Abstract
Drosophila telomeres are maintained as a result of transpositions of specialized telomeric retrotransposons. The abundance of telomeric retroelement transcripts, as well as the frequency of their transpositions onto the chromosome ends, is controlled by a PIWI-interacting RNA (piRNA) pathway. In our recent report, we demonstrate strong evidence of piRNA-mediated transcriptional silencing of telomeric repeats in the Drosophila germline. Telomerase-generated repeats serve as a platform for recruiting specialized DNA-binding proteins which are involved in chromosome end protection and in the telomere length control. No specific proteins are known to bind to heterogeneous long sequences of the Drosophila telomeric retrotransposons. The importance of the piRNA silencing mechanism in the formation of telomeric chromatin along the region of the retrotransposon array will be discussed. We propose that Drosophila telomeric retrotransposon HeT-A serves as a template for the piRNA-mediated assembly of the specific protein complex, which is functionally similar to the recruiting of the DNA-binding telomeric proteins by the telomerase-generated repeats. The role of the piRNA pathway components in the assembly of the telomere capping complex was recently unveiled. Taken together, these data elucidate the importance of the piRNA pathway in the Drosophila telomere homeostasis.
Keywords: Chromatin, Drosophila, germline, piRNA, PIWI, retrotransposon, telomere
Drosophila Telomeric Sequences are Targets of piRNAs
In most eukaryotes, telomere maintenance is provided by the telomerase activity which generates an array of short 6–9 nucleotides repeats using the RNA template encoded by a cellular gene. Drosophila telomeres are elongated by transpositions of specialized telomeric retrotransposons, HeT-A, TART and TAHRE, onto chromosome ends where they are found in mixed head-to-tail arrays.1,2 In this case, the RNA template for telomere elongation is encoded by the telomeric sequences themselves. Drosophila telomere elongation via assistance of retrotransposition of mobile elements represents a striking example of adaptation of selfish genetic elements to the realization of the vital cellular function.
Silencing of mobile elements in germ cells depends on a distinct class of RNAs called PIWI-interacting RNAs3 associated with Argonaute proteins from the PIWI subfamily.4 piRNA pathway mutations lead to overexpression and mobilization of retrotransposons in the germline.4,5 Drosophila telomeric retroelements, as well as other parasitic elements, were shown to be targets of the piRNA-mediated silencing pathway.6-8 The expression and transposition frequency of the telomeric retroelements are negatively regulated by the Spn-E and Aubergine components of the piRNA pathway in the female germline.6 In other words, the increased telomeric element expression in mutant flies is correlated with an increased frequency of their transposition and, consequently, in telomere elongation.6 This assay was performed using broken terminally deleted chromosomes. However, spn-E or aub mutant lines do not have detectably greater numbers of HeT-A and TART in their genomes.6,9 This may indicate that truncated chromosome ends are more sensitive to telomeric element attachments than native telomeres. Recent studies revealed an increase in HeT-A copy number in the genomes of armi and ago3 piRNA pathway mutant stocks.9 These data provide strong evidence of the involvement of the piRNA pathway in the control of Drosophila telomere length. Components of telomeric chromatin also play an important role in the regulation of Drosophila telomere elongation, most likely through the limitation of accessibility of the chromosome end for transpositions. It was shown that mutations in the gene for heterochromatic protein 1 (HP1), a chromatin protein and a component of the telomere cap complex, increase both the abundance of HeT-A and TART RNA and their frequency of transposition to broken ends.10 Lines heterozygous for HP1 mutations show an increase in telomeric retrotransposon copy number.10 The germline-specific HP1 homolog Rhino is required for production of piRNAs and transposon silencing, including telomeric elements.11 rhi mutants also have longer telomeres in their genomes.11 These data imply that the control of the natural telomere length is more complex and depends both on telomere chromatin components and the piRNA pathway. It is tempting to speculate that interplay between both mechanisms provides proper telomere functioning.
