Abstract
Transposable elements (retrotransposons and DNA transposons) comprise a large proportion of animal genomes, for example 20% in D. melanogaster, 36% in X. tropicalis and 45% in humans. After invading a new genome, the transposable element increases its copy number and subsequently accumulates mutations. These may eventually result in inactive copies. Until recent days transposons have been considered “junk” DNA and no clear function have been assigned for this important amount of information on genomes.
Keywords: regulated expression, Tc1, transposon, Xenopus
Expression of Transposable Elements from the Tc1/Mariner Family during Early Development
The regulated expression of different families of retrotransposons and DNA transposons has been described in different tissues and species. However, a detailed analysis of the transcripts or their temporal and spatial expression patterns has been reported in only a few cases. During Drosophila embryogenesis, the differential temporal expression of retrotransposons has been characterized.3 Spatial differential expression of the retrotransposon 412 has been described, specifically in the gonadal mesoderm.4 In mouse, retrotransposons make a high contribution to the pool of maternal mRNAs in early embryos, and the expression of these elements is developmentally regulated.5 In Xenopus, the 1A11 retrotransposon-like element is specifically expressed in the mesoderm and its expression is regulated by FGF.6 Likewise, the expression of the retrotransposon family Xretpos is restricted to ventro-posterior regions during development.7
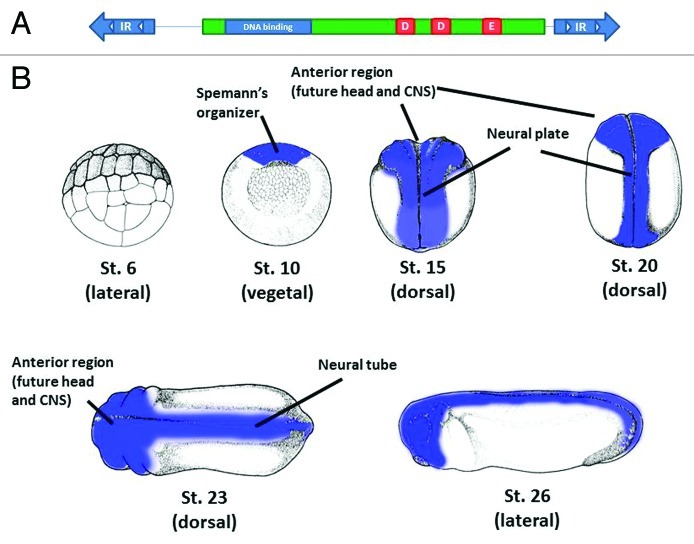
We extended these observations by showing that a DNA transposable element of the Tc1/mariner family is differentially expressed during X. tropicalis development.8,9 The general structure of Tc1/mariner elements is shown in Figure 1A. Transcripts for some Tc1-like elements have been detected in other EST databases,10-16 but no detailed analyses has been performed. Our studies showed that the Tc1-like element Tc1–2_Xt mRNA (named according to the RepeatMasker nomenclature) is specifically expressed at the gastrula stage in the Spemann’s organizer. This region is required for the proper dorso-ventral and anterior-posterior patterning of the embryo and the neuroectoderm, the tissue that will give rise to the nervous system (Fig. 1B). Then as development progresses, Tc1–2_Xt mRNA is found restricted to neural tissue regions. Both Tc1–2_Xt sense and antisense strands present similar expression patterns. The length of the mRNAs suggests that these transcripts are not included in other genes. Transcripts from Tc1–2_Xt are detected mainly after zygotic transcription has begun. piRNAs are specifically derived from Tc1–2_Xt, and analysis of the expression of selected Tc1–2_Xt-derived piRNAs suggests that these elements control its temporal expression.
Figure 1. Putative structure of Tc1–2_Xt transposable element and expression during development. (A) The sequence analysis of the 116 copies of the Tc1–2_Xt transposable element in the X. tropicalis genome showed the typical structure of Tc1-like elements. The length of this element is 1,581 bases. It is flanked by two inverted repeats (IR) containing two direct repeats (white triangles) for the binding of the transposase. The transposase ORF (green) contains a putative DNA binding domain (blue) and the catalytic triad (red, aspartic-aspartic-glutamic residues (DDE)). Five copies in the genome could code for an intact transposase. (B) Schematic representation of the expression of Tc1–2_Xt during X. tropicalis early development. The expression of Tc1–2_Xt is shown in blue for early stages of X. tropicalis development. No expression is detected before the beggining of the zygotic transcription (st.6 is shown as an example, lateral view). Expression is clearly detected from gastrula stage (st.10) in the Spemann’s organizer and then in anterior and neural tissues. Views are indicated in brackets. CNS, central nervous system. Figures were downloaded from http://www.xenbase.org/anatomy/static/NF/NF-all.jsp and modified according to the expression pattern obtained by in situ hybridization.
