn Gulf Coast Consortium for Chemical Genomics Robert A. Welch Collaborative Grant Program, Eli & Edith Broad Foundation, and grants from the National Institutes of Health NIAID (R01AI088748), NIDDK (R01DK084509, K01DK076549; R21-DK078032–01), NHLBI (R01-HL059130, R01-HL091876, R01 HL095463, P01 HL075443–06A) and 1UL1RR029876–01.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/19250
References
- 1.Rudenskaya GN, Pupov DV. Cysteine proteinases of microorganisms and viruses. Biochemistry (Mosc) 2008;73:1–13. doi: 10.1134/S000629790801001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKerrow JH, Engel JC, Caffrey CR. Cysteine protease inhibitors as chemotherapy for parasitic infections. Bioorg Med Chem. 1999;7:639–44. doi: 10.1016/S0968-0896(99)00008-5. [DOI] [PubMed] [Google Scholar]
- 3.Satchell KJ. MARTX, multifunctional autoprocessing repeats-in-toxin toxins. Infect Immun. 2007;75:5079–84. doi: 10.1128/IAI.00525-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheahan KL, Cordero CL, Satchell KJ. Autoprocessing of the Vibrio cholerae RTX toxin by the cysteine protease domain. EMBO J. 2007;26:2552–61. doi: 10.1038/sj.emboj.7601700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egerer M, Giesemann T, Jank T, Satchell KJ, Aktories K. Auto-catalytic cleavage of Clostridium difficile toxins A and B depends on cysteine protease activity. J Biol Chem. 2007;282:25314–21. doi: 10.1074/jbc.M703062200. [DOI] [PubMed] [Google Scholar]
- 6.Pei J, Lupardus PJ, Garcia KC, Grishin NV. CPDadh: a new peptidase family homologous to the cysteine protease domain in bacterial MARTX toxins. Protein Sci. 2009;18:856–62. doi: 10.1002/pro.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reineke J, Tenzer S, Rupnik M, Koschinski A, Hasselmayer O, Schrattenholz A, et al. Autocatalytic cleavage of Clostridium difficile toxin B. Nature. 2007;446:415–9. doi: 10.1038/nature05622. [DOI] [PubMed] [Google Scholar]
- 8.Prochazkova K, Satchell KJ. Structure-function analysis of inositol hexakisphosphate-induced autoprocessing of the Vibrio cholerae multifunctional autoprocessing RTX toxin. J Biol Chem. 2008;283:23656–64. doi: 10.1074/jbc.M803334200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lupardus PJ, Shen A, Bogyo M, Garcia KC. Small molecule-induced allosteric activation of the Vibrio cholerae RTX cysteine protease domain. Science. 2008;322:265–8. doi: 10.1126/science.1162403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egerer M, Giesemann T, Herrmann C, Aktories K. Autocatalytic processing of Clostridium difficile toxin B. Binding of inositol hexakisphosphate. J Biol Chem. 2009;284:3389–95. doi: 10.1074/jbc.M806002200. [DOI] [PubMed] [Google Scholar]
- 11.Prochazkova K, Shuvalova LA, Minasov G, Voburka Z, Anderson WF, Satchell KJ. Structural and molecular mechanism for autoprocessing of MARTX toxin of Vibrio cholerae at multiple sites. J Biol Chem. 2009;284:26557–68. doi: 10.1074/jbc.M109.025510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pruitt RN, Chagot B, Cover M, Chazin WJ, Spiller B, Lacy DB. Structure-function analysis of inositol hexakisphosphate-induced autoprocessing in Clostridium difficile toxin A. J Biol Chem. 2009;284:21934–40. doi: 10.1074/jbc.M109.018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreimeyer I, Euler F, Marckscheffel A, Tatge H, Pich A, Olling A, et al. Autoproteolytic cleavage mediates cytotoxicity of Clostridium difficile toxin A. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:253–62. doi: 10.1007/s00210-010-0574-x. [DOI] [PubMed] [Google Scholar]
- 14.Guttenberg G, Papatheodorou P, Genisyuerek S, Lü W, Jank T, Einsle O, et al. Inositol hexakisphosphate-dependent processing of Clostridium sordellii lethal toxin and Clostridium novyi alpha-toxin. J Biol Chem. 2011;286:14779–86. doi: 10.1074/jbc.M110.200691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DuPont HL. The search for effective treatment of Clostridium difficile infection. N Engl J Med. 2011;364:473–5. doi: 10.1056/NEJMe1013236. [DOI] [PubMed] [Google Scholar]