In different organisms, short RNAs were shown to be implicated in the posttranscriptional degradation of mRNA and/or transcriptional repression of the homologous locus. In the Drosophila model, piRNAs were shown to be essential for the post-transcriptional retrotransposon mRNA degradation in the germline,12 however no direct evidence of the piRNA-mediated transcriptional silencing was obtained. In our recent study, we initially wished to address the mechanism of piRNA-mediated silencing of telomeric retrotransposons. This problem is of great interest because, in the case of transcriptional silencing, it might concern the formation of the telomeric chromatin which is involved in meiotic and mitotic telomere behavior.
piRNAs Mediate Transcriptional Silencing of Telomeric Retrotransposons
An siRNA-mediated spreading of heterochromatin is involved in the centromeric and telomeric repeat silencing in yeast.13,14 In this case, short RNAs homologous to the centromeric and subtelomeric repeats guide histone methyltransferase to the target locus to methylate lysine 9 of histone H3 (H3K9) with subsequent binding of the yeast HP1 homolog Swi6. In Drosophila, it is still unknown whether piRNAs affect the transcriptional state of retrotransposons. To address this question in our study we decided to directly estimate the transcriptional status of retroelements in the germline of piRNA pathway mutants rather than to look for the histone marks of known chromatin modifying pathways in attempt to connect them with the piRNA pathway. In fact, considering that different transposable element subfamilies may have principally distinct mechanisms of the control of their expression and chromatin structure, we do not exclude the possibility that the mechanism of the piRNA-mediated chromatin protein recruitment depends on genomic context of the target sequences.
We explored different approaches to estimate transcriptional activity of the telomeric retroelements in the piRNA pathway mutants.15 Nuclear run-on assay on ovarian tissues was applied to estimate the density of transcriptionally active RNA-polymerase complexes at the loci of interest. An increase in the nascent transcripts emerging from telomeric loci as well as from some other retrotransposons was found. This observation was strengthened by the fact of the increase of retrotransposon sequence association with two histone H3 modifications (dimethylation of lysines 4 and 79) known to be linked to the RNA polymerase II activity. Using combined DNA/RNA FISH, we obtained visual confirmation of the piRNA-mediated transcriptional regulation of the telomeric retrotransposons. Namely, nascent HeT-A and TART transcripts accumulate at the sites of transcription at the telomeres in the piRNA pathway mutants. These data provided strong evidence that piRNAs impact transcriptional status of their target loci. Thus, transposon defense in the Drosophila germline is a combination of the piRNA-mediated post-transcriptional and transcriptional silencing. On the one hand, it seems that telomeric retrotransposons along with parasitic transposons are the robust targets of this defense system. On the other hand, these retroelements form telomeres suggesting an additional role for the piRNA pathway in the telomere homeostasis, probably, in the piRNA-mediated telomeric chromatin assembly.
In the absence of telomerase generated repeats, Drosophila telomeric retrotransposons may be considered as a platform for the specific telomeric protein binding. The most plausible candidate for this role is HeT-A. HeT-A element is the main structural component of telomeres present at each chromosome arm in contrast to TART and TAHRE which are represented by a few copies. The total HeT-A copy number is estimated to be ~30 per genome, which is comparable with the copy number of other retrotransposon families.16,17 However, the dramatic effect of HeT-A overexpression in the piRNA pathway mutants (up to 1,000-fold) considerably differs from the effects on other retrotransposons which expression increases in 5–50 times.15 According to the run-on analysis, the HeT-A transcriptional rate is increased up to 60-fold as a result of the piRNA pathway disruption.15 It looks like piRNAs trigger assembly of the higher order inhibition protein complex at the HeT-A sequences. Prolonged 3′UTRs of HeT-A elements were proposed to serve as a platform for the protein binding to form a specific telomeric chromatin.18 What is known about chromatin structure of the telomeric retrotransposon array?
