Abstract
We recently discovered that the major mammalian bile acid, taurocholate, accelerated polarity in primary rat hepatocytes. Taurocholate increased cellular cAMP and signals through an Epac-Rap1-MEK-LKB1-AMPK pathway for its polarity effect. This review discusses possible mechanisms for how taurocholate affects different cell polarity factors, particularly AMPK, and thereby regulates events that generate polarity. These include tight junction formation, apical trafficking, recycling endosome dynamics, and cytoskeleton rearrangement. We also discuss whether the effects of taurocholate are mediated by other LKB1 downstream kinases, such as Par1 and NUAK1.
Keywords: apical trafficking, bile acid, bile canalicular formation, hepatocyte, polarity, tight junction
Introduction
Hepatocytes are the major epithelial cells in the liver, comprise 70–80% of the liver's cytoplasmic mass, and are polarized. The polarized surfaces of hepatocytes consist of a basolateral domain facing the circulation and an apical domain that forms the bile canaliculus, the smallest branch of the biliary tree. Tight junction proteins, including occludin, claudin and ZO-1, seal the apical domains of two adjacent hepatocytes, helping to create the bile canaliculus.1,2 A major function of the liver is biliary secretion which requires hepatocyte polarization. Loss of polarity causes bile secretory failure (cholestasis) and subsequent liver damage due to bile acid retention.1 The mechanisms controlling hepatocyte polarization are partially understood. Structurally they include cytoskeletal, tight junctional and intracellular trafficking components.3-8
Taurocholate, the major bile acid, is synthesized from cholesterol in hepatocytes and secreted into the canaliculus by ABCB11 (BSEP), an ATP binding cassette (ABC) transporter which couples ATP hydrolysis to transport.9 Approximately 85% of bile acids are absorbed and transported back to the liver via the enterohepatic circulation.10 The traditional function of bile acids is to emulsify dietary fat;11 however, bile acids are also signaling molecules,12,13 which are involved in many signaling pathways including increasing cellular cAMP10,12 activating protein kinase C,14 nuclear farnesoid X receptor (FXR) and pregnane X receptors (PXR),10,12 PI3K/AKT/glycogen synthase kinase 3 (GSK3),15 and also enhance liver regeneration.16 These properties of bile acids and their polarized secretion suggest that hepatocytes respond to bile acid. Recent work has shown that bile acids, including taurocholate regulate hepatocyte polarization.17 Here, we discuss possible mechanisms.
Taurocholate Accelerates Hepatocyte Polarity in Primary Hepatocyte Sandwich Culture
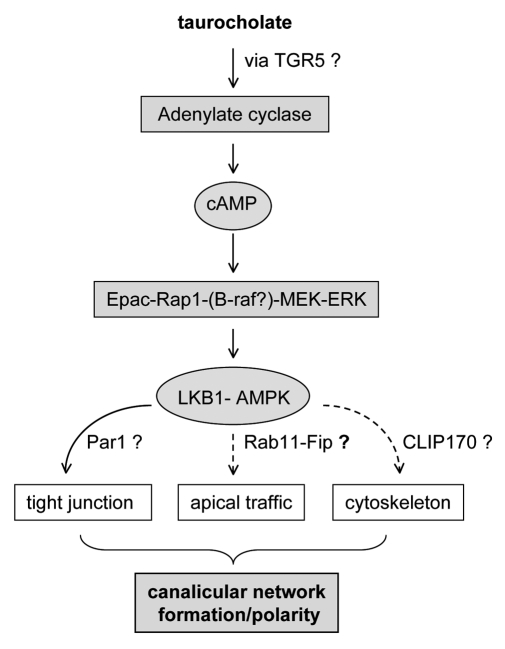
Recent work has revealed a novel and important role for bile acids in hepatocyte polarity.17 Addition of taurocholate to rat hepatocytes in a collagen sandwich culture system acceclerated polarization.17 Using pharmacological activators and inhibitors as well as dominant negative constructs, we discovered that the taurocholate effect on bile canalicular formation requires activtion of adenyl cyclase and cAMP downstream kinase, Epac (Exchange Protein Activated by cAMP), and signaling through the downstream kinases Rap1-MEK pathway resulting in activation of LKB1 (Liver Kinase B1) and AMPK (AMP-activated Protein Kinase) (Fig. 1). This study links taurocholate with Epac and LKB1-AMPK, the key cellular metobolic kinases. Mass spectroscopic study of lysates of cultured hepatocytes revealed detectable levels of endogenous bile acids that increased by day 3 in culture, which correlates with canalicular development. These observations suggest that endogenous bile acids may participate in normal polarity development in hepatocytes. Interestingly, during liver development, the fetal hepatocytes are not fully polarized and only have very small canaliculi, and bile acid synthesis is sparse. However, hepatocytes rapidly polarize shortly after birth in parallel with increased bile acid syntheses in the liver.