In the X. tropicalis genome, 72% of transposable elements correspond to DNA transposons17 with seven families of Tc1-like elements characterized.18 Our analyses for these elements, albeit not detailed, have shown that several of these families are transcribed during X. tropicalis development.9
Interestingly, we could not find Tc1–2_Xt in X. laevis by using RT-PCR or in situ hybridization. Whether this is explained because this element invaded the X. tropicalis genome after both species diverged, or to a high divergence in the sequence in the X. laevis genome is not known. The availability of the X. laevis genome could be very useful to study these alternatives. However, in X. laevis, two Tc1-like elements have been described in the genome, TXr and TXz.19 Our studies have demonstrated that TXr and TXz are also expressed during X. laevis development. Importantly, the expression pattern of these elements is very similar to Tc1–2_Xt in X. tropicalis and both strands are also expressed.
At present, we do not know how the expression of these elements is controlled and whether one or several loci are being transcribed. The presence of regulatory elements controlling gene expression in the sequences of these Tc1-like elements has not been studied. The expression can be regulated by endogenous promoters controlling neural-specific genes. However, in contrast to the expression of Tc1 elements in C. elegans, which occurs by fortuitous read-through transcription,15 most Tc1–2_Xt RNA is not included in other protein-coding transcripts, for which read-through transcription is unlikely to explain the bulk of Tc1–2_Xt RNAs. Another alternative is the presence of clusters of several copies of transposable elements under the control of a single promoter. The transcription of clusters of transposable elements to generate piRNAs has been described in Drosophila.20 It is possible that these clusters can also be regulated during development to produce specific expression patterns. Another explanation is that transcription is ubiquitous, and that Tc1–2_Xt RNAs are degraded in ventral and posterior regions by a piRNA dependent-mechanism. In any case, the specific temporal and spatial expression of all these elements strongly suggests that they could play a role during nervous system development.
Possible Roles of Transposable Elements as Non-Coding RNAs
In the X. tropicalis genome, 111 out of 116 Tc1–2_Xt copies contain frame-shifts and mutations rendering putative transposase-inactive copies of the element. Consistently, the analysis of 20 Tc1–2_Xt cDNAs from gastrula stage embryos indicates that none of them codes for an active transposase. These results suggest that Tc1–2_Xt could play a role as a non-coding RNA. In mouse, the transcription of the retrotransposon SINE B2 is necessary for gene activation in the growth hormone locus.21 In this case, the expression of this retrotransposon regulates the formation of a euchromatin/heterochromatin boundary, resulting in the expression of genes in proximal regions. It is still unknown whether this is the case for Tc1–2_Xt expression, but we can propose that some of the Tc1–2_Xt loci may be regulating chromatin architecture, allowing the expression of genes involved in the formation of the nervous system.
Considering that piRNAs derived from Tc1–2_Xt were detected, another possible function is to generate piRNAs. The expression of both strands is consistent with the ping-pong model of amplification of piRNAs.20,22 If this is the case, it is worth noting that the Tc1–2_Xt RNA is stable enough to be detected by RT-PCT, RNA gel blot and in situ hybridization, suggesting that the piRNAs produced are most likely not enough to degrade all of the Tc1–2_Xt RNA during early development. In addition, the expression of sense and antisense strands is specifically detected in neural tissues, and therefore, the amplification of piRNAs could occur in these tissues. Alternatively, piRNAs derived from Tc1–2_Xt may not be involved in the degradation of Tc1–2_Xt itself. Rather than that, they may regulate endogenous genes that contain sites complementary to piRNAs. Although our analysis for two Tc1–2_Xt-derived piRNAs showed that all the piRNA sequences in the genome are included only in Tc1–2_Xt sequences, the possibility that other Tc1–2_Xt-derived piRNAs map to genes cannot be ruled out. For example, piRNAs derived from transposable elements regulate the expression of endogenous genes and allow the clearance of maternal mRNAs during early Drosophila development.23 Finally, recently it has been published that piRNAs derived from non-repetitive regions have a role in spine morphogenesis in the central nervous system in mice.24 Therefore, Tc1–2_Xt -derived piRNAs may have a role in Xenopus neural physiology. Similar scenarios can be proposed for TXr and TXz in X. laevis.