The retrotransposon array exhibits euchromatic characteristics since P-element insertions into this region are not silenced in somatic tissues.19 This domain has the histone marks which are associated with actively transcribed genes; however, no actively elongating RNA polymerase II isoform was detected in this region when polytene chromosomes of larval salivary glands were inspected.20 This fact is in agreement with the observation that HeT-A transcripts are not detected in this tissue.21,22 There is no data concerning telomeric chromatin state in the germ cells. From our recent results it may be deduced that in germ cells telomeric retrotransposon repeats display the features of an open chromatin as well.15 We have shown that expression of the telomeric HeT-A/yellow reporter originated as a result of HeT-A attachment to the truncated yellow gene is regulated at transcriptional level by the piRNA pathway. Additionally, we have tested the Drosophila lines in which the attachments of telomeric elements have occurred ~100–1,000 bp upstream of the yellow promoter to the terminal deleted X chromosome. In these cases, no changes of the yellow expression were observed in the piRNA pathway mutants. This fact indicates that the inactive chromatin, if to assume its formation at the telomeric retrotransposon sequences via the piRNA assistance, does not spread onto the adjacent yellow promoter region. One may suggest that piRNAs mediate sequence-specific binding of the inhibition protein complex locally at the HeT-A promoter in the germ cells rather than heterochromatinization along telomeric arrays (Fig. 1). It should be noted here, that HeT-A promoter is located on the very 3′ end of the retrotransposon and drives transcription of the downstream element.23 Drosophila telomeres contain a mixture of the complete and 5′ truncated HeT-A copies; the last of them possesses an active promoter. For example, there are five full-size and seven truncated HeT-As distributed throughout the fourth chromosome telomere of the stock sequenced by the Genome Project.16 Of course, it is considerably less than the copy number of the telomerase-generated repeats, however, enough to be considered as a putative platform for the telomere protein binding. Components of the transcriptional initiation complex may be considered as a putative link between piRNAs and inhibition of the transcription (Fig. 2). However, little is known about the transcription factors involved in the telomeric element expression. The PROD protein was shown to bind HeT-A 3′ UTR causing a transcriptional repression of the HeT-A expression.24 This