Figure 1. Signal pathway by which taurocholate accelerates hepatocyte polarity.
Possible Effect on Tight Junction Assembly
How LKB1-AMPK regulates polarization in primary hepatocyte culture is not known. Several studies suggest that AMPK regulates the tight junction assembly required for polarity.18,19 Inhibition of AMPK by overexpression of dominant negative-AMPK resulted in loss of tight junction structure and polarity.18,20 AMPK regulates myosin light chain (MLC) which may affect the actin cytoskeleton that is involved in tight junction formation.21 In addition, AMPK activation occurs in parallel with mitochondrial fusion, increased mitochondrial potential and ATP levels. These observations suggest that mitochondria are important in the effect of AMPK on metabolism and polarization. Thus, AMPK can regulate tight junction assembly through small GTPases directly or indirectly by altering the cellular energy status. The small GTPase RhoA promotes junction formation and apical constriction,22 and involves myosin II signaling.23 Another small GTPase, Rab13, is involved in tight junction assembly.24 In addition, Epac downstream Rap1 also regulates junction formation.25
Effect on Apical Trafficking/Recycling
Knockdown of Rab11a or overexpression of myosin Vb motorless tail domain prevented canalicular formation, suggesting that Rab11a is required for canalicular formation.8 This study revealed that polarization of hepatocytes requires recruitment of Rab11a and myosin Vb for targeting ABC transporters (e.g ABCB11) to the apical plasma membrane. The recycling pathway for ABCB11 was initially characterized in WIFB cells, a hybrid of rat hepatoma and human fibroblast that retain a spherical canalicular structure, and confirmed in hepatocytes in collagen sandwich cultures. Overexpression of dominant negative-Rab11A or motorless tail domain of myosin Vb inhibited trafficking of ABCB11 to the canalicular membrane; ABCB11 accumulated intracellularly and this was associated with loss of polarization.26 These observations confirm that Rab11a and myosin Vb dependent apical traffic is essential for hepatic polarity. Using FRAP (Fluorescent Recovery After Photobleaching) analysis, our study revealed that taurocholate enhances delivery of ABCB11 to the canalicular membrane in primary hepatocytes. Moreover, our recent FRAP studies of hepatocytes from LKB1 knockout mice showed loss of taurocholate-enhanced ABCB11 trafficking to the apical membrane, suggesting that taurocholate enhancement of ABCB11 trafficking is LKB1 dependent. How taurocholate facilitates apical protein trafficking is under investigation. After activation by taurocholate, LKB1-AMPK may interact with Rab11a either directly or indirectly via Rab11a-Fip1 proteins (Rab11 family-interacting protein 1). Rab11a-Flp1 is required for the endosomal recycling process. Rab11a-Fip1 has constitutive AMPK phosphorylation sites. LKB1-AMPK may interact with Rab11a-Fip1 to regulate Rab11a activity and modulate apical protein trafficking. We are currently studying possible interactions of AMPK and Rab11a-FIPs proteins.