Possible Roles of Transposable Elements as Active Copies
Although all the Tc1–2_Xt cDNA copies we analyzed do not contain a functional transposase open reading frame, 5 out of 116 Tc1–2_Xt complete sequences in the genome could putatively code for an open reading frame containing the catalytic triad and perhaps an active transposase (Fig. 1A). We don’t know if these five copies contain all the other residues required for transposition, such us the binding to DNA domains. However, the presence of active copies in the genome and its possible expression cannot be ruled out.
Interestingly, work from the Gage laboratory showed that endogenous LINE-1 retrotransposition can occur during mouse development.25 In addition, LINE-1 retrotransposition in the vicinity of neural genes can alter the expression of these genes in neural precursor cells. Furthermore, the same group showed that LINE-1 retrotranspositions can also occur in neural progenitor cells isolated from human fetal brain, suggesting that de novo LINE-1 retrotransposition events may occur in the human brain.26 These events produce mosaicism in the neurons due to different genomic modifications on different neurons in the same individual. This has been suggested as a novel mechanism involved in the generation of the astonishing neuronal diversity required for nervous system formation.25-27 Therefore, the specific expression of Tc1–2_Xt (and TXr and TXz) in dorsal and neural tissues allows speculation about a similar role in Xenopus. As active elements for transposition, these transposons could be involved in the generation of heterogeneity during Xenopus nervous system development.
Experimental Approaches to Study the Role of Transposable Elements during Development
In this section we will briefly discuss possible experimental approaches to evaluate transposition of Tc1 elements during nervous system development and determine its possible contribution to produce neuronal diversity.
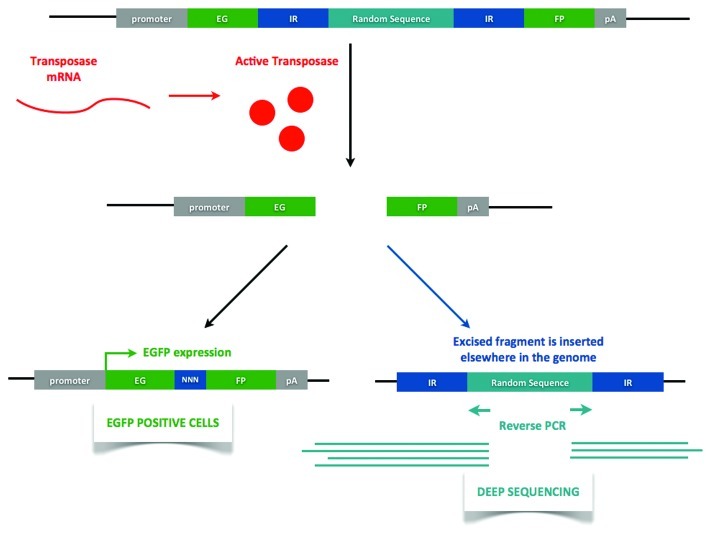
One of the first questions is to determine whether expression of an active DNA transposase occurs during early development. After cloning the possible candidates, transposase activity must first be determined by in vitro assays.28 For this, a plasmid reporter for excision events needs to be prepared. Based upon the white-peach allele studied in Drosophila,29 we devised the plasmidial DNA excision reporter construct shown in Figure 2. The egfp open reading frame contains an insertion of a random sequence (same length as the transposase) in between of the inverted repeats the putative transposase recognizes. Therefore, when co-expressed with the transposase mRNA in an exogenous system (e.g., cell culture), successful translation into an active transposase would render EGFP+ cells. Excision footprints would have to be devised in frame with the egfp gene.