- 16.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, et al. OPT-80-003 Clinical Study Group Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 17.Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 18.Lyras D, O’Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–9. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–3. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 20.Savidge TC, Pan WH, Newman P, O’brien M, Anton PM, Pothoulakis C. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology. 2003;125:413–20. doi: 10.1016/S0016-5085(03)00902-8. [DOI] [PubMed] [Google Scholar]
- 21.Riegler M, Sedivy R, Pothoulakis C, Hamilton G, Zacherl J, Bischof G, et al. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Invest. 1995;95:2004–11. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693–703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 23.Puri AW, Lupardus PJ, Deu E, Albrow VE, Garcia KC, Bogyo M, et al. Rational design of inhibitors and activity-based probes targeting Clostridium difficile virulence factor TcdB. Chem Biol. 2010;17:1201–11. doi: 10.1016/j.chembiol.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen A, Lupardus PJ, Gersch MM, Puri AW, Albrow VE, Garcia KC, et al. Defining an allosteric circuit in the cysteine protease domain of Clostridium difficile toxins. Nat Struct Mol Biol. 2011;18:364–71. doi: 10.1038/nsmb.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oezguen N, Schein CH, Peddi SR, Power TD, Izumi T, Braun W. A “moving metal mechanism” for substrate cleavage by the DNA repair endonuclease APE-1. Proteins. 2007;68:313–23. doi: 10.1002/prot.21397. [DOI] [PubMed] [Google Scholar]
- 26.Navaratnarajah CK, Oezguen N, Rupp L, Kay L, Leonard VH, Braun W, et al. The heads of the measles virus attachment protein move to transmit the fusion-triggering signal. Nat Struct Mol Biol. 2011;18:128–34. doi: 10.1038/nsmb.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savidge TC, Urvil P, Oezguen N, Ali K, Choudhury A, Acharya V, et al. Host S-nitrosylation inhibits clostridial small molecule-activated glucosylating toxins. Nat Med. 2011;17:1136–41. doi: 10.1038/nm.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letcher AJ, Schell MJ, Irvine RF. Do mammals make all their own inositol hexakisphosphate? Biochem J. 2008;416:263–70. doi: 10.1042/BJ20081417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohn L, Meyer AS, Rasmussen SK. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J Zhejiang Univ Sci B. 2008;9:165–91. doi: 10.1631/jzus.B0710640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu B, Pothoulakis C, Castagliuolo I, Nikulasson Z, LaMont JT. Nitric oxide inhibits rat intestinal secretion by Clostridium difficile toxin A but not Vibrio cholerae enterotoxin. Gastroenterology. 1996;111:409–18. doi: 10.1053/gast.1996.v111.pm8690206. [DOI] [PubMed] [Google Scholar]
- 31.Ng J, Hirota SA, Gross O, Li Y, Ulke-Lemee A, Potentier MS, et al. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology. 2010;139:542–52, 552.e1-3. doi: 10.1053/j.gastro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamler JS, Hess DT. Nascent nitrosylases. Nat Cell Biol. 2010;12:1024–6. doi: 10.1038/ncb1110-1024. [DOI] [PubMed] [Google Scholar]
- 34.Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JV, Snowman AM, et al. GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol. 2010;12:1094–100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laver JR, Stevanin TM, Messenger SL, Lunn AD, Lee ME, Moir JW, et al. Bacterial nitric oxide detoxification prevents host cell S-nitrosothiol formation: a novel mechanism of bacterial pathogenesis. FASEB J. 2010;24:286–95. doi: 10.1096/fj.08-128330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–58. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 37.Flamant M, Aubert P, Rolli-Derkinderen M, Bourreille A, Neunlist MR, Mahé MM, et al. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut. 2011;60:473–84. doi: 10.1136/gut.2010.229237. [DOI] [PubMed] [Google Scholar]
- 38.Costantini TW, Bansal V, Krzyzaniak M, Putnam JG, Peterson CY, Loomis WH, et al. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1308–18. doi: 10.1152/ajpgi.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary video file supplied by authors.
Supplementary video file supplied by authors.
Supplementary video file supplied by authors.
Supplementary video file supplied by authors.
Supplementary PDF file supplied by authors.