Effect on Cytoskeleton
AMPK phosphorylates microtubule plus end protein CLIP-170, which is essential for polymerization and depolymerization of microtubules. AMPK induced phosphorylation of CLIP-170 is important for lamellipodium formation and adhesion maturation, which is important for cell polarity.27 Taurocholate may activate AMPK-CLIP170 thereby inducing cytoskeleton arrangement for polarization. In addition, proteomic studis suggest that AMPK can interact with β 2 tubulin and gamma actin, indicating a role for AMPK in cytoskeleton arrangement.28 Moreover, microtubule plus-ends can interact with the actin cytoskeleton which is required for apical protein trafficking/recycling. Recycling endosomes containing ABCB11, move along microtubules and are eventually transferred to an actin-based system. Therefore, taurocholate may accelerate apical traffic through activation of AMPK-CLIP170. Whether taurocholate increases phosphorylation of CLIP-170 is under examination in our lab.
LKB1 Downstream Kinases Par1 and NUAK1 do not Mediate the Taurocholate Effect
In addition to AMPK, LKB1 also activates Par1, a downstream kinase involved in polarization.29 Hepatocytes lost polarity when they were infected with dominant negative Par-1-GFP adenovirus. However, taurocholate did not rescue polarity in dominant negative-Par1 positive cells, suggesting that taurocholate may not affect the LKB1-Par1 pathway.
LKB1 phosphorylates and activates the AMPK-related kinase NUAK1 resulting in increased phosphorylation of MLC2 (myosin light chain 2) and activation of myosin II.30 Since myosin II is required for actin cytoskeleton arrangements as cells polarize,3,7 we also tested whether NUAK1 mediates the taurocholate effect on hepatocyte polarity. BX795, an inhibitor of NUAK1 (5 to 100 nM, IC50 = 5 nM), did not affect taurocholate accelerated canalicular formation in hepatocytes, suggesting that taurocholate may not signal through NUAK1 after activation of LKB1 for its effect on canalicular formation.
The mechanism whereby taurocholate activates adenyl cyclase in hepatocytes is uncertain. In other cells bile acids activate adenyl cyclase by interacting with the G-protein coupled receptor, TGR-5; however, it is controversial whether hepatocytes express TGR-5. We are investigating TGR5 function in hepatoyctes using TGR5 knockout mouse liver and specific TGR-5 activator, INF-777.
Conclusion
Bile acids play multiple roles in many cellular events. Taurocholate has a novel role in hepatocyte polarity linked to its effect on cellular energy metabolism, through AMPK. Given their diverse pharmacological effects, bile acids may accelerate hepatocyte polarity through multiple downstream effectors that regulate tight junction formation, apical trafficking and cytoskeleton rearrangements occurring during cell polarization (Fig. 1). Given their novel role in hepotocyte polarity, bile acids may be potential therapeutics for polarity relevant disorders.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/18087
References
- 1.Vinken M, Papeleu P, Snykers S, De Rop E, Henkens T, Chipman JK, et al. Involvement of cell junctions in hepatocyte culture functionality. Crit Rev Toxicol. 2006;36:299–318. doi: 10.1080/10408440600599273. [DOI] [PubMed] [Google Scholar]
- 2.Kojima T, Yamamoto T, Murata M, Chiba H, Kokai Y, Sawada N. Regulation of the blood-biliary barrier: interaction between gap and tight junctions in hepatocytes. Med Electron Microsc. 2003;36:157–64. doi: 10.1007/s00795-003-0220-5. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–47. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Boulan E, Salas PJ. External and internal signals for epithelial cell surface polarization. Annu Rev Physiol. 1989;51:741–54. doi: 10.1146/annurev.ph.51.030189.003521. [DOI] [PubMed] [Google Scholar]
- 5.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol. 2003;5:287–93. doi: 10.1038/ncb0403-287. [DOI] [PubMed] [Google Scholar]
- 7.Hoekstra D, Tyteca D. van ISC. The subapical compartment: a traffic center in membrane polarity development. J Cell Sci. 2004;117:2183–92. doi: 10.1242/jcs.01217. [DOI] [PubMed] [Google Scholar]