Figure 2. Transposition reporter construct. The reporter construct contains an egfp open reading frame with an insertion of a random sequence (same length as the original transposase). When transposase mRNA is present (either exogenous, when working in vitro, or endogenous, when in vivo) and an active transposase is being translated, transposition will occur, rendering EGFP+ cells. Note that transposition footprint is in frame with the egfp gene. The random sequence insertion sites can then be characterized using deep sequencing of reverse PCR amplicons. IR- inverted repeat. EGFP-enhanced green fluorescent protein. pA-polyadenylation signal. NNN-transposition footprint.
Experiments to demonstrate in vivo transposition could be performed. For this purpose, transgenic Xenopus embryos containing the reporter construct in Figure 2 can be obtained.30 A tissue-specific promoter would allow us to follow transposition in the central nervous system. The presence of an endogenously active transposase would render EGFP+ cells that we could observe in later developmental stages, such as stage 50 tadpoles. Furthermore, it would be possible to characterize transposon insertion sites in neural tissues of individual tadpoles using deep sequencing of reverse PCR amplicons (see Fig. 2). The comparison of insertion sites in neural tissues with non-neural tissues could be an indicator of in vivo transposition events.
All of these approaches can be useful to determine whether transposition events occur during Xenopus development. Although host cells contain mechanisms to avoid the expression of transposable elements, they could also be using these elements for cellular functions. A proper balance must exist to control these positive and negative effects of transposable elements. Our work has shown that the specific expression of Tc1 elements in neural tissues and suggests that transposable elements may play a role during the formation of the nervous system in vertebrates.
Acknowledgments
F.F. was a CONICYT Ph.D. fellow. This work was funded by FONDECYT grants 1080158 and 1110400.
Footnotes
Previously published online: www.landesbioscience.com/journals/mge/article/18550
References
- 1.Malone CD, Hannon GJ. Small RNAs as guardians of the genome. Cell. 2009;136:656–68. doi: 10.1016/j.cell.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girard A, Hannon GJ. Conserved themes in small-RNA-mediated transposon control. Trends Cell Biol. 2008;18:136–48. doi: 10.1016/j.tcb.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkhurst SM, Corces VG. Developmental expression of Drosophila melanogaster retrovirus-like transposable elements. EMBO J. 1987;6:419–24. doi: 10.1002/j.1460-2075.1987.tb04771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brookman JJ, Toosy AT, Shashidhara LS, White RA. The 412 retrotransposon and the development of gonadal mesoderm in Drosophila. Development. 1992;116:1185–92. doi: 10.1242/dev.116.4.1185. [DOI] [PubMed] [Google Scholar]
- 5.Peaston AE, Evsikov AV, Graber JH, de Vries WN, Holbrook AE, Solter D, et al. Retrotransposons regulate host genes in mouse oocytes and preimplantation embryos. Dev Cell. 2004;7:597–606. doi: 10.1016/j.devcel.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Greene JM, Otani H, Good PJ, Dawid IB. A novel family of retrotransposon-like elements in Xenopus laevis with a transcript inducible by two growth factors. Nucleic Acids Res. 1993;21:2375–81. doi: 10.1093/nar/21.10.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shim S, Lee SK, Han JK. A novel family of retrotransposons in Xenopus with a developmentally regulated expression. Genesis. 2000;26:198–207. doi: 10.1002/(SICI)1526-968X(200003)26:3<198::AID-GENE5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Faunes F, Sanchez N, Castellanos J, Vergara IA, Melo F, Larrain J. Identification of novel transcripts with differential dorso-ventral expression in Xenopus gastrula using serial analysis of gene expression. Genome Biol. 2009;10:R15. doi: 10.1186/gb-2009-10-2-r15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faunes F, Sanchez N, Moreno M, Olivares GH, Lee-Liu D, Almonacid L, et al. Expression of Transposable Elements in Neural Tissues during Xenopus Development. PLoS ONE. 2011;6:e22569. doi: 10.1371/journal.pone.0022569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park EC, Hayata T, Cho KW, Han JK. Xenopus cDNA microarray identification of genes with endodermal organ expression. Dev Dyn. 2007;236:1633–49. doi: 10.1002/dvdy.21167. [DOI] [PubMed] [Google Scholar]