protein of the unknown function which is present at the centric heterochromatin, as well as at numerous euchromatic sites, was proposed to be required for changing the telomeric repeat chromatin structure. These fragmentary data are not sufficient to propose a possible candidate for the role of a piRNA partner in the HeT-A transcriptional complex. We believe that future studies will allow revealing such components.
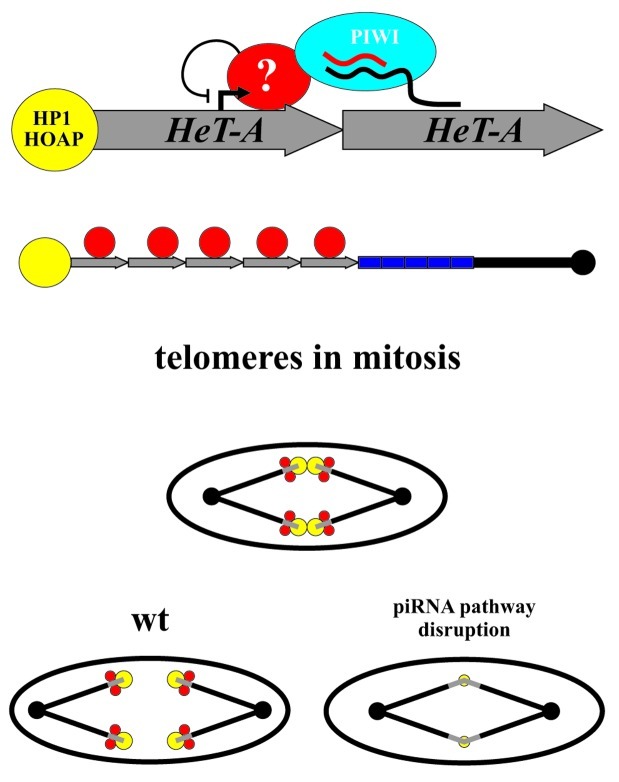
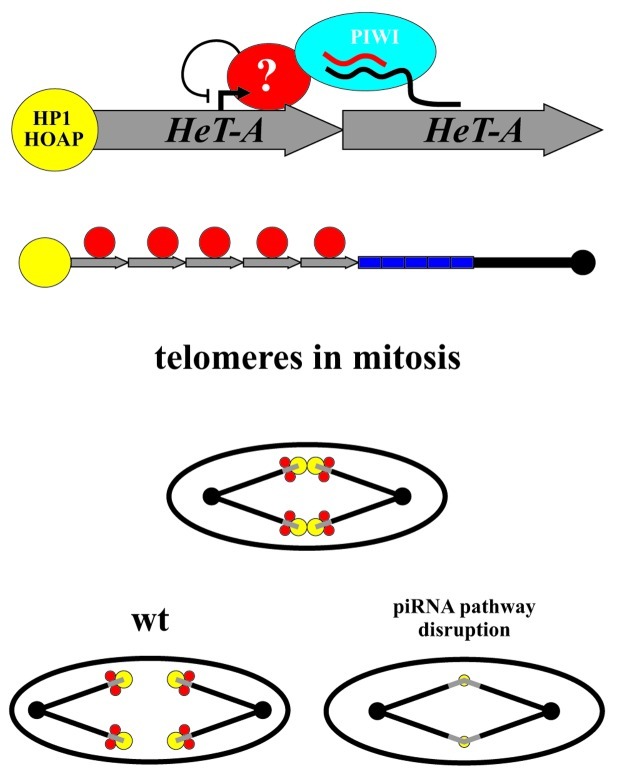
Figure 1. Mechanism of the piRNA-mediated silencing of the telomeric repeats. (A) Schematic representation of the two possible scenarios of the piRNA-mediated telomeric chromatin assembly is shown. Heterochromatin spreading or binding of the transcriptional inhibitors might be responsible for the piRNA-mediated transcriptional silencing of the telomeric repeats. (B) A scheme of the experiment indicating that the putative inhibition protein complexes recruited to the HeT-A promoter via the assistance of piRNAs do not spread into adjacent regions. We have studied effect of the piRNA pathway disruption on the expression of the yellow gene located at the end of the terminally deleted chromosome. Attachment of the HeT-A to the truncated yellow gene results in the appearance of the fused HeT-A/yellow transcript which expression is downregulated at transcriptional level by the piRNA pathway (upper scheme). When HeT-A attaches upstream of the yellow promoter, yellow expression is not affected by the piRNAs (lower scheme).
Figure 2. Putative role of the piRNA pathway in Drosophila telomere functioning. piRNA/PIWI complex is proposed to mediate binding of the transcriptional inhibitors at the HeT-A promoter (red filled circles). At the same time, piRNA pathway components are involved in the telomere capping protein recruitment (yellow filled circles). Thus, telomeric protein complex is formed via the assistance of piRNAs providing telomere segregation during mitosis (in wild type, wt). Telomere fusions in early embryogenesis are observed as a result of piRNA pathway disruption.
Fission yeast employs two independent mechanisms to maintain gene silencing at telomeres. A chromatin-remodeling complex is recruited to yeast telomeres via the interaction with telomeric repeat binding proteins Ccq1 and Taz1 or the RNAi machinery that acts through repeat-like sequences embedded within subtelomeric regions.14 It is tempting to speculate that HeT-A-specific piRNAs facilitate assembly of the telomeric protein complex on the HeT-A sequences providing telomere functioning in the absence of telomerase generated repeats.
piRNAs and Telomere Capping
aub and armi piRNA pathway mutations lead to telomere fusions during the cleavage division stage in early embryos, which suggests that these components are required for telomere resolution.9 These mutations also reduce the HOAP and HP1 cap components binding to telomeres thus disrupting the assembly of the telomere protection complex. A subpopulation of telomere-specific piRNAs was proposed to direct assembly of the telomere cap.9 However, in Drosophila, chromosome ends are capped by a sequence-independent manner providing cap formation in the absence of telomeric retrotransposon sequences at terminally deleted chromosomes both in somatic and germinal cells.15,25 Most likely, piRNAs provide a redundant pathway for the telomere capping protein recruitment. In this case, telomere fusions during mitosis in the aub and armi mutants could not be explained exclusively by disruption of the telomere capping. The piRNA-mediated chromatin formation at the retrotransposon array is proposed to be important for the mitotic telomere behavior (Fig. 2). Besides, subtelomeric sequences also produce piRNAs4 and may be considered as a putative platform for the piRNA-mediated chromatin assembly. In this context, it would be interesting to look at the behavior of the terminally deleted chromosomes, which lack both telomeric retrotransposons and subtelomeric repeats, in the piRNA pathway mutants. Which of the telomeric sequences are really involved in the meiotic and mitotic telomere behavior, and if the piRNA pathway plays a role in this process are challenging but important questions in the understanding of the telomere homeostasis.
Acknowledgments
This work was supported by grants to A.K. from the Russian Academy of Sciences program for Molecular and Cell Biology and the Russian Foundation for Basic Researches (09-04-00305). We thank Edward M. Perkins for checking of English spelling.
Footnotes
Previously published online: www.landesbioscience.com/journals/mge/article/18301
References
- 1.Pardue ML, DeBaryshe PG. Retrotransposons provide an evolutionarily robust non-telomerase mechanism to maintain telomeres. Annu Rev Genet. 2003;37:485–511. doi: 10.1146/annurev.genet.38.072902.093115. [DOI] [PubMed] [Google Scholar]
- 2.Abad JP, De Pablos B, Osoegawa K, De Jong PJ, Martin-Gallardo A, Villasante A. TAHRE, a novel telomeric retrotransposon from Drosophila melanogaster, reveals the origin of Drosophila telomeres. Mol Biol Evol. 2004;21:1620–4. doi: 10.1093/molbev/msh180. [DOI] [PubMed] [Google Scholar]
- 3.Aravin AA, Hannon GJ, Brennecke J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science. 2007;318:761–4. doi: 10.1126/science.1146484. [DOI] [PubMed] [Google Scholar]
- 4.Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Klattenhoff C, Theurkauf W. Biogenesis and germline functions of piRNAs. Development. 2007;135:3–9. doi: 10.1242/dev.006486. [DOI] [PubMed] [Google Scholar]
- 6.Savitsky M, Kwon D, Georgiev P, Kalmykova A, Gvozdev V. Telomere elongation is under the control of the RNAi-based mechanism in the Drosophila germline. Genes Dev. 2006;20:345–54. doi: 10.1101/gad.370206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shpiz S, Kwon D, Rozovsky Y, Kalmykova A. rasiRNA pathway controls antisense expression of Drosophila telomeric retrotransposons in the nucleus. Nucleic Acids Res. 2009;37:268–78. doi: 10.1093/nar/gkn960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shpiz S, Kwon D, Uneva A, Kim M, Klenov M, Rozovsky Y, et al. Characterization of Drosophila telomeric retroelement TAHRE: transcription, transpositions, and RNAi-based regulation of expression. Mol Biol Evol. 2007;24:2535–45. doi: 10.1093/molbev/msm205. [DOI] [PubMed] [Google Scholar]
- 9.Khurana JS, Xu J, Weng Z, Theurkauf WE. Distinct functions for the Drosophila piRNA pathway in genome maintenance and telomere protection. PLoS Genet. 2010;6:e1001246. doi: 10.1371/journal.pgen.1001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savitsky M, Kravchuk O, Melnikova L, Georgiev P. Heterochromatin protein 1 is involved in control of telomere elongation in Drosophila melanogaster. Mol Cell Biol. 2002;22:3204–18. doi: 10.1128/MCB.22.9.3204-3218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klattenhoff C, Xi H, Li C, Lee S, Xu J, Khurana JS, et al. The Drosophila HP1 homolog Rhino is required for transposon silencing and piRNA production by dual-strand clusters. Cell. 2009;138:1137–49. doi: 10.1016/j.cell.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim AK, Tao L, Kai T. piRNAs mediate posttranscriptional retroelement silencing and localization to pi-bodies in the Drosophila germline. J Cell Biol. 2009;186:333–42. doi: 10.1083/jcb.200904063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpe TA, Kidner C, Hall IM, Teng G, Grewal SI, Martienssen RA. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–7. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- 14.Sugiyama T, Cam HP, Sugiyama R, Noma K, Zofall M, Kobayashi R, et al. SHREC, an effector complex for heterochromatic transcriptional silencing. Cell. 2007;128:491–504. doi: 10.1016/j.cell.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 15.Shpiz S, Olovnikov I, Sergeeva A, Lavrov S, Abramov Y, Savitsky M, Kalmykova A. Mechanism of the piRNA-mediated silencing of Drosophila telomeric retrotransposons. Nucleic Acids Res. 2011;39:8703–11. doi: 10.1093/nar/gkr552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George JA, DeBaryshe PG, Traverse KL, Celniker SE, Pardue ML. Genomic organization of the Drosophila telomere retrotransposable elements. Genome Res. 2006;16:1231–40. doi: 10.1101/gr.5348806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaminker JS, Bergman CM, Kronmiller B, Carlson J, Svirskas R, Patel S, Frise E, Wheeler DA, Lewis SE, Rubin GM, Ashburner M, Celniker SE. The transposable elements of the Drosophila melanogaster euchromatin: a genomics perspective. Genome Biol 20023:RESEARCH0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danilevskaya ON, Lowenhaupt K, Pardue ML. Conserved subfamilies of the Drosophila HeT-A telomere-specific retrotransposon. Genetics. 1998;148:233–42. doi: 10.1093/genetics/148.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biessmann H, Prasad S, Semeshin VF, Andreyeva EN, Nguyen Q, Walter MF, et al. Two distinct domains in Drosophila melanogaster telomeres. Genetics. 2005;171:1767–77. doi: 10.1534/genetics.105.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreyeva EN, Belyaeva ES, Semeshin VF, Pokholkova GV, Zhimulev IF. Three distinct chromatin domains in telomere ends of polytene chromosomes in Drosophila melanogaster Tel mutants. J Cell Sci. 2005;118:5465–77. doi: 10.1242/jcs.02654. [DOI] [PubMed] [Google Scholar]
- 21.George JA, Pardue ML. The promoter of the heterochromatic Drosophila telomeric retrotransposon, HeT-A, is active when moved into euchromatic locations. Genetics. 2003;163:625–35. doi: 10.1093/genetics/163.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walter MF, Biessmann H. Expression of the telomeric retrotransposon HeT-A in Drosophila melanogaster is correlated with cell proliferation. Dev Genes Evol. 2004;214:211–9. doi: 10.1007/s00427-004-0400-x. [DOI] [PubMed] [Google Scholar]
- 23.Danilevskaya ON, Arkhipova IR, Traverse KL, Pardue ML. Promoting in tandem: the promoter for telomere transposon HeT-A and implications for the evolution of retroviral LTRs. Cell. 1997;88:647–55. doi: 10.1016/S0092-8674(00)81907-8. [DOI] [PubMed] [Google Scholar]
- 24.Török T, Benitez C, Takacs S, Biessmann H. The protein encoded by the gene proliferation disrupter (prod) is associated with the telomeric retrotransposon array in Drosophila melanogaster. Chromosoma. 2007;116:185–95. doi: 10.1007/s00412-006-0090-4. [DOI] [PubMed] [Google Scholar]
- 25.Fanti L, Dorer DR, Berloco M, Henikoff S, Pimpinelli S. Heterochromatin protein 1 binds transgene arrays. Chromosoma. 1998;107:286–92. doi: 10.1007/s004120050310. [DOI] [PubMed] [Google Scholar]