- 8.Wakabayashi Y, Dutt P, Lippincott-Schwartz J, Arias IM. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc Natl Acad Sci USA. 2005;102:15087–92. doi: 10.1073/pnas.0503702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, et al. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem. 1998;273:10046–50. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- 10.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–93. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65:2461–83. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen A, Bouscarel B. Bile acids and signal transduction: role in glucose homeostasis. Cell Signal. 2008;20:2180–97. doi: 10.1016/j.cellsig.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25:1419–25. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Looby E, Long A, Kelleher D, Volkov Y. Bile acid deoxycholate induces differential subcellular localisation of the PKC isoenzymes beta 1, epsilon and delta in colonic epithelial cells in a sodium butyrate insensitive manner. Int J Cancer. 2005;114:887–95. doi: 10.1002/ijc.20803. [DOI] [PubMed] [Google Scholar]
- 15.Fang Y, Studer E, Mitchell C, Grant S, Pandak WM, Hylemon PB, et al. Conjugated bile acids regulate hepatocyte glycogen synthase activity in vitro and in vivo via Galphai signaling. Mol Pharmacol. 2007;71:1122–8. doi: 10.1124/mol.106.032060. [DOI] [PubMed] [Google Scholar]
- 16.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–6. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 17.Fu D, Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Bile acid stimulates hepatocyte polarization through a cAMP-Epac-MEK-LKB1-AMPK pathway. Proc Natl Acad Sci USA. 2011;108:1403–8. doi: 10.1073/pnas.1018376108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci USA. 2007;104:819–22. doi: 10.1073/pnas.0610157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci USA. 2006;103:17272–7. doi: 10.1073/pnas.0608531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu D, Wakabayashi Y, Ido Y, Lippincott-Schwartz J, Arias IM. Regulation of bile canalicular network formation and maintenance by AMP-activated protein kinase and LKB1. J Cell Sci. 2010;123:3294–302. doi: 10.1242/jcs.068098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitonaka T, Muramatsu Y, Sugiyama S, Mizuno T, Nishida Y. Essential roles of myosin phosphatase in the maintenance of epithelial cell integrity of Drosophila imaginal disc cells. Dev Biol. 2007;309:78–86. doi: 10.1016/j.ydbio.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, et al. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol. 2011;13:159–66. doi: 10.1038/ncb2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong K, Van Keymeulen A, Bourne HR. PDZRhoGEF and myosin II localize RhoA activity to the back of polarizing neutrophil-like cells. J Cell Biol. 2007;179:1141–8. doi: 10.1083/jcb.200706167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marzesco AM, Dunia I, Pandjaitan R, Recouvreur M, Dauzonne D, Benedetti EL, et al. The small GTPase Rab13 regulates assembly of functional tight junctions in epithelial cells. Mol Biol Cell. 2002;13:1819–31. doi: 10.1091/mbc.02-02-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bos JL, de Rooij J, Reedquist KA. Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol. 2001;2:369–77. doi: 10.1038/35073073. [DOI] [PubMed] [Google Scholar]
- 26.Wakabayashi Y, Fu D, Lippincott-Schwartz J, Arias IM. Rab11A and myosin VB are required for canalicular network formation and ABC transporter cycling in primary rat hepatocytes. Hepatology. 2009;50:378A. [Google Scholar]
- 27.Nakano A, Kato H, Watanabe T, Min KD, Yamazaki S, Asano Y, et al. AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol. 2010;12:583–90. doi: 10.1038/ncb2060. [DOI] [PubMed] [Google Scholar]
- 28.Tuerk RD, Thali RF, Auchli Y, Rechsteiner H, Brunisholz RA, Schlattner U, et al. New candidate targets of AMP-activated protein kinase in murine brain revealed by a novel multidimensional substrate-screen for protein kinases. J Proteome Res. 2007;6:3266–77. doi: 10.1021/pr070160a. [DOI] [PubMed] [Google Scholar]
- 29.Cohen D, Fernandez D, Lazaro-Dieguez F, Musch A. The serine/threonine kinase Par1b regulates epithelial lumen polarity via IRSp53-mediated cell-ECM signaling. J Cell Biol. 2011;192:525–40. doi: 10.1083/jcb.201007002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zagórska A, Deak M, Campbell DG, Banerjee S, Hirano M, Aizawa S, et al. New roles for the LKB1-NUAK pathway in controlling myosin phosphatase complexes and cell adhesion. Sci Signal. 2010;3:ra25. doi: 10.1126/scisignal.2000616. [DOI] [PubMed] [Google Scholar]