- 11.Nandi S, Peatman E, Xu P, Wang S, Li P, Liu Z. Repeat structure of the catfish genome: a genomic and transcriptomic assessment of Tc1-like transposon elements in channel catfish (Ictalurus punctatus) Genetica. 2007;131:81–90. doi: 10.1007/s10709-006-9115-4. [DOI] [PubMed] [Google Scholar]
- 12.de Boer JG, Yazawa R, Davidson WS, Koop BF. Bursts and horizontal evolution of DNA transposons in the speciation of pseudotetraploid salmonids. BMC Genomics. 2007;8:422. doi: 10.1186/1471-2164-8-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arends HM, Jehle JA. Sequence analysis and quantification of transposase cDNAs of transposon TCp3.2 in Cydia pomonella larvae. Arch Insect Biochem Physiol. 2006;63:135–45. doi: 10.1002/arch.20149. [DOI] [PubMed] [Google Scholar]
- 14.Krasnov A, Koskinen H, Afanasyev S, Molsa H. Transcribed Tc1-like transposons in salmonid fish. BMC Genomics. 2005;6:107. doi: 10.1186/1471-2164-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sijen T, Plasterk RH. Transposon silencing in the Caenorhabditis elegans germ line by natural RNAi. Nature. 2003;426:310–4. doi: 10.1038/nature02107. [DOI] [PubMed] [Google Scholar]
- 16.Göttgens B, Barton LM, Grafham D, Vaudin M, Green AR. Tdr2, a new zebrafish transposon of the Tc1 family. Gene. 1999;239:373–9. doi: 10.1016/S0378-1119(99)00390-X. [DOI] [PubMed] [Google Scholar]
- 17.Hellsten U, Harland RM, Gilchrist MJ, Hendrix D, Jurka J, Kapitonov V, et al. The genome of the Western clawed frog Xenopus tropicalis. Science. 2010;328:633–6. doi: 10.1126/science.1183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinzelle L, Pollet N, Bigot Y, Mazabraud A. Characterization of multiple lineages of Tc1-like elements within the genome of the amphibian Xenopus tropicalis. Gene. 2005;349:187–96. doi: 10.1016/j.gene.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Lam WL, Seo P, Robison K, Virk S, Gilbert W. Discovery of amphibian Tc1-like transposon families. J Mol Biol. 1996;257:359–66. doi: 10.1006/jmbi.1996.0168. [DOI] [PubMed] [Google Scholar]
- 20.Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 21.Lunyak VV, Prefontaine GG, Nunez E, Cramer T, Ju BG, Ohgi KA, et al. Developmentally regulated activation of a SINE B2 repeat as a domain boundary in organogenesis. Science. 2007;317:248–51. doi: 10.1126/science.1140871. [DOI] [PubMed] [Google Scholar]
- 22.Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, et al. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science. 2007;315:1587–90. doi: 10.1126/science.1140494. [DOI] [PubMed] [Google Scholar]
- 23.Rouget C, Papin C, Boureux A, Meunier AC, Franco B, Robine N, et al. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature. 2010;467:1128–32. doi: 10.1038/nature09465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee EJ, Banerjee S, Zhou H, Jammalamadaka A, Arcila M, Manjunath BS, et al. Identification of piRNAs in the central nervous system. RNA. 2011;17:1090–9. doi: 10.1261/rna.2565011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muotri AR, Chu VT, Marchetto MC, Deng W, Moran JV, Gage FH. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature. 2005;435:903–10. doi: 10.1038/nature03663. [DOI] [PubMed] [Google Scholar]
- 26.Coufal NG, Garcia-Perez JL, Peng GE, Yeo GW, Mu Y, Lovci MT, et al. L1 retrotransposition in human neural progenitor cells. Nature. 2009;460:1127–31. doi: 10.1038/nature08248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muotri AR, Zhao C, Marchetto MC, Gage FH. Environmental influence on L1 retrotransposons in the adult hippocampus. Hippocampus. 2009;19:1002–7. doi: 10.1002/hipo.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark KJ, Carlson DF, Leaver MJ, Foster LK, Fahrenkrug SC. Passport, a native Tc1 transposon from flatfish, is functionally active in vertebrate cells. Nucleic Acids Res. 2009;37:1239–47. doi: 10.1093/nar/gkn1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medhora M, Maruyama K, Hartl DL. Molecular and functional analysis of the mariner mutator element Mos1 in Drosophila. Genetics. 1991;128:311–8. doi: 10.1093/genetics/128.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–